Higher effector T-cell numbers and their rejuvenated cytotoxic function accompanies favorable clinical responses to ibrutinib-rituximab.
Enhanced CD8+ T-cell lytic synapse activity during ibrutinib-rituximab therapy can be exploited using the bispecific antibody glofitamab.
Visual Abstract
Bruton tyrosine kinase inhibitors (BTKis) that target B-cell receptor signaling have led to a paradigm shift in chronic lymphocytic leukemia (CLL) treatment. BTKis have been shown to reduce abnormally high CLL-associated T-cell counts and the expression of immune checkpoint receptors concomitantly with tumor reduction. However, the impact of BTKi therapy on T-cell function has not been fully characterized. Here, we performed longitudinal immunophenotypic and functional analysis of pretreatment and on-treatment (6 and 12 months) peripheral blood samples from patients in the phase 3 E1912 trial comparing ibrutinib-rituximab with fludarabine, cyclophosphamide, and rituximab (FCR). Intriguingly, we report that despite reduced overall T-cell counts; higher numbers of T cells, including effector CD8+ subsets at baseline and at the 6-month time point, associated with no infections; and favorable progression-free survival in the ibrutinib-rituximab arm. Assays demonstrated enhanced anti-CLL T-cell killing function during ibrutinib-rituximab treatment, including a switch from predominantly CD4+ T-cell:CLL immune synapses at baseline to increased CD8+ lytic synapses on-therapy. Conversely, in the FCR arm, higher T-cell numbers correlated with adverse clinical responses and showed no functional improvement. We further demonstrate the potential of exploiting rejuvenated T-cell cytotoxicity during ibrutinib-rituximab treatment, using the bispecific antibody glofitamab, supporting combination immunotherapy approaches.
Introduction
E1912 was the first frontline phase 3 study to compare ibrutinib-based therapy (ibrutinib with rituximab) with the chemoimmunotherapy fludarabine, cyclophosphamide, and rituximab (FCR).1 Long-term follow-up has demonstrated superior progression-free survival (PFS) and overall survival for ibrutinib-rituximab relative to those for FCR.2 Nevertheless, clinical challenges remain, including the current need for continuous therapy, tolerability, and residual and progressive disease. Immunotherapy represents a powerful combination or alternative therapy to tackle resistant disease and deepen responses.3,4 However, T-cell exhaustion is a major barrier for optimal immunotherapy.5,6 Dysfunctionality of T cells is characterized by increased chronic lymphocytic leukemia (CLL)-associated T-cell subsets expressing inhibitory checkpoint molecules.7 Furthermore, helper CD4+ T cells have a tumor-promoting capacity, whereas impaired immune synapse formation contributes to suppressed CD8+ T-cell cytotoxicity.8 Correlative studies have revealed that BTKis reduce abnormally high T-cell numbers and checkpoint receptor expression while reducing malignant B cells.9-13 However, the impact of BTKis on T-cell function and association with clinical response is less well defined. Here, we leverage pretreatment and on-treatment peripheral blood patient samples serially collected from the E1912 trial and report on the impact of ibrutinib-rituximab vs FCR on T cells using immune monitoring functional assays.
Study design
Samples from patients with CLL
Viable peripheral blood mononuclear cells at baseline and 6-, 12-, and 18-month time points from the Eastern Cooperative Oncology Group–American College of Radiology Imaging Network (ECOG-ACRIN)-E1912 Cancer Research Group (supplemental Figure 1, available on the Blood website) were biobanked for longitudinal comparative immune analysis. supplemental Table1,2 summarizes the samples studied (ibrutinib-rituximab, n = 89; FCR, n = 62).
Immune monitoring and functional assays are detailed in supplemental Methods.
All patient samples were obtained after written informed consent, in accordance with the declaration of Helsinki, and approved by the National Research Ethics Committees, ECOG-ACRIN and the National Institutes of Health.
Results and discussion
T-cell monitoring and correlation with PFS, MRD, and infections
We initially investigated the impact of therapy on T cells and explored the association with clinical outcome (Figure 1A). Flow cytometry measured the absolute numbers of naive (CD45RA+/CCR7+), central memory (CD45RA−/CCR7+), effector memory (TEM; CD45RA−/CCR7−) and terminally differentiated effector memory (CD45RA+/CCR7−) subsets in patients at baseline and 6- and 12-month treatment time points (supplemental Table 3). This analysis revealed a reduction in the majority of CD4+ and CD8+ T-cell subsets, including naive and effectors, during ibrutinib-rituximab treatment (Figure 1B; supplemental Figure 2A,C), consistent with T-cell normalization, as previously reported for monotherapy.10,13 Expectedly, we observed a marked decrease of subsets after FCR, with evidence of immune reconstitution at 12 months (Figure 1B; supplemental Figure 2B,D).14 The frequencies of subsets remained relatively stable during ibrutinib-rituximab treatment, whereas FCR caused naive and central memory subsets to contract, whereas TEM expanded (supplemental Figure 2E-F). Both therapies reduced the number of regulatory T cells, T helper 17 (TH17) cells, and natural killer (NK) cells compared with those at baseline, but an increased the regulatory T-cell to CD4 ratio after FCR was observed (supplemental Figure 3A-C).15 Strikingly, patients on ibrutinib-rituximab with higher T-cell numbers, including PD-1+ effector CD8+ and CD4+ subsets, at baseline had longer PFS (Figure 1D,F), suggesting the importance of an existent but exhausted immune response before therapy. Interestingly, higher levels of PD-L1–expressing CLL cells at baseline correlated with favorable PFS (Figure 1D,F; multivariable analysis in supplemental Table 4). Furthermore, an elevated frequency of effector CD8+ T cells at the 6-month ibrutinib-rituximab time point associated with favorable PFS, whereas no association was detectable at 12 months (Figure 1D,F). Conversely, higher T-cell numbers correlated with worse PFS in the FCR arm, whereas increased NK-cell frequency at baseline associated with favorable outcome (supplemental Figure 4A). Consistent with tumor-mediated exhaustion, greater numbers of PD-1+ and PD-L1+ CD8+ T-cell subsets associated with higher measurable residual disease (MRD) during ibrutinib-rituximab treatment (supplemental Figure 5A). In contrast, elevated frequencies of T-cell subsets not expressing checkpoint molecules, including CD8+ terminally differentiated effector memory and NK cells, correlated with low MRD during ibrutinib-rituximab treatment, in keeping with reduced exhaustion. An association between T cells and MRD was less evident in the FCR arm, except for increased checkpoint-expressing T cells at 12 months, which correlated with higher MRD (supplemental Figure 5B). Ibrutinib’s inhibition of interleukin-2–inducible T-cell kinase (ITK) enhanced TH1 polarization,16,17 but both therapeutic arms reduced TH1 and TH2 numbers and TH1:TH2 ratios (supplemental Figure 6A-B). Nevertheless, an increased frequency of TH2 and CD4:CD8 ratio (baseline and 6 months) associated with unfavorable PFS and incidence of infection, respectively, during ibrutinib-rituximab treatment (Figure 1F-G). However, increased effector CD8+ T-cell numbers and CD16+ NK cells at 6 months were associated with no infections during ibrutinib-rituximab treatment (Figure 1G). Conversely, increased T cells after FCR correlated with infections (supplemental Figure 4B). In sum, higher CD8+ T-cell numbers at baseline and early on-therapy, associated with favorable clinical responses, whereas PD-1+ and PD-L1+ subsets associated with greater MRD during ibrutinib-rituximab treatment.
Higher CD8+T-cell numbers at baseline and early on-therapy associate with favorable PFS and no infections with ibrutinib-rituxumab. (A) Schematic representation of the E1912 trial and biobanked peripheral blood mononuclear cell samples collected at baseline (B/L) and 6- (6M) and 12- (12M) month time points for correlative T-cell analysis. PFS, infection, and MRD clinical outcome data were collected. (B) Absolute numbers of CD8+ TEM cell subsets (CD45RA−CCR7−) for ibrutinib-rituximab (n = 86 patients) and FCR (n = 50) at the time points indicated. Patient data are presented as Box and whiskers (10-90 percentile; log scale) plots. (C) Percentage of PD-1+ CD8+ TEM subsets during ibrutinib-rituximab (n = 86) or FCR (n = 50) treatments. (B-C) Data are given as the mean ± standard error of the mean; statistical analysis between time points were assessed using the Wilcoxon signed-rank test. (D) Tabular schematic summary of the significant correlations (Cox model) between higher immune subset levels (flow cytometry, median values used as cut-off point) and PFS for patients on ibrutinib-rituximab (n = 88 patients with 13 experiencing disease progression). Green rows (correlations with hazard ratio [HR] values < 1) indicate higher immune subsets associated with longer PFS, whereas higher immune subsets associating with shorter PFS (HR > 1) are highlighted in blue rows. Confidence intervals (95%) and P values are shown. (E) Schematic summary of the significant correlations (Wilcoxon test) between immune subsets and infection (any infection) during ibrutinib-rituximab treatment (n = 88 patients). Negative t statistics (t.stat) indicate higher immune subset levels in patients who did not develop infection (green rows). In contrast, correlations with a positive t.stat indicate higher immune subset levels in patients who developed infection (blue rows). (F) Kaplan-Meier curves of immune subsets associated to good prognosis for the ibrutinib-rituximab arm. Higher levels of percentage PD-L1+ CD19+ cells (high: 12 progression events per 43 patients and low: 1 progression event per 43 patients), absolute number of PD-1+CD8+ TEM (high: 3 progression events per 43 patients and low: 10 progression events per 43 patients), and PD-1+CD4+ T cells (high: 3 progression events per 43 patients and low: 10 progression events per 43 patients) at baseline associate with longer PFS. Higher percentage of CD8+ TEM (high: 3 progression events per 42 patients and low: 10 progression events per 43 patients) at the 6-month time point associate with longer PFS. Absolute number data are referred to as “ab.” P values indicated. ∗P < .05; ∗∗P < .01; ∗∗∗∗P < .0001. n/s, not significant.
Higher CD8+T-cell numbers at baseline and early on-therapy associate with favorable PFS and no infections with ibrutinib-rituxumab. (A) Schematic representation of the E1912 trial and biobanked peripheral blood mononuclear cell samples collected at baseline (B/L) and 6- (6M) and 12- (12M) month time points for correlative T-cell analysis. PFS, infection, and MRD clinical outcome data were collected. (B) Absolute numbers of CD8+ TEM cell subsets (CD45RA−CCR7−) for ibrutinib-rituximab (n = 86 patients) and FCR (n = 50) at the time points indicated. Patient data are presented as Box and whiskers (10-90 percentile; log scale) plots. (C) Percentage of PD-1+ CD8+ TEM subsets during ibrutinib-rituximab (n = 86) or FCR (n = 50) treatments. (B-C) Data are given as the mean ± standard error of the mean; statistical analysis between time points were assessed using the Wilcoxon signed-rank test. (D) Tabular schematic summary of the significant correlations (Cox model) between higher immune subset levels (flow cytometry, median values used as cut-off point) and PFS for patients on ibrutinib-rituximab (n = 88 patients with 13 experiencing disease progression). Green rows (correlations with hazard ratio [HR] values < 1) indicate higher immune subsets associated with longer PFS, whereas higher immune subsets associating with shorter PFS (HR > 1) are highlighted in blue rows. Confidence intervals (95%) and P values are shown. (E) Schematic summary of the significant correlations (Wilcoxon test) between immune subsets and infection (any infection) during ibrutinib-rituximab treatment (n = 88 patients). Negative t statistics (t.stat) indicate higher immune subset levels in patients who did not develop infection (green rows). In contrast, correlations with a positive t.stat indicate higher immune subset levels in patients who developed infection (blue rows). (F) Kaplan-Meier curves of immune subsets associated to good prognosis for the ibrutinib-rituximab arm. Higher levels of percentage PD-L1+ CD19+ cells (high: 12 progression events per 43 patients and low: 1 progression event per 43 patients), absolute number of PD-1+CD8+ TEM (high: 3 progression events per 43 patients and low: 10 progression events per 43 patients), and PD-1+CD4+ T cells (high: 3 progression events per 43 patients and low: 10 progression events per 43 patients) at baseline associate with longer PFS. Higher percentage of CD8+ TEM (high: 3 progression events per 42 patients and low: 10 progression events per 43 patients) at the 6-month time point associate with longer PFS. Absolute number data are referred to as “ab.” P values indicated. ∗P < .05; ∗∗P < .01; ∗∗∗∗P < .0001. n/s, not significant.
Ibrutinib-rituximab promotes CD8+ synapses and immunotherapy-triggered killing function
Next, we characterized the cytolytic function of therapy-reshaped T cells against baseline CLL cells (Figure 2A-B). T cells from both 6- and 12-month ibrutinib-rituximab time points showed enhanced killing function compared with those at pretreatment levels. In contrast, T cells after FCR showed no cytolytic improvement. Notably, patients who experienced grade 3 infections during ibrutinib-rituximab treatment showed lower anti-CLL T-cell cytotoxic function (Figure 2C). Hypothesizing altered T-cell:CLL interactions, we then performed conjugation assays. T cells during ibrutinib-rituximab treatment showed augmented formation of polarized F-actin synapses with baseline CLL cells (Figure 2D; supplemental Figure 7A). In comparison, T cells after FCR exhibited distinctly nonpolarized synapses (Figure 2E). Given the opposing roles of patient CD4+ and CD8+ T cells,8 we examined these subsets and detected an increased frequency of granzyme B+ CD8+ T-cell:CLL synapses at both ibrutinib-rituximab time points compared with that at baseline, in which CD4+ T-cell:CLL synapses dominated. This switch in the CD4+:CD8+ synapse balance was not detected after FCR (Figure 2F-G; supplemental Figure 7B-D). Interestingly, in keeping with protumoral CD4+ T cells, increased formation of CD4+ T-cell:CLL F-actin–positive synapses at baseline correlated with unfavorable PFS and grade 3 infections during ibrutinib-rituximab administration (Figure 2H; supplemental Figure 7E). Together, these data demonstrate that ibrutinib-rituximab promotes previously exhausted CD8+ T-cell activity, which could provide a gateway for immunotherapy.
Ibrutinib-rituximab promotes CD8+ T-cell lytic synapse activity and supports immunotherapy-triggered anti-CLL killing function. (A) Illustration of the autologous cytotoxicity assay using anti-CD3/-CD28–activated T cells (cytolytic T lymphocytes [CTLs]) from B/L, 6M, and 12M time points mixed with target B/L CLL B cells (pulsed with superantigen as a model antigen) with flow-based quantification of T-cell killing function. (B) T-cell–mediated CLL cell death comparing T cells purified from B/L, 6M, and 12M time point samples (n = 30 patients per treatment arm). Data at 6M and 12M were normalized to B/L levels to generate fold change values for each patient. (C) The association between patient’s T-cell killing function (12M ibrutinib-rituximab time point, n = 30) and infection status during ibrutinib-rituximab therapy (no infections vs grade 2 or 3 infections) (Wilcoxon test, P = .01). (D-E) Representative confocal medial optical section and 3-dimensional (3D) volume–rendered images of T-cell:CLL conjugates formed between patient T cells (B/L, 6M, and 12M on-ibrutinib-rituximab [D] or FCR [E]) interacting with autologous B/L CLL B cells (blue, CMAC dyed). Bar charts: quantitative relative recruitment index (RRI) analysis of F-actin polarization (red, rhodamine phalloidin) in T-cell:CLL conjugates (n = 50 patients per treatment arm). (F) Box and violin plots (minimum-maximum) showing the percentage of CD4+ or CD8+ T-cell:CLL conjugates formed from the total T-cell:CLL conjugates in B/L, 6M, and 12M ibrutinib-rituximab time point samples (n = 15 patients). Representative confocal images of CD8+ (white) and CD4+ (green) T-cell conjugates with CLL B cells (blue) at B/L vs on ibrutinib-rituximab therapy. (G) Representative confocal 3D volume–rendered images of granzyme B (GrB; white) expression at CD8+ T-cell synapses, comparing ibrutinib-rituximab and FCR 12M time point samples. (H) Kaplan-Meier curve showing the association between the strength of polarized F-actin CD4+ T-cell:CLL immune synapse interactions in patient B/L samples and their PFS outcomes during ibrutinib-rituximab administration. Median F-actin RRI values were used as a cut-off point to determine weak (<median RRI) vs strong (>median RRI) CD4+ T-cell synapses (n = 52 patients). Patients’ showing strong CD4+ T-cell:CLL immune synapses at B/L showed significantly adverse PFS (9 progression events per 29 patients) compared with patients showing weak CD4+ T-cell:CLL interactions (1 progression event per 23 patients). (Cox model, P = .01; HR, 9.14; 95% confidence interval, 1.15-72.47). Bar chart: F-actin RRI analysis of CD4+ T-cell:CLL conjugates at B/L and representative 3D volume–rendered confocal images comparing patients who progressed (n = 7) with those who did not (progression free, n = 7) during ibrutinib-rituximab therapy. (I) Illustration of the cytotoxicity assay after ex vivo treatment of purified T cells (B/L, 6M, and 12M time points) and B/L CLL cells with anti–PD-1 (αPD-1) or anti–PD-L1 (αPD-L1) blocking antibodies (10 μg/mL) or isotype controls. (J-K) T-cell killing function against autologous B/L CLL cells examining T cells at B/L or at the 6-month ibrutinib-rituximab (orange) or FCR (blue) time points after ex vivo treatment with (J) αPD-1 or isotype control (indicated using “−”) (B/L: n = 6, ibrutinib-rituximab: n = 13, and FCR: n = 15) or (K) αPD-L1 or isotype control (−) (B/L: n = 6, ibrutinib-rituximab: n = 23, and FCR: n = 13). (L) Illustration of the autologous cytotoxicity assay incorporating the CD20 × CD3 glofitamab or a nonbinding antibody control. (M-N) T-cell–mediated CLL cell death using purified T cells from B/L, 6M, 12M, or 18M ibrutinib-rituximab (M) or FCR (N) time points against target B/L CLL B cells after ex vivo treatment with glofitamab (0.01 μg/mL) or nonbinding antibody control (indicated as “−”) (B/L, n = 13; ibrutinib-rituximab 6M and 12M,n = 6; ibrutinib-rituximab 18M, n = 5; FCR 6M and 12M, n = 7; and FCR 18M, n = 5 patient samples). Data for all cytotoxicity assay timepoints were normalized to isotype antibody control for panels J-K or nonbinding antibody control for panels M-N treated sample levels and presented as fold change data for each immunotherapy treated patient sample. Wilcoxon signed-rank test for panels B,D-E,J-K,M-N, multiple comparisons mixed effect analysis of variance for panel F and Mann-Whitney U test for panel H. Mann-Whitney U test was used to compare cell death among CD20-TCB–treated conditions at B/L, 6M, 12M, and 18M. Original magnification ×63; scale bars, 10 μm. Bar chart data are presented as mean ± standard error of the mean. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. CMAC, CellTracker Blue (7-amino-4-chloromethylcoumarin); TCB, T-cell bispecific antibody.
Ibrutinib-rituximab promotes CD8+ T-cell lytic synapse activity and supports immunotherapy-triggered anti-CLL killing function. (A) Illustration of the autologous cytotoxicity assay using anti-CD3/-CD28–activated T cells (cytolytic T lymphocytes [CTLs]) from B/L, 6M, and 12M time points mixed with target B/L CLL B cells (pulsed with superantigen as a model antigen) with flow-based quantification of T-cell killing function. (B) T-cell–mediated CLL cell death comparing T cells purified from B/L, 6M, and 12M time point samples (n = 30 patients per treatment arm). Data at 6M and 12M were normalized to B/L levels to generate fold change values for each patient. (C) The association between patient’s T-cell killing function (12M ibrutinib-rituximab time point, n = 30) and infection status during ibrutinib-rituximab therapy (no infections vs grade 2 or 3 infections) (Wilcoxon test, P = .01). (D-E) Representative confocal medial optical section and 3-dimensional (3D) volume–rendered images of T-cell:CLL conjugates formed between patient T cells (B/L, 6M, and 12M on-ibrutinib-rituximab [D] or FCR [E]) interacting with autologous B/L CLL B cells (blue, CMAC dyed). Bar charts: quantitative relative recruitment index (RRI) analysis of F-actin polarization (red, rhodamine phalloidin) in T-cell:CLL conjugates (n = 50 patients per treatment arm). (F) Box and violin plots (minimum-maximum) showing the percentage of CD4+ or CD8+ T-cell:CLL conjugates formed from the total T-cell:CLL conjugates in B/L, 6M, and 12M ibrutinib-rituximab time point samples (n = 15 patients). Representative confocal images of CD8+ (white) and CD4+ (green) T-cell conjugates with CLL B cells (blue) at B/L vs on ibrutinib-rituximab therapy. (G) Representative confocal 3D volume–rendered images of granzyme B (GrB; white) expression at CD8+ T-cell synapses, comparing ibrutinib-rituximab and FCR 12M time point samples. (H) Kaplan-Meier curve showing the association between the strength of polarized F-actin CD4+ T-cell:CLL immune synapse interactions in patient B/L samples and their PFS outcomes during ibrutinib-rituximab administration. Median F-actin RRI values were used as a cut-off point to determine weak (<median RRI) vs strong (>median RRI) CD4+ T-cell synapses (n = 52 patients). Patients’ showing strong CD4+ T-cell:CLL immune synapses at B/L showed significantly adverse PFS (9 progression events per 29 patients) compared with patients showing weak CD4+ T-cell:CLL interactions (1 progression event per 23 patients). (Cox model, P = .01; HR, 9.14; 95% confidence interval, 1.15-72.47). Bar chart: F-actin RRI analysis of CD4+ T-cell:CLL conjugates at B/L and representative 3D volume–rendered confocal images comparing patients who progressed (n = 7) with those who did not (progression free, n = 7) during ibrutinib-rituximab therapy. (I) Illustration of the cytotoxicity assay after ex vivo treatment of purified T cells (B/L, 6M, and 12M time points) and B/L CLL cells with anti–PD-1 (αPD-1) or anti–PD-L1 (αPD-L1) blocking antibodies (10 μg/mL) or isotype controls. (J-K) T-cell killing function against autologous B/L CLL cells examining T cells at B/L or at the 6-month ibrutinib-rituximab (orange) or FCR (blue) time points after ex vivo treatment with (J) αPD-1 or isotype control (indicated using “−”) (B/L: n = 6, ibrutinib-rituximab: n = 13, and FCR: n = 15) or (K) αPD-L1 or isotype control (−) (B/L: n = 6, ibrutinib-rituximab: n = 23, and FCR: n = 13). (L) Illustration of the autologous cytotoxicity assay incorporating the CD20 × CD3 glofitamab or a nonbinding antibody control. (M-N) T-cell–mediated CLL cell death using purified T cells from B/L, 6M, 12M, or 18M ibrutinib-rituximab (M) or FCR (N) time points against target B/L CLL B cells after ex vivo treatment with glofitamab (0.01 μg/mL) or nonbinding antibody control (indicated as “−”) (B/L, n = 13; ibrutinib-rituximab 6M and 12M,n = 6; ibrutinib-rituximab 18M, n = 5; FCR 6M and 12M, n = 7; and FCR 18M, n = 5 patient samples). Data for all cytotoxicity assay timepoints were normalized to isotype antibody control for panels J-K or nonbinding antibody control for panels M-N treated sample levels and presented as fold change data for each immunotherapy treated patient sample. Wilcoxon signed-rank test for panels B,D-E,J-K,M-N, multiple comparisons mixed effect analysis of variance for panel F and Mann-Whitney U test for panel H. Mann-Whitney U test was used to compare cell death among CD20-TCB–treated conditions at B/L, 6M, 12M, and 18M. Original magnification ×63; scale bars, 10 μm. Bar chart data are presented as mean ± standard error of the mean. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. CMAC, CellTracker Blue (7-amino-4-chloromethylcoumarin); TCB, T-cell bispecific antibody.
Ibrutinib is known to reduce PD-1 expression on patient T cells.9,10,18 Here, we detected a reduced frequency of PD-1–expressing T-cell subsets during ibrutinib-rituximab administration as well as PD-L1–expressing T cells except for CD8+ TEM at 6 months (Figure 1D-E; supplemental Figure 8). In contrast, T-cell PD-1/PD-L1 expression was relatively unaffected after FCR. This prompted us to investigate for a checkpoint blockade in our cytotoxicity assay (Figure 2I-K; supplemental Figure 9). Both ibrutinib-rituximab– and FCR-exposed T cells were insensitive to anti–PD-1. However, anti–PD-L119 increased anti-CLL T-cell cytotoxicity only at the 6-month ibrutinib-rituximab time point, suggesting a narrow window for the checkpoint blockade activity.17 This led us to investigate whether the T-cell–engaging bispecific antibody glofitamab (CD20 × CD3)20 could trigger improved cytolytic responses. T cells from all ibrutinib-rituximab time points tested up to 18 months showed increased anti-CLL T-cell killing after treatment with glofitamab, compared with those at baseline (Figure 2L-N). However, T cells after FCR did not respond to glofitamab, including at the later time point. Overall, these data support the ability of ibrutinib-based therapy to enhance T-cell–mediated cytotoxicity induced by bispecific immunotherapy.
In summary, our data highlight the importance of T cells during ibrutinib-rituximab therapy, with higher T-cell numbers and rejuvenated cytotoxicity accompanying favorable clinical responses. Our exploratory findings that increased levels of PD-1–expressing T cells as well as PD-L1–expressing CLL cells before therapy associate with longer PFS suggests that ibrutinib-rituximab appears to capitalize on T-cell–mediated immune surveillance in patients. Strikingly, opposing associations were found in the chemoimmunotherapy arm, and T cells showed no functional improvement after FCR. Previous studies have reported CD8+ T clonotype expansion during ibrutinib therapy,21,22 likely reflecting active immunosurveillance. Taken together, tumor debulking and alleviation of T-cell exhaustion during BTKi-based therapy9-13 may promote CD8+ T-cell activity. The switch from CD4+ T-cell:CLL interactions at baseline to CD8+ lytic synapses during ibrutinib-rituximab therapy supports this concept. Although ibrutinib-rituximab did not increase TH1 numbers, we do not exclude ITK inhibition contributing to beneficial immunomodulation.18 Furthermore, our longitudinal assays designed to evaluate changes in T-cell cytolytic function with therapy, revealed that revitalized cytotoxicity during ibrutinib-rituximab therapy could be maximized with glofitamab, further supporting combination immunotherapy approaches.23-25 Overall, this report underscores the importance of trial-associated science to understand how BTKis modulate T cells and supports the development of immunotherapy-based therapies.
Acknowledgments
The authors thank the Nikon Imaging Facility at King’s College London. This study was coordinated, in part, by the ECOG-ACRIN Cancer Research Group (Peter J. O'Dwyer and Mitchell D. Schnall, joint group chairs).
This study was supported by the National Institutes of Health, National Cancer Institute awards RO1CA193541, U10CA180820, UG1CA232760, UG1CA233290, U10CA180794, and RO1CA251801.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: D.P. designed, performed experimental work, analyzed data, and wrote the manuscript; X.V.W. performed data and association analysis; C.E.L. and N.I. performed experimental work; G.D.R. contributed to image analysis; T.D.S., N.E.K., and M.S.T. designed and supervised the study and managed data; S.H., M.B., and C.K. provided the bispecific antibodies and advised on the study; A.G.R. designed and supervised the study and wrote the manuscript.
Conflict-of-interest disclosure: S.H., M.B., and C.K. are employees of and declare patents and stock ownership with Roche Glycart AG. A.G.R. has received research support from Roche Glycart AG to Institution as part of a research collaboration. The remaining authors declare no competing financial interests.
Correspondence: Alan G. Ramsay, Lymphoma Immunology, Innovation Hub, Guy’s Cancer Centre, Great Maze Pond, London SE1 9RT, United Kingdom; email: alan.ramsay@kcl.ac.uk.
References
Author notes
Presented in abstract form at the 60th annual meeting of the American Society of Hematology, San Diego, CA, 1 to 4 December 2018.
Original data and protocols are available on request from the corresponding author, Alan G. Ramsay (alan.ramsay@kcl.ac.uk).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

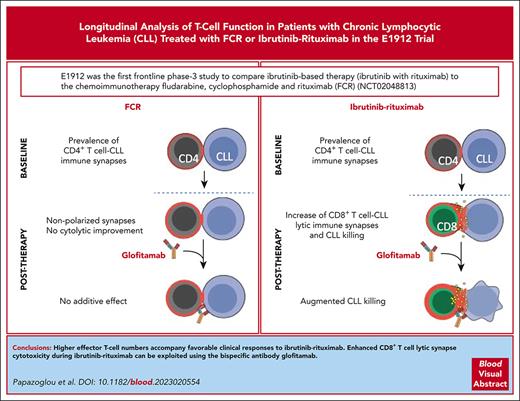
![Higher CD8+T-cell numbers at baseline and early on-therapy associate with favorable PFS and no infections with ibrutinib-rituxumab. (A) Schematic representation of the E1912 trial and biobanked peripheral blood mononuclear cell samples collected at baseline (B/L) and 6- (6M) and 12- (12M) month time points for correlative T-cell analysis. PFS, infection, and MRD clinical outcome data were collected. (B) Absolute numbers of CD8+ TEM cell subsets (CD45RA−CCR7−) for ibrutinib-rituximab (n = 86 patients) and FCR (n = 50) at the time points indicated. Patient data are presented as Box and whiskers (10-90 percentile; log scale) plots. (C) Percentage of PD-1+ CD8+ TEM subsets during ibrutinib-rituximab (n = 86) or FCR (n = 50) treatments. (B-C) Data are given as the mean ± standard error of the mean; statistical analysis between time points were assessed using the Wilcoxon signed-rank test. (D) Tabular schematic summary of the significant correlations (Cox model) between higher immune subset levels (flow cytometry, median values used as cut-off point) and PFS for patients on ibrutinib-rituximab (n = 88 patients with 13 experiencing disease progression). Green rows (correlations with hazard ratio [HR] values < 1) indicate higher immune subsets associated with longer PFS, whereas higher immune subsets associating with shorter PFS (HR > 1) are highlighted in blue rows. Confidence intervals (95%) and P values are shown. (E) Schematic summary of the significant correlations (Wilcoxon test) between immune subsets and infection (any infection) during ibrutinib-rituximab treatment (n = 88 patients). Negative t statistics (t.stat) indicate higher immune subset levels in patients who did not develop infection (green rows). In contrast, correlations with a positive t.stat indicate higher immune subset levels in patients who developed infection (blue rows). (F) Kaplan-Meier curves of immune subsets associated to good prognosis for the ibrutinib-rituximab arm. Higher levels of percentage PD-L1+ CD19+ cells (high: 12 progression events per 43 patients and low: 1 progression event per 43 patients), absolute number of PD-1+CD8+ TEM (high: 3 progression events per 43 patients and low: 10 progression events per 43 patients), and PD-1+CD4+ T cells (high: 3 progression events per 43 patients and low: 10 progression events per 43 patients) at baseline associate with longer PFS. Higher percentage of CD8+ TEM (high: 3 progression events per 42 patients and low: 10 progression events per 43 patients) at the 6-month time point associate with longer PFS. Absolute number data are referred to as “ab.” P values indicated. ∗P < .05; ∗∗P < .01; ∗∗∗∗P < .0001. n/s, not significant.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/143/1/10.1182_blood.2023020554/4/m_blood_bld-2023-020554-gr1.jpeg?Expires=1765893182&Signature=d0IeFkB5GYBcRLPiOZsmIL8fz1l2M16Dbj3Vevz3gbP8bV4Zt-P-VQ--YLRG4UxvvR4VP7Yt7O2ZrvBNBB9GaGxuen3cyqCjfGgVr0jDFh7IBM4al2A5jWS-lGA6m0jvQK4itrAFvSaANoFWi6AO0QMNAu7D3f8dwjyvOQbYwLotQxOEciyTtq0EjuzVmgcSeZNNxoWD32e-l443xIraP8rifdFvMXtYH0mTzmqbfMxFJ4WXzlA3ZW0zy164PXM~APL1JFI4DMhFos~~qqEoRdUV4Etb2M~ZhO-Drzl9zF5kYYe1mB-ppC-9LDLba3Z7VMST6WkNW4tdNBeaxygWdQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
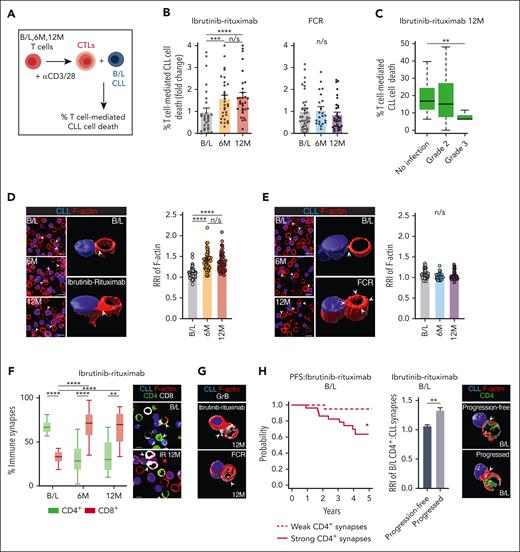
![Ibrutinib-rituximab promotes CD8+ T-cell lytic synapse activity and supports immunotherapy-triggered anti-CLL killing function. (A) Illustration of the autologous cytotoxicity assay using anti-CD3/-CD28–activated T cells (cytolytic T lymphocytes [CTLs]) from B/L, 6M, and 12M time points mixed with target B/L CLL B cells (pulsed with superantigen as a model antigen) with flow-based quantification of T-cell killing function. (B) T-cell–mediated CLL cell death comparing T cells purified from B/L, 6M, and 12M time point samples (n = 30 patients per treatment arm). Data at 6M and 12M were normalized to B/L levels to generate fold change values for each patient. (C) The association between patient’s T-cell killing function (12M ibrutinib-rituximab time point, n = 30) and infection status during ibrutinib-rituximab therapy (no infections vs grade 2 or 3 infections) (Wilcoxon test, P = .01). (D-E) Representative confocal medial optical section and 3-dimensional (3D) volume–rendered images of T-cell:CLL conjugates formed between patient T cells (B/L, 6M, and 12M on-ibrutinib-rituximab [D] or FCR [E]) interacting with autologous B/L CLL B cells (blue, CMAC dyed). Bar charts: quantitative relative recruitment index (RRI) analysis of F-actin polarization (red, rhodamine phalloidin) in T-cell:CLL conjugates (n = 50 patients per treatment arm). (F) Box and violin plots (minimum-maximum) showing the percentage of CD4+ or CD8+ T-cell:CLL conjugates formed from the total T-cell:CLL conjugates in B/L, 6M, and 12M ibrutinib-rituximab time point samples (n = 15 patients). Representative confocal images of CD8+ (white) and CD4+ (green) T-cell conjugates with CLL B cells (blue) at B/L vs on ibrutinib-rituximab therapy. (G) Representative confocal 3D volume–rendered images of granzyme B (GrB; white) expression at CD8+ T-cell synapses, comparing ibrutinib-rituximab and FCR 12M time point samples. (H) Kaplan-Meier curve showing the association between the strength of polarized F-actin CD4+ T-cell:CLL immune synapse interactions in patient B/L samples and their PFS outcomes during ibrutinib-rituximab administration. Median F-actin RRI values were used as a cut-off point to determine weak (<median RRI) vs strong (>median RRI) CD4+ T-cell synapses (n = 52 patients). Patients’ showing strong CD4+ T-cell:CLL immune synapses at B/L showed significantly adverse PFS (9 progression events per 29 patients) compared with patients showing weak CD4+ T-cell:CLL interactions (1 progression event per 23 patients). (Cox model, P = .01; HR, 9.14; 95% confidence interval, 1.15-72.47). Bar chart: F-actin RRI analysis of CD4+ T-cell:CLL conjugates at B/L and representative 3D volume–rendered confocal images comparing patients who progressed (n = 7) with those who did not (progression free, n = 7) during ibrutinib-rituximab therapy. (I) Illustration of the cytotoxicity assay after ex vivo treatment of purified T cells (B/L, 6M, and 12M time points) and B/L CLL cells with anti–PD-1 (αPD-1) or anti–PD-L1 (αPD-L1) blocking antibodies (10 μg/mL) or isotype controls. (J-K) T-cell killing function against autologous B/L CLL cells examining T cells at B/L or at the 6-month ibrutinib-rituximab (orange) or FCR (blue) time points after ex vivo treatment with (J) αPD-1 or isotype control (indicated using “−”) (B/L: n = 6, ibrutinib-rituximab: n = 13, and FCR: n = 15) or (K) αPD-L1 or isotype control (−) (B/L: n = 6, ibrutinib-rituximab: n = 23, and FCR: n = 13). (L) Illustration of the autologous cytotoxicity assay incorporating the CD20 × CD3 glofitamab or a nonbinding antibody control. (M-N) T-cell–mediated CLL cell death using purified T cells from B/L, 6M, 12M, or 18M ibrutinib-rituximab (M) or FCR (N) time points against target B/L CLL B cells after ex vivo treatment with glofitamab (0.01 μg/mL) or nonbinding antibody control (indicated as “−”) (B/L, n = 13; ibrutinib-rituximab 6M and 12M,n = 6; ibrutinib-rituximab 18M, n = 5; FCR 6M and 12M, n = 7; and FCR 18M, n = 5 patient samples). Data for all cytotoxicity assay timepoints were normalized to isotype antibody control for panels J-K or nonbinding antibody control for panels M-N treated sample levels and presented as fold change data for each immunotherapy treated patient sample. Wilcoxon signed-rank test for panels B,D-E,J-K,M-N, multiple comparisons mixed effect analysis of variance for panel F and Mann-Whitney U test for panel H. Mann-Whitney U test was used to compare cell death among CD20-TCB–treated conditions at B/L, 6M, 12M, and 18M. Original magnification ×63; scale bars, 10 μm. Bar chart data are presented as mean ± standard error of the mean. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. CMAC, CellTracker Blue (7-amino-4-chloromethylcoumarin); TCB, T-cell bispecific antibody.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/143/1/10.1182_blood.2023020554/4/m_blood_bld-2023-020554-gr2in.jpeg?Expires=1765893182&Signature=qjnprEAiYeOZUBxSlUEWMq17O4Lr0Ofh4we1SDeSPYpL4gwR1ZR2deYWAq6WmJwvn~dvitgiP9ZrhTcIxUKzyl6iaFizJwv4WJE2FlZ2PTtn4gmQPFy2hNJdvDFjEqu-zhQy~LMqr3nqDvAqOC6c5An59ICgyQBgYxdrQs87k538VwgddhpUIxZbJSpH61MzMPaE145PrQaS~Qx5ipZ0Z85VffGFscQNm1~1HmPctiHzfBzfSja4Z6US5q1CP8OmoqTDIKW3ulWBcnktdb9UKZH-uvp5IbpG5SpSOtAxdt~75a0lzqpXevbpM8oYCaKLyKr30Mu6Hy9cFj1E2URGsA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal