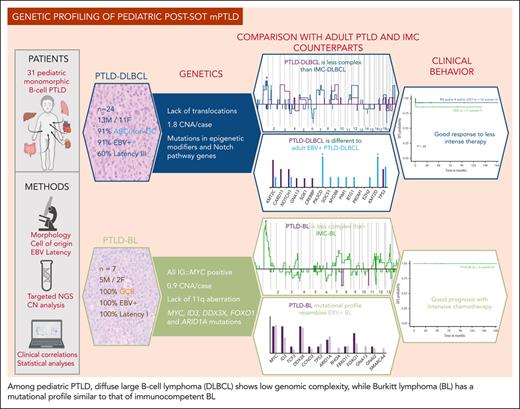
Key Points
The mutational profile of pediatric PTLD-BL resembles immunocompetent EBV-positive BL, suggesting the need of intensive therapy.
Pediatric PTLD-DLBCL is genetically less complex than the adult PTLD-DLBCL and pediatric immunocompetent DLBCL.
Abstract
Posttransplant lymphoproliferative disorders (PTLDs) represent a broad spectrum of lymphoid proliferations, frequently associated with Epstein-Barr virus (EBV) infection. The molecular profile of pediatric monomorphic PTLDs (mPTLDs) has not been elucidated, and it is unknown whether they display similar genetic features as their counterpart in adult and immunocompetent (IMC) pediatric patients. In this study, we investigated 31 cases of pediatric mPTLD after solid organ transplantation, including 24 diffuse large B-cell lymphomas (DLBCLs), mostly classified as activated B cell, and 7 cases of Burkitt lymphoma (BL), 93% of which were EBV positive. We performed an integrated molecular approach, including fluorescence in situ hybridization, targeted gene sequencing, and copy number (CN) arrays. Overall, PTLD-BL carried mutations in MYC, ID3, DDX3X, ARID1A, or CCND3 resembling IMC-BL, higher mutational burden than PTLD-DLBCL, and lesser CN alterations than IMC-BL. PTLD-DLBCL showed a very heterogeneous genomic profile with fewer mutations and CN alterations than IMC-DLBCL. Epigenetic modifiers and genes of the Notch pathway were the most recurrently mutated in PTLD-DLBCL (both 28%). Mutations in cell cycle and Notch pathways correlated with a worse outcome. All 7 patients with PTLD-BL were alive after treatment with pediatric B-cell non-Hodgkin lymphoma protocols, whereas 54% of patients with DLBCL were cured with immunosuppression reduction, rituximab, and/or low-dose chemotherapy. These findings highlight the low complexity of pediatric PTLD-DLBCL, their good response to low-intensity treatment, and the shared pathogenesis between PTLD-BL and EBV-positive IMC-BL. We also suggest new potential parameters that could help in the diagnosis and the design of better therapeutic strategies for these patients.
Introduction
Posttransplant lymphoproliferative disorders (PTLDs) are relatively common complications after solid organ transplantation (SOT) or hematopoietic stem cell transplantation and represent a major cause of morbidity and mortality.1 Pediatric transplantation recipients are at an increased risk of developing PTLD, in part because of Epstein-Barr virus (EBV) seronegativity at the time of transplantation.1 There are 3 main EBV latency patterns described in B cells, through which the virus is able to transit, distinguished by different viral gene expression profiles.2 Characteristically, PTLDs show a latency III pattern.3
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of monomorphic PTLD (mPTLD) in both adult and pediatric patients, whereas Burkitt lymphoma (BL) is a less frequent form, with aggressive clinical presentation and different pathological and prognostic characteristics.4,5 These 2 entities are also the most frequent mature B-cell non-Hodgkin lymphomas (B-NHL) in immunocompetent (IMC) children and adolescents, and their genetic landscapes have been extensively studied.6-10 In detail, sporadic BL is characterized by t(8;14)(q24;q32)/IGH::MYC translocation and recurrent mutations in ID3, CCND3, MYC, TP53, and TCF3.6-8 In addition, EBV-associated BL, including the endemic variant (eBL), displays less frequent mutations in those genes and a higher incidence of ARID1A and RHOA mutations.11-15 In contrast, pediatric and young adult DLBCL lacks BCL2 and BCL6 primary aberrations, but similar to adults, has recurrent 2p16/REL gains, 19p13/CD70 homozygous deletions, and mutations in SOCS1 and KMT2D.9,10
Despite the clear morphological similarities of mPTLD with lymphomas in IMC patients, few studies have systematically analyzed their biology and genetics beyond their relationship to EBV infection. The genetic profile of PTLD-BL has not been unraveled yet, whereas the few studies that describe the molecular profile of PTLD-DLBCL have been conducted with adult patients.16-18 In this sense, adult PTLD-DLBCL carries recurrent alterations in the JAK-STAT pathway,18 lower genetic complexity of germinal center (GC) B-cell like (GCB) PTLD-DLBCL than IMC-DLBCL, and the absence of mutations in key genes characteristic of IMC-DLBCL lymphomagenesis such as SOCS1, and genes involved in the NF-κB signaling pathway as CARD11.16,17 Moreover, patients with EBV-positive (EBV+) PTLD-DLBCL carry a lower mutational burden than the patients who are EBV-negative (EBV−).16 In addition, gains of 3q, 5p, 8q and 11p, and 1p36, 12p, and 17p13 losses have been described in adult PTLD-DLBCL.19-21
The lack of information in pediatric mPTLD also raises the question of whether large/high-grade B-cell lymphoma with 11q aberration (LBCL/HG-11q) and large B-cell lymphoma with IRF4 translocation (LBCL-IRF4), 2 recently described entities predominantly occurring in children, are represented in this setting.22,23 Of note, the mutational profile of these diseases has been well characterized in the IMC population. LBCL/HG-11q lacks BL-related mutations but displays recurrent mutations in BTG2, DDX3X, ETS1, EP300, GNA13, and NFRKB,24,25 whereas LBCL-IRF4 carries frequent alterations in IRF4 and CARD11 genes.9
In terms of clinical management, there is no standard of care (SOC) for mPTLD in children, a significant gap sharpened by the fact that these patients are particularly vulnerable owing to the risk of toxicity and rejection and susceptibility to infections. Reduction of immunosuppression (RIS) is generally the first treatment step, followed by rituximab in combination (or not) with chemotherapy.26,27
This study aimed to delineate for the first time, to our knowledge, the specific genetic, clinical, and pathological features of pediatric mPTLD after SOT and to clarify whether the pathogenesis of these lymphomas is the same as in IMC patients. A thorough morphological review and molecular characterization of these cases, together with a correlation between these aspects and clinical parameters, might help select better treatment strategies for this challenging population.
Methods
Patients and samples
In total, 31 SOT-related pediatric (≤18 years old) patients with mPTLD at diagnosis were recruited from pediatric transplantation Spanish national reference centers in the context of a centralized review supported by the Sociedad Española de Hematología y Oncología Pediátricas. All cases were centrally reviewed by 4 hematopathologists (N.C.-d.-A., B.G.-F., O.B., and E.C.) and classified according to the revised 4th edition of the World Health Organization Classification 2017 criteria.28 Available clinical data, details on transplantation, and mPTLD management were retrospectively collected (supplemental Table 1, available on the Blood website).
All formalin-fixed and paraffin-embedded (FFPE) samples investigated contained more than 60% of neoplastic cells. DNA and RNA from FFPE materials were extracted using the Qiagen AllPrep DNA/RNA FFPE kit (Qiagen, Hilden, Germany). Polymerase chain reaction amplifications for the detection of clonal immunoglobulin heavy chain (IGH) gene rearrangements were performed according to BIOMED-2 protocols.29 This study was approved by the Hospital Clinic of Barcelona Review Board (HCB/2018/0365) and was performed in accordance with the Declaration of Helsinki.
Immunohistochemical studies, EBV infection, and cell of origin (COO) determination
The phenotypical profile was studied using standard immunohistochemistry protocols on an automated platform (Ventana BenchmarkUltra, Roche, Basel, Switzerland). EBV infection, latency pattern, and replicative phase were determined using in situ hybridization (Epstein-Barr virus-encoded small RNA) and immunohistochemistry (LMP-1, EBNA-2 [Epstein-Barr virus nuclear antigen 2], ZEBRA) (supplemental Methods; supplemental Table 2). Cases were classified as GC and non-GC subtypes according to the Hans algorithm.30 In addition, COO classification was performed using Lymph2Cx assay (NanoString Technologies, Seattle, WA).31
Fluorescence in situ hybridization (FISH)
FISH analyses were performed using standard protocols. Breaks at BCL2, PAX5, BCL6, MYC, IGK, IRF4 loci, t(8;14) and t(2;8) fusions, and 11q alterations were analyzed using commercial (Metasystems, Altlußheim, Germany; Empire Genomics, Williamsville, NY; Agilent Technologies, Santa Clara, CA; Abbot, Chicago, IL; ZytoVision, Bremerhaven, Germany) or homemade FISH probes.32
DNA copy number alterations (CNAs) analysis
CNAs were examined in 16 PTLD-DLBCL and 7 PTLD-BL cases using the OncoScan array (Thermo Fisher Scientific, Waltham, MA) following standard protocols (supplemental Methods). Gains, losses, and CN neutral–loss of heterozygosity regions were evaluated using Nexus Biodiscovery v9.0 software (Biodiscovery, Hawthorne, CA). Previously published CN data of pediatric IMC-BL,33 IMC-DLBCL,9 and adult PTLD-DLBCL21,34 were used for comparison.
Targeted NGS and mutational analysis
Samples from 25 patients with mPTLD, with material available, were included in the mutational analysis. The custom next generation sequencing (NGS) panel interrogated 167 B-cell lymphoma–related genes (supplemental Table 3), including those needed to perform the LymphGen prediction algorithm.35 SureSelectXT Target Enrichment System Capture NGS strategy library (Agilent Technologies) was used before sequencing on a MiSeq equipment (Illumina, San Diego, CA) (supplemental Methods). Pathway enrichment analysis was performed defining the contribution of each gene based on previous literature (supplemental Methods).36 Previously published mutational profiles of adult PTLD-DLBCL,17,18 pediatric and young adult IMC-DLBCL,9 and pediatric sporadic and eBL,6,11-13 were used for comparisons.
Statistical methods
Disease-free survival (DFS) was defined as the time from diagnosis until lymphoma disease–related death and event-free survival (EFS) was established as the time to progression, death of any cause, organ loss, or secondary malignancy. The Kaplan–Meier method was used to estimate the DFS and EFS distributions, whereas the log-rank test37 was used to assess differences. Differences in the distribution of individual parameters among patient subsets were analyzed using Fisher exact test for categorized variables and the Student t test for continuous variables. Nonparametric tests were applied when necessary. The P values for multiple comparisons were adjusted using the Benjamini–Hochberg correction procedure (false discovery rate). A P = .05 cutoff value was considered significant unless otherwise indicated. Statistical analyses were conducted using R software v4.1.2.
Results
Clinical features
Of the 31 recruited patients, 18 were male and 13 were female. The mean age at the time of lymphoma diagnosis was 8.7 years (range 2-17). Kidney and liver were the most frequently transplanted organs (in 11 and 10 patients, respectively), followed by the heart (8 patients), intestine (2 cases), and lung (1 case). Twenty-four cases presented with extranodal disease, and the most frequently affected site was the gastrointestinal tract (21/29) (supplemental Figure 3). Five patients had stage IV disease (4 had bone marrow involvement and 1 had both central nervous system and bone marrow infiltration). The mean time from transplantation to PTLD diagnosis was 35 months (range 2-170). Fifteen out of 31 patients (48%) had early-onset PTLD diagnosed in the first year after the transplantation, whereas only 6% (2/31) had very late-onset PTLD (≥10 years). Clinicopathological features are detailed in Table 1 and supplemental Table 1.
Clinicopathological characteristics of 31 pediatric patients with B-cell mPTLD
| . | PTLD-DLBCL, n = 24 . | PTLD-BL, n = 7 . | Total, n = 31 . |
|---|---|---|---|
| Mean age (range, years old) | 8 (2-17) | 12 (6-16) | 9 (2-17) |
| Male | 13/24 (54%) | 5/7 (71%) | 18/31 (58%) |
| Female | 11/24 (46%) | 2/7 (29%) | 13/31 (42%) |
| Localization | |||
| Extranodal involvement | 17/23 (74%) | 6/7 (86%) | 23/30 (77%) |
| Gastrointestinal tract | 17/23 (74%) | 4/6 (67%) | 21/29 (72%) |
| Stage | |||
| Stage I | 1/19 (5%) | 1/7 (14%) | 2/26 (8%) |
| Stage II | 3/19 (16%) | 0/7 (0%) | 3/26 (11%) |
| Stage III | 14/19 (74%) | 2/7 (29%) | 16/26 (62%) |
| Stage IV | 1/19 (5%) | 4/7 (57%) | 5/26 (19%) |
| COO (NanoString and/or Hans algorithm)∗ | |||
| GCB | 2/22 (9%) | 7/7 (100%) | 9/29 (31%) |
| ABC/non-GCB | 20/22 (91%) | 0/7 (0%) | 20/29 (69%) |
| EBER hybridization positive | 21/23 (91%) | 7/7 (100%) | 28/30 (93%) |
| EBV replication | 6/11 (55%) | 0/5 (0%) | 6/16 (38%) |
| Latency pattern | |||
| Latency I | 2/20 (10%) | 6/6 (100%) | 8/26 (31%) |
| Latency II | 6/20 (30%) | 0/6 (0%) | 6/26 (23%) |
| Latency III† | 12/20 (60%) | 0/6 (0%) | 12/26 (46%) |
| Mean time from the transplantation to PTLD diagnosis (range, months) | 24 (2-141) | 74.4 (32-170) | 35 (2-170) |
| Early-onset PTLD | 15/24 (63%) | 0/7 (0%) | 15/31 (48%) |
| Alive with no evidence of disease | 17/24 (71%) | 7/7 (100%) | 24/31 (77%) |
| Died of disease | 5/24 (21%) | 0/7 (0%) | 5/31 (16%) |
| Fulminant PTLD | 3/5 (60%) | 0/0 (0%) | 3/5 (60%) |
| Follow-up (median) (range) | 1.3 y (10 d-14.1 y) | 5.2 y (2.1-22.6 y) | 2.7 y (10 d-22.6 y) |
| 5y-DFS | 78.9% | 100% | 83.7% |
| . | PTLD-DLBCL, n = 24 . | PTLD-BL, n = 7 . | Total, n = 31 . |
|---|---|---|---|
| Mean age (range, years old) | 8 (2-17) | 12 (6-16) | 9 (2-17) |
| Male | 13/24 (54%) | 5/7 (71%) | 18/31 (58%) |
| Female | 11/24 (46%) | 2/7 (29%) | 13/31 (42%) |
| Localization | |||
| Extranodal involvement | 17/23 (74%) | 6/7 (86%) | 23/30 (77%) |
| Gastrointestinal tract | 17/23 (74%) | 4/6 (67%) | 21/29 (72%) |
| Stage | |||
| Stage I | 1/19 (5%) | 1/7 (14%) | 2/26 (8%) |
| Stage II | 3/19 (16%) | 0/7 (0%) | 3/26 (11%) |
| Stage III | 14/19 (74%) | 2/7 (29%) | 16/26 (62%) |
| Stage IV | 1/19 (5%) | 4/7 (57%) | 5/26 (19%) |
| COO (NanoString and/or Hans algorithm)∗ | |||
| GCB | 2/22 (9%) | 7/7 (100%) | 9/29 (31%) |
| ABC/non-GCB | 20/22 (91%) | 0/7 (0%) | 20/29 (69%) |
| EBER hybridization positive | 21/23 (91%) | 7/7 (100%) | 28/30 (93%) |
| EBV replication | 6/11 (55%) | 0/5 (0%) | 6/16 (38%) |
| Latency pattern | |||
| Latency I | 2/20 (10%) | 6/6 (100%) | 8/26 (31%) |
| Latency II | 6/20 (30%) | 0/6 (0%) | 6/26 (23%) |
| Latency III† | 12/20 (60%) | 0/6 (0%) | 12/26 (46%) |
| Mean time from the transplantation to PTLD diagnosis (range, months) | 24 (2-141) | 74.4 (32-170) | 35 (2-170) |
| Early-onset PTLD | 15/24 (63%) | 0/7 (0%) | 15/31 (48%) |
| Alive with no evidence of disease | 17/24 (71%) | 7/7 (100%) | 24/31 (77%) |
| Died of disease | 5/24 (21%) | 0/7 (0%) | 5/31 (16%) |
| Fulminant PTLD | 3/5 (60%) | 0/0 (0%) | 3/5 (60%) |
| Follow-up (median) (range) | 1.3 y (10 d-14.1 y) | 5.2 y (2.1-22.6 y) | 2.7 y (10 d-22.6 y) |
| 5y-DFS | 78.9% | 100% | 83.7% |
ABC, activated B cell; EBER, Epstein-Barr virus-encoded small RNAs.
Unclassified cases by NanoString analysis were classified according to Hans algorithm results.
In the absence of LMP-1 assessability, 2 cases with latency pattern IIb-III are included in latency pattern III group.
At PTLD diagnosis, EBV was detectable in blood in 23 out of 27 patients with available information, and PTLD was secondary to EBV primary infection in 47% (7/15) of the patients with known seronegativity before the transplantation.
Pathological characteristics
Individual descriptions of clinicopathological features, COO, and EBV latency patterns of patients with mPTLD are detailed in supplemental Table 4.
Twenty-four cases were classified as DLBCL and 7 as BL (Figure 1). Of note, 2 BL cases had less monotonous cytology (p28 and p29) (supplemental Figure 1), and 1 BL case (p38) (supplemental Figure 2) showed DLBCL morphology. However, all 3 cases harbored typical phenotypic and molecular features and were, therefore, classified as BL. IGH gene rearrangements analysis identified a clonal peak in 23 out of 24 mPTLDs. In case p27 IGH-FR3 was polyclonal, no further analyses of IGH-FR1 and IGH-FR2 could be performed because of DNA quality.
Most DLBCL cases showed a non-GC phenotype (18/19, 95%), including 4 DLBCL cases with plasmacytic differentiation, in line with Lymph2Cx results that showed an activated B cell signature in 71% (15/21), followed by unclassified in 19%, and GCB in 10%. The 7 BLs were classified as GCB by either 1 of the 2 methods.
Epstein-Barr virus-encoded small RNA was positive in 28 out of 30 cases. A complete EBV latency pattern was determined in 26 cases. All 7 BLs were EBV+, and all 6 studied cases had a latency pattern I. In contrast, 21 out of 23 DLBCL cases were EBV+, 60% of which had latency III. Viral replication was identified in 6 out of 16 analyzed cases, 5 of which were among the latency pattern III group (Table 1).
FISH results
MYC rearrangements were detected in all 7 BLs. Six cases carried t(8;14)(q24;q32) translocation confirmed by IGH::MYC dual fusion FISH probe, whereas t(2;8)(p11.2;q24) rearrangement was identified in case p28 using IGK::MYC dual fusion probe. Furthermore, no 11q alterations or chromosomal rearrangements involving IRF4, PAX5, BCL2, or BCL6 were observed in the 29, 29, 29, 13, and 11 analyzed cases, respectively (supplemental Figure 3).
CNA profile
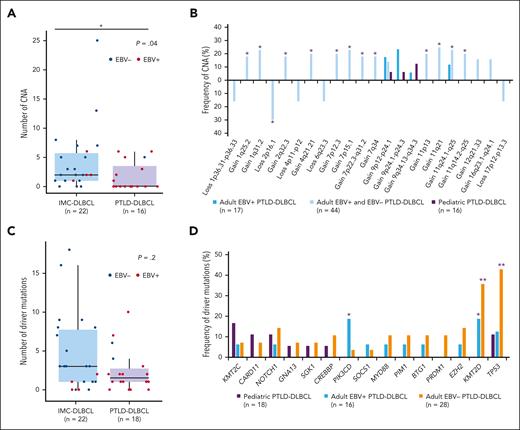
Eleven out of the 23 pediatric mPTLD studied cases displayed CNAs (mean 1.5 alt/case; range 0-7) (Figure 2A; supplemental Table 5). Seven out of the 16 (44%) PTLD-DLBCL cases showed CNAs (mean 1.8 alt/case), with gains of 3p and 9q21.11-q34.3 (3 cases each; 19%) being the most frequent. Four out of 7 (57%) PTLD-BL cases displayed CNAs (mean 0.9 alt/case) with no common CNA among those cases. None of the 23 investigated cases showed the characteristic 11q alteration found in LBCL/HG-11q.
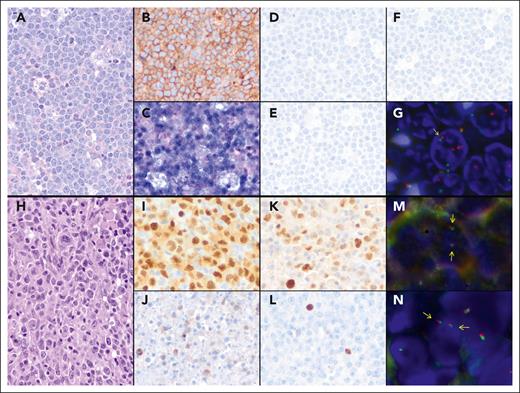
Morphological, immunohistochemical, and genetic features of 2 prototypical post-SOT mPTLD, BL (case p11), and DLBCL cases (case p10), respectively. Case p11: (A) hematoxylin and eosin, original magnification at ×400. (B) Tumor cells are diffusely positive for CD10 (immunostain, original magnification at ×400). (C) In situ hybridization for EBV RNA is positive in tumor cells (in situ hybridization, original magnification at ×400). (D-F) Neoplastic cells are negative for LMP1 (D), EBNA2 (E), and ZEBRA (F) immunostains, indicating latency pattern I (immunostains, original magnification at ×400). (G) FISH using MYC Break-Apart Probe (BAP) shows a signal constellation of 1 colocalized signal (yellow arrow) and 2 split signals (green and red arrows) in accordance with the gene rearrangement. Case p10: (H) DLBCL with focal plasmacytic differentiation (hematoxylin and eosin, original magnification at ×400). (I) Atypical cells are diffusely positive for MUM1 (immunostain, original magnification at ×400). (J-L) LMP1 (J), EBNA2 (K), and ZEBRA (L) immunostains show positive tumor cells, reflecting a pattern III of latency (immunostains, original magnification at ×400). (M-N) FISH using BAP for MYC (M) and IRF4 (N) genes show a normal pattern with 2 colocalizations in each nucleus for both hybridizations (yellow arrows).
Morphological, immunohistochemical, and genetic features of 2 prototypical post-SOT mPTLD, BL (case p11), and DLBCL cases (case p10), respectively. Case p11: (A) hematoxylin and eosin, original magnification at ×400. (B) Tumor cells are diffusely positive for CD10 (immunostain, original magnification at ×400). (C) In situ hybridization for EBV RNA is positive in tumor cells (in situ hybridization, original magnification at ×400). (D-F) Neoplastic cells are negative for LMP1 (D), EBNA2 (E), and ZEBRA (F) immunostains, indicating latency pattern I (immunostains, original magnification at ×400). (G) FISH using MYC Break-Apart Probe (BAP) shows a signal constellation of 1 colocalized signal (yellow arrow) and 2 split signals (green and red arrows) in accordance with the gene rearrangement. Case p10: (H) DLBCL with focal plasmacytic differentiation (hematoxylin and eosin, original magnification at ×400). (I) Atypical cells are diffusely positive for MUM1 (immunostain, original magnification at ×400). (J-L) LMP1 (J), EBNA2 (K), and ZEBRA (L) immunostains show positive tumor cells, reflecting a pattern III of latency (immunostains, original magnification at ×400). (M-N) FISH using BAP for MYC (M) and IRF4 (N) genes show a normal pattern with 2 colocalizations in each nucleus for both hybridizations (yellow arrows).
CN landscape of pediatric mPTLD and comparisons with previously published cohorts. (A) Global CN profile of 23 B-cell post-SOT mPTLD. x-axis represents chromosomes from 1 to Y and p to q. The y-axis indicates the frequency of each genomic alteration among the analyzed cases; CN gains are represented in blue, and CN losses are depicted in red. Chromosomal bands of regions altered in more than 10% of cases are indicated in the plot. (B-C) CN profile comparison with previously published data on BL33 (B) and DLBCL9 (C) in IMC patients. Asterisks indicate significant differences between both groups according to Fisher exact test raw P < .1, considering a minimum number of altered cases n = 3.
CN landscape of pediatric mPTLD and comparisons with previously published cohorts. (A) Global CN profile of 23 B-cell post-SOT mPTLD. x-axis represents chromosomes from 1 to Y and p to q. The y-axis indicates the frequency of each genomic alteration among the analyzed cases; CN gains are represented in blue, and CN losses are depicted in red. Chromosomal bands of regions altered in more than 10% of cases are indicated in the plot. (B-C) CN profile comparison with previously published data on BL33 (B) and DLBCL9 (C) in IMC patients. Asterisks indicate significant differences between both groups according to Fisher exact test raw P < .1, considering a minimum number of altered cases n = 3.
In addition, we compared those CN profiles with the ones observed in IMC patients. Of note, pediatric PTLD-BL had lower genetic complexity than IMC-sporadic BL33 (0.9 alt/case vs 6.3 alt/case; P < .005) and lacked the 1q23.2-q25.3 gains characteristic of BL (Figure 2B). Similarly, PTLD-DLBCL had less CNA than IMC-DLBCL9 (1.8 alt/case vs 4.4 alt/case; P < .05) (Figures 2C and 3A) and adult PTLD-DLBCL21,34 (Figure 3B), with the absence of CNAs frequently observed in IMC-DLBCL,9 such as telomeric 1q31.3-q42.13 gains and losses of 6q21 and 19p13.3 (Figure 2C).
CN and mutational landscape of pediatric PTLD-DLBCL and comparison with previously published series. Comparison between pediatric PTLD-DLBCL, pediatric and young adult IMC-DLBCL,9 and adult PTLD-DLBCL17,18,21,34 in terms of (A-B) CNA and (C-D) mutational frequencies. The vertical axis of panels A and C represents the number of alterations and the 2 groups are separated in the x-axis (PTLD-DLBCL represented in purple and IMC-DLBCL represented in blue). The asterisk in panel A marks significant differences between both groups according to Wilcoxon rank-sum test P < .05. The vertical axis of panels B,D represents the frequency of alteration (%), with the different CNAs in panel B and genes in panel D represented in the horizontal axis. Asterisks in panel B mark significant differences between adult and pediatric PTLD-DLBCL, according to Fisher exact test P < .1 and in panel D indicate significant differences between adult, either EBV+ or negative, and pediatric PTLD-DLBCL, according to Fisher exact test ∗P < .1 and ∗∗P < .05.
CN and mutational landscape of pediatric PTLD-DLBCL and comparison with previously published series. Comparison between pediatric PTLD-DLBCL, pediatric and young adult IMC-DLBCL,9 and adult PTLD-DLBCL17,18,21,34 in terms of (A-B) CNA and (C-D) mutational frequencies. The vertical axis of panels A and C represents the number of alterations and the 2 groups are separated in the x-axis (PTLD-DLBCL represented in purple and IMC-DLBCL represented in blue). The asterisk in panel A marks significant differences between both groups according to Wilcoxon rank-sum test P < .05. The vertical axis of panels B,D represents the frequency of alteration (%), with the different CNAs in panel B and genes in panel D represented in the horizontal axis. Asterisks in panel B mark significant differences between adult and pediatric PTLD-DLBCL, according to Fisher exact test P < .1 and in panel D indicate significant differences between adult, either EBV+ or negative, and pediatric PTLD-DLBCL, according to Fisher exact test ∗P < .1 and ∗∗P < .05.
Identification of mutational profiles by targeted NGS
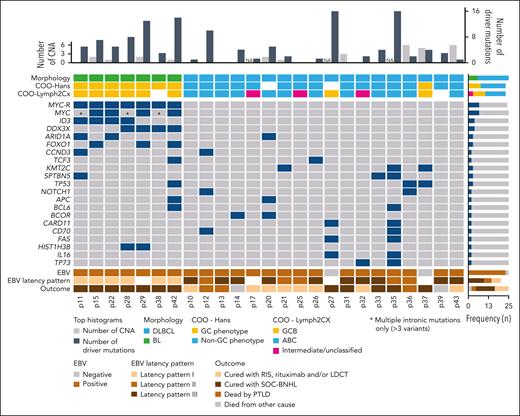
Eighteen DLBCL and 7 BL samples were analyzed using NGS (mean coverage 2586×, range 402-7256×). After filtering, 187 somatic variants were detected in 23 out of 25 (range 0-28) mPTLD cases, with 127 of them predicted as drivers (mean 5.5 driver mutation/case) (supplemental Methods; supplemental Table 6).
The mutational burden of PTLD-BL was higher than PTLD-DLBCL (7.8 vs 4.4 mutations, P = .03). All 7 PTLD-BL cases carried MYC mutations. ID3 and DDX3X variants were seen in 4, FOXO1 in 3, ARID1A in 2, and CCND3 in 1 case (Figure 4). Four cases harbored exonic MYC driver mutations, 3 of which carried further multiple intronic mutations. Three cases had only multiple intronic mutations.
Molecular CN and mutational information on 25 B-cell post-SOT mPTLD cases. Each column corresponds to a case, the gray bars in the top histogram depicts the number of CNAs while steel blue bars represent the number of driver mutations. Each row of the bottom plot represents a gene, where the dark blue color marks driver mutations. Only genes with driver mutations in more than 2 cases are represented. Blue in the MYC-R row indicates MYC rearrangement was detected using FISH using MYC BAP and in cases p11, p15, p22, p29, p38, and p42, also using IGH::MYC DF probe, whereas in the case of p28, IGK::MYC DF probe was used. ABC, activated B cell; NA, not available.
Molecular CN and mutational information on 25 B-cell post-SOT mPTLD cases. Each column corresponds to a case, the gray bars in the top histogram depicts the number of CNAs while steel blue bars represent the number of driver mutations. Each row of the bottom plot represents a gene, where the dark blue color marks driver mutations. Only genes with driver mutations in more than 2 cases are represented. Blue in the MYC-R row indicates MYC rearrangement was detected using FISH using MYC BAP and in cases p11, p15, p22, p29, p38, and p42, also using IGH::MYC DF probe, whereas in the case of p28, IGK::MYC DF probe was used. ABC, activated B cell; NA, not available.
In comparison with previously published EBV+ and EBV− BL cohorts, both sporadic and endemic subtypes11-13 revealed that PTLD-BL displays a mutational frequency closer to EBV+ BL, with a lower incidence of TCF3, CCND3, TP53, and SMARCA4 mutations and a higher incidence of ARID1A and FOXO1 (supplemental Figure 4A). Furthermore, the recently defined IC-BL (ID3/CCND3) and DGG-BL (DDX3X/GNA13/GNAI2) molecular genetic subgroups seem to be represented in our PTLD-BL cohort (4 and 3 cases, respectively) (supplemental Figure 4B).15
Differently, the mutational profile of the 18 PTLD-DLBCLs was very heterogeneous with lower complexity than their counterparts in IMC patients9 when 94 commonly investigated genes were considered (2.4 vs 4.6 mutation/case, P = .2) (Figure 3C; supplemental Table 7). The most recurrently mutated gene was KMT2C in 3 cases (17%), followed by mutations in SPTNB5, TP53, NOTCH1, BCOR, CARD11, CD70, FAS, IL16, and TP73 (2 cases each; 11%). The 3 KMT2C mutations were spread throughout the coding region of the gene (exons 36 and 43), whereas 3 NOTCH1 mutations were detected in 2 cases, both carrying a mutation predicted to truncate the protein in its PEST domain. Of note, PTLD-DLBCL lacked the characteristic mutations of SOCS1, MYD88, PIM1, BTG1, EZH2, and PRDM1 genes observed in pediatric and young adult IMC-DLBCL (supplemental Table 7).9
Pathway enrichment analysis showed that in line with IMC-DLBCL, genes involved in epigenetic modifications were one of the most recurrently affected (5 cases). In contrast, and unlike IMC-DLBCL, PTLD-DLBCL carried frequent mutations in Notch and MAPK pathways (5 and 2 cases, respectively), whereas lacked alterations affecting the JAK-STAT pathway (supplemental Figure 5). To discern whether these PTLD-DLBCL cases could belong to previously defined genetic subtypes of DLBCL,35LymphGen algorithm was applied to 18 PTLD-DLBCL cases. Only 2 cases (11%) were classified as N1, whereas the rest remained undetermined. These data suggest that this group of pediatric PTLD-DLBCLs, mainly EBV+ (91%), does not fit in any of the established genetic DLBCL subtypes.
Clinical outcome and prognostic value of clinical and molecular features
Four out of the 7 patients with PTLD-BL (57%) presented with stage IV. All of them underwent RIS and received chemotherapy according to the SOC for B-NHL. All 7 patients were alive and in complete remission at the last follow-up (mean follow-up 8.7 years; range 2-22 years) (Table 1; supplemental Table 1).
Different from PTLD-BL, the treatment strategy of PTLD-DLBCL was more heterogeneous. In detail, 7 out of 24 cases were initially treated following first-line B-NHL protocols. The remaining cases received a less aggressive regimen, mainly consisting of RIS (12 cases) and/or rituximab (12 cases). In 5 patients, low-dose chemotherapy (LDCT) was added (supplemental Table 1).
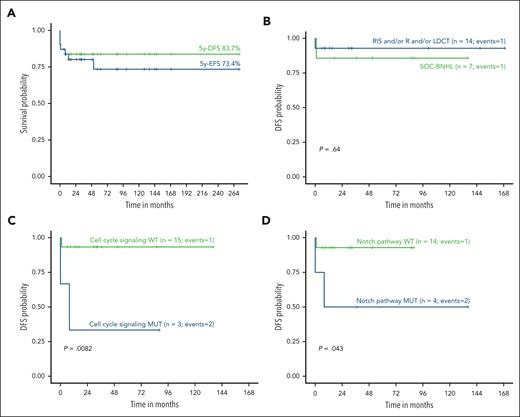
The 5-year EFS (5y-EFS) rate was 73% in the whole cohort. Seven patients died, of which 5 were related to lymphoma (5y-DFS 84%; Figure 5A). Of note, all deceased patients had PTLD-DLBCL, and 3 of them (with clinical information available) were at an advanced stage (III-IV) at diagnosis. Only 1 out of 14 patients with PTLD-DLBCL treated with less aggressive therapy (RIS, rituximab, and/or LDCT) died because of lymphoma (5y-DFS 93%). This survival rate is in line with the one observed in patients who received intense chemotherapy (6/7 patients, 86%) (Figure 5B). Two of the patients died of F-PTLD before any therapy could be initiated, and there was no information available on the treatment received by the remaining case.
Survival analyses and correlations with treatment and molecular features. (A) EFS and DFS probabilities within the complete cohort. (B) DFS probability of patients with PTLD-DLBCL treated with RIS and/or rituximab (R) and/or LDCT compared with that of cases treated with SOC-BNHL. (C) DFS probability of PTLD-DLBCL patients according to mutations affecting cell cycle signaling and (D) Notch pathway.
Survival analyses and correlations with treatment and molecular features. (A) EFS and DFS probabilities within the complete cohort. (B) DFS probability of patients with PTLD-DLBCL treated with RIS and/or rituximab (R) and/or LDCT compared with that of cases treated with SOC-BNHL. (C) DFS probability of PTLD-DLBCL patients according to mutations affecting cell cycle signaling and (D) Notch pathway.
Discussion
PTLD is a major complication in the recipients of both SOT and hematopoietic stem cell transplantation and occurs in up to 20% of patients, depending on the series.5,38 In this study, to the best of our knowledge, we report for the first time an integrative genetic and molecular characterization of a large series of mPTLDs in children and adolescents who received a SOT. Our results show relevant differences in the molecular landscape of these tumors with both PTLD in adults and DLBCL in IMC pediatric patients. Moreover, we show different molecular and clinical features between the 24 PTLD-DLBCL cases and 7 PTLD-BL cases included in the study, stressing the need for an adequate morphological and molecular diagnosis.
Overall, all 7 PTLD-BL cases had a MYC rearrangement and, unlike PTLD-DLBCL, had a GCB phenotype and EBV latency pattern I, fully resembling IMC-BL. However, morphology ranged from typical BL cytology to less monotonous cytology or even DLBCL morphology. Nevertheless, all cases harbored typical phenotypic and molecular features of BL, highlighting the relevance of latency and COO determination in those cases. Furthermore, all 7 cases depicted the mutational profile described in patients with IMC-BL. Specifically, MYC mutations, either exonic or multiple intronic, were detected in all BL samples, followed by frequent mutations in ID3 and DDX3X (4/7 cases each; 57%) and ARID1A (2/7 cases; 29%), with all 7 BL patients harboring, at least, one of these mutations. Our PTLD-BL also displayed lower genetic complexity than IMC-BL,33 with the absence of the characteristic 1q gains, which could be related to the EBV infection, as previously reported.15
Previous studies have observed differences in the mutational profile between EBV− and EBV+ BL, the latter being associated with recurrent mutations in ARID1A and RHOA and lower incidence of mutations in ID3, CCND3, or SMARCA4.11-13,15 Our 7 PTLD-BL cases were EBV positive, an expected higher frequency than that reported in sporadic and HIV-related BL39 and similar to eBL.40 All 6 investigated PTLD-BL cases had an EBV latency pattern I, typically seen in BL,12,41 unlike other types of PTLD in which latency type III is the most prevalent.3,42 We have also compared the mutational profile of previously published EBV+ and EBV− BL cohorts11-13 with our cases having a mutational distribution closer to EBV+ BL.4,5 Similarly, our cases seem to belong to the recently defined molecular subgroups highly represented in a large series of EBV+ BL tumors (DGG-BL and IC-BL) by whole-exome sequencing.15 Despite the limited number of our PTLD-BL series, our data also confirm the lower mutational burden in IC-BL–like cases than those with DGG-BL characteristics (6 vs 13 mutations/case) and the overrepresentation of male patients within DGG-BL.
Clinically, PTLD-BL showed a longer interval between transplantation and lymphoma onset compared with PTLD-DLBCL (Table 1) and had a more aggressive presentation (4/7 were at stage IV) than PTLD-DLBCL (1/24). All patients with PTLD-BL were alive and in complete remission at the last follow-up after treatment with RIS and chemotherapy following SOC for mature B-cell NHL (rituximab included in 4/7).
In line with this, previous studies have highlighted that PTLD-BL required chemotherapy in most cases.26,27,43 Moreover, a phase 2 trial proved the use of LDCT combined with rituximab to be effective in pediatric mPTLD after SOT, with a 2-year rate of overall survival and EFS being 90% and 76%, respectively.26 In this study from Gross et al, 4 out of 5 patients with PTLD-BL were long-term survivors. However, MYC status was not known in all cases. Another nonrandomized prospective multicenter trial studied a response-adapted sequential immunochemotherapy in PTLD after SOT observing a 2-year overall survival and EFS of 86% and 67%, respectively. In half of the patients, chemotherapy could be avoided after responding to rituximab induction. Of note, in this study, 86% of PTLD-BL cases required chemotherapy.27 All these observations claimed that PTLD-BL should be considered an independent entity and would benefit from intensive therapeutic regimes, therefore, its distinction from other mPTLDs is relevant.
In contrast, our data show that the genetic features of pediatric PTLD-DLBCL are very heterogeneous. Latency pattern III was the most common among PTLD-DLBCL cases, though patterns II and I were also observed in 30% and 10% of the cases respectively, in line with previous literature on mPTLD.3 Seven out of the 16 samples evaluated showed CNAs, with the most common alterations being gains of 3p and 9q21.11-q34.3. This CN landscape lacks characteristic alterations specific to pediatric and young adult DLBCL in IMC cases, such as 1q31.3-q42.13 gains and losses of 6q21 and 19p13.3;9 and it is also different from the one observed in adult PTLD-DLBCL, with the absence of most CNAs reported in those cases.21,34 The mutational profile was very diverse as well, with KMT2C being the most recurrently mutated gene (3 cases; 17%), followed by mutations in NOTCH1 or TP53 genes (2 cases each; 11%), among others. In terms of global mutational burden, PTLD-DLBCL showed a lower mutational load than IMC counterparts, lacking typically found SOCS1 mutations, and the profile of affected pathways was enriched with alterations in epigenetic modifiers and Notch signaling. EBNA-2 has been described to use the Notch pathway to immortalize B cells.44 In our series, none of the 10 EBNA-2 positive cases with mutational information harbored mutations in the Notch pathway, whereas 40% (2/5) of the EBV+ PTLD-DLBCL, EBNA-2 negative cases had mutations in the Notch pathway, supporting an alternative role for EBNA-2 and Notch in the oncogenesis of PTLD-DLBCL.45
The mutational landscape of pediatric PTLD-DLBCL was different from the one reported in adult PTLD-DLBCL, either EBV+ or EBV− cases, with a lack of mutations in KMT2D and PIK3CD and a lower frequency of TP53 mutations. This lack of recurrent genomic alterations in pediatric PTLD-DLBCL sheds light on the biology of these tumors, although, to confirm these observations, analyses in independent series by whole-exome or whole-genome approaches need to be performed.
Regarding clinical aspects, in our series, the patients with PTLD-DLBCL who were treated with less intensive schemes (14/24; 58%) had a 5-year DFS of 93%, and there was only 1 lymphoma-related death. Furthermore, among the 7 patients treated with B-NHL–specific strategies (5y-DFS 86%), there was 1 toxic death, stressing the vulnerability of this fragile population and the need of adapting treatments to reduce toxicities.
In summary, the different genetic backgrounds of pediatric PTLD-DLBCL in comparison with adult PTLD-DLBCL and IMC children and young adults, and the response to different treatment strategies, suggest that perhaps less intensive schemes with only RIS and rituximab or LDCT could be a therapeutic option in selected patients. In addition, correlation analyses between molecular features and clinical outcomes revealed that alterations in the cell cycle signaling and Notch pathway were associated with a worse outcome. The real impact of these alterations as potential biomarkers needs to be further investigated.
Moreover, previous studies in adult PTLD have reported the association of EBV latency pattern III and replication with significantly shorter survival and early PTLD onset.3 In pediatric mPTLD, patients with EBV latency III and viral replication presented a shorter time from transplantation to PTLD diagnosis (8.2 vs 47.6 months; P = .004) and a tendency toward a worse prognosis (2y-DFS 60 vs 82%; P=.33) (supplemental Figure 6B). Although the EBV latency pattern is currently not available for clinicians, its inclusion in the design of future therapeutic strategies should be considered.
The incidence of LBCL/HG-11q and LBCL-IRF4 in pediatric mPTLD had not been previously investigated. Remarkably, Ferreiro et al46 described an overrepresentation of LBCL/HG-11q in a subset of molecularly defined adult BL in the posttransplantation setting. Interestingly, in our pediatric mPTLD, no 11q aberrations were identified by FISH and/or CN arrays. Similarly, although 20 mPTLD cases with DLBCL diagnosis expressed IRF4/MUM1, none of the 23 investigated cases displayed IRF4 rearrangements. These results suggest the rarity of LBCL/HG-11q and LBCL-IRF4 in pediatric mPTLD, although larger series of cases should validate these observations. In addition, PAX5 alterations, previously reported to occur in B-NHL in the posttransplantation setting and associated with unfavorable outcomes,3,47,48 were absent in pediatric mPTLD, suggesting that PAX5 is not a key gene related to lymphomagenesis in these patients.
To our knowledge, we performed a multidisciplinary genome-wide analysis of a large series of pediatric SOT–associated mPTLD for the first time, observing distinct molecular profiles among PTLD-BL and PTLD-DLBCL cases. Furthermore, PTLD-BL showed similarities with EBV+ IMC-BL, whereas PTLD-DLBCL was genetically different compared with adult PTLD and IMC-DLBCL counterparts.
Moreover, although the low number of patients included and the diverse therapeutic approaches in our series are limitations, our analyses support previous evidence stating that PTLD-DLBCL in SOT recipients can respond to less intensive treatment.26,27 Further prospective studies should be undertaken to corroborate our findings and help in defining more personalized therapeutic strategies for these patients.
Acknowledgments
The authors thank the centers of the Sociedad Española de Hematología y Oncología Pediátricas that submitted cases for centralized review and to Silvia Martín, Helena Suarez, Nuria Orellana, and Silvia Ruiz for their excellent technical and administrative assistance. The authors thank Adela Rodriguez from Hospital Clínic for helping to confirm the nondeleterious effect of BRCA2 mutations. The authors are indebted to the Institut d´Investigacions Biomèdiques August Pi I Sunyer (IDIBAPS) Genomics Core Facility, HCB-IDIBAPS Biobank-Tumor Bank, and Biobanc de l’HospitaI Infantil Sant Joan de Déu, Hospital Universitario Virgen del Rocío-Instituto de Biomedicina de Sevilla Biobank (Instituto de Salud Carlos III (ISCIII)-Red de Biobancos PT13/0010/0056), all 3 integrated into the National Network Biobanks of ISCIII for the sample and data procurement. This work was developed at the Centro Esther Koplowitz, Barcelona, Spain.
This work was funded by Fondo de Investigaciones Sanitarias ISCIII through the projects “Miguel Servet Program MSII18/0015” and “PI18/00471” (I.S.) and European Regional Development Fund “Una manera de hacer Europa,” Asociación Española Contra el Cáncer (AECC CICPFI6025SALA), Generalitat de Catalunya Suport Grups de Recerca (2017-SGR-1107 and 2021-SGR-01293 [I.S.]). J.E.R.-Z. was supported by a fellowship from Generalitat de Catalunya AGAUR FI-DGR 2017 (2017 FI_B01004). N.G. has been continuously supported by Acció instrumental d’incorporació de científics i tecnòlegs PERIS (SLT002/16/00336 and SL017/20/000204) from Generalitat de Catalunya. E.C. is an academia researcher of the Institució Catalana de Recerca i Estudis Avançats of the Generalitat de Catalunya.
Authorship
Contribution: J.S.-V. performed research, analyzed data, and wrote the manuscript; N.C.-d.-A. reviewed and interpreted pathological data, analyzed data, and wrote the manuscript; P.G.-G. reviewed and interpreted clinical data, analyzed data, and wrote the manuscript; J.E.R.-Z., M.L.-G., S.M., D.C., N.G., and I.M.-G. performed research and analyzed data; F.D.-C., J.M., M.G.-P., E.G.-F., M.L., G.F., B.G.-F., and E.C. reviewed and interpreted pathological data; M.A., C.G.-C., I.A., A.F., J.V.-A., S.G.-M., B.G., and V.C. reviewed and interpreted clinical data; O.B. performed morphological diagnosis, analyzed data, and wrote the manuscript; I.S. performed research, analyzed data, designed research, and wrote the manuscript; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Olga Balagué, Pathology department, Hematopathology Unit, Hospital Clinic de Barcelona, Villarroel 170, 08036 Barcelona, Spain; e-mail: obalague@clinic.cat; and Itziar Salaverria, Institut d´Investigacions Biomèdiques August Pi I Sunyer, Rosselló 153, 08036 Barcelona, Spain; e-mail: isalaver@recerca.clinic.cat.
References
Author notes
∗J.S.-V., N.C.-d.-A., P.G.-G., O.B., and I.S. contributed equally to the study.
Copy-number and sequencing data are available at the Gene Expression Omnibus repository and European Nucleotide Archive under accession numbers GSE198253 and ERP134862, respectively.
Data are available on request from the corresponding authors, Olga Balagué (obalague@clinic.cat) and Itziar Salaverria (isalaver@recerca.clinic.cat).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal