Lineage-specific assessment of residual disease could identify patients who can safely cease therapy.
Detection of BCR::ABL1 DNA in granulocytes and T cells at TKI cessation is a better predictor of relapse than those in total leukocytes.
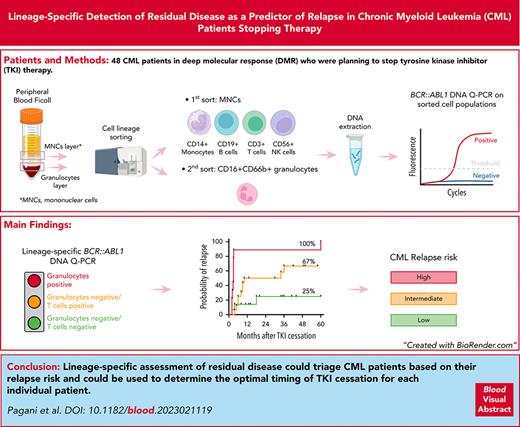
Visual Abstract
Patients with chronic myeloid leukemia who are eligible for treatment-free remission (TFR) may still relapse after tyrosine kinase inhibitor (TKI) cessation. There is a need for accurate predictors of outcome to enable patients with a favorable profile to proceed while avoiding futile attempts. Sensitive detection of residual disease in total leukocytes at treatment cessation is associated with relapse but is not highly discriminatory, likely because it is a composite measure of residual leukemia derived from different cell lineages, whereas only some lineages are relevant for relapse. We prospectively measured BCR::ABL1 DNA as a predictive yes/no binary test in 5 cellular fractions from 48 patients meeting conventional criteria for TKI discontinuation. The median BCR::ABL1 DNA level was higher in granulocytes and T cells, but not in other lineages, in patients who relapsed. Among the 40 patients undergoing their first TFR attempt, we defined 3 groups with differing relapse risk: granulocyte-positive group (100%), granulocyte-negative/T-cell–positive group (67%), and granulocyte-negative /T-cell–negative group (25%). These data show the critical importance of lineage-specific assessment of residual disease in the selection of patients who can attempt to achieve TFR with a high expectation of success and, concurrently, defer patients who have a high probability of relapse.
Introduction
Treatment-free remission (TFR) is the current therapeutic goal for patients with chronic myeloid leukemia (CML). TFR is achieved when a patient remains in major molecular response (MMR; BCR::ABL1 ≤ 0.1%) long term after discontinuing tyrosine kinase inhibitor (TKI) therapy. Recommended minimum eligibility criteria include ≥3 years on TKI treatment and ≥2 years in deep molecular response (DMR; BCR::ABL1 ≤ 0.01%).1 Approximately 50% of the patients who meet these criteria and cease TKIs will sustain TFR.2 To date, there are no accurate predictors of TFR to guide the optimal timing of stopping TKI therapy. Shorter BCR::ABL1-halving time upon first-line TKI initiation and longer total duration of TKI therapy or DMR before stopping TKI are associated with a higher probability of TFR,3-7 as is the DMR defined by digital polymerase chain reaction (PCR) for BCR::ABL1 messenger RNA (mRNA).5,8-12 However, low-level measurable residual disease by digital PCR in total leukocytes is still associated with a ≥32% probability of TFR,9,13 possibly because leukocytes comprise a mixture of granulocytic and lymphoid lineages, which may differ in their association with relapse risk. Digital PCR for BCR::ABL1 mRNA is typically dichotomized with an empirically determined threshold to define higher relapse risk.
Using fluorescence-activated cell sorting followed by patient-specific BCR::ABL1 DNA quantitative real-time PCR (qPCR),14-16 we previously showed that up to 10% of mature T and B lymphocytes at diagnosis are derived from the CML clone and may persist at low levels in stable TFR, mainly in long-lived naive B cells.17 Conversely, BCR::ABL1 DNA was never detected in granulocytes from patients in stable TFR.17 Hence, in this study, we tested whether prospective lineage-specific measurement of residual disease at the time of stopping TKI is a more accurate predictor of relapse.
Study design
Peripheral blood (80-100 mL) was collected from 48 patients with CML in DMR who were planning to stop TKI therapy. Lineage-specific BCR::ABL1 DNA qPCR was performed as previously described.17 Briefly, mononuclear cells were separated from granulocytes by Ficoll density gradient centrifugation. CD3+CD14– T cells, CD14+CD3– monocytes, CD3–CD14–CD19+CD56– B cells, and CD3–CD14–CD19–CD56+ natural killer (NK) cells were purified from the mononuclear cell layer by fluorescence-activated cell sorting. CD16+CD66b+ granulocytes were sorted from the granulocyte layer (supplemental Figure 1; supplemental Methods, available on the Blood website). DNA was extracted, and patient-specific BCR::ABL1 nested qPCR was performed using 10-μg amplifiable DNA (detection limit, 10–6.2; 1 in 2 × 106 cells; samples below this value were defined as undetectable or negative; supplemental Figure 2). Molecular relapse was defined as the loss of MMR on a single test.18 Patients were considered evaluable for the outcome if they remained in TFR for ≥6 months or relapsed at any time. Univariate Cox proportional hazards regression was performed to identify variables associated with TFR outcome (Tables 1 and 2), and variables with P value <.05 were selected for multivariate analysis.
Univariate Cox regression analysis of variables investigated as potential predictors of TFR
| Variable . | No. of patients . | Univariate analysis at 24 mo . | No. of patients . | Univariate analysis at 60 mo . | ||||
|---|---|---|---|---|---|---|---|---|
| β coefficient . | Hazard ratio (95% CI) . | P . | β coefficient . | Hazard ratio (95% CI) . | P . | |||
| Gender | 40 | 40 | ||||||
| Male | 19 | Ref | 19 | Ref | ||||
| Female | 21 | 0.111 | 1.117 (0.462-2.702) | .806 | 21 | 0.17 | 1.185 (0.519-2.707) | .687 |
| Age at TFR attempt, y | 40 | 0.026 | 1.027 (0.986-1.069) | .196 | 40 | 0.017 | 1.017 (0.981-1.055) | .36 |
| Sokalscore | 36 | 36 | ||||||
| High | 8 | Ref | 8 | Ref | ||||
| Intermediate | 14 | 0.94 | 2.56 (0.695-9.432) | .158 | 14 | 0.753 | 2.123 (1.264-0.206) | .206 |
| Low | 14 | −0.104 | 0.901 (0.215-3.784) | .887 | 14 | −0.164 | 0.849 (0.236-3.049) | .802 |
| EUTOS score | 36 | 36 | ||||||
| High | 4 | Ref | 4 | Ref | ||||
| Low | 32 | −0.08 | 0.923 (0.211-4.045) | .915 | 32 | −0.296 | 0.744 (1.214-2.588) | .642 |
| Transcript type | 40 | 40 | ||||||
| e13a2 | 16 | Ref | 16 | Ref | ||||
| e14a2 | 18 | −1.106 | 0.331 (0.117-0.980) | .046 | 18 | −0.86 | 0.423 (0.162-0 1.103) | .078 |
| Both e13a2 and e14a2 | 6 | 0.435 | 1.544 (0.525-4.542) | .43 | 6 | 0.398 | 1.49 (0.511-4.340) | .465 |
| Duration of TKI therapy | 40 | 0.019 | 1.02 (0.913-1.139) | .731 | 40 | 0.03 | 1.031 (0.927-1.146) | .579 |
| Duration of MR4, y | 40 | −0.027 | 0.973 (0.807-1.174) | .971 | 40 | −0.005 | 0.995 (0.837-1.183) | .955 |
| Duration of MR4.5, y | 40 | −0.11 | 0.896 (0.719-1.116) | .327 | 40 | −0.078 | 0.925 (0.756-1.131) | .448 |
| TKI ceased | 40 | 40 | ||||||
| Dasatinib | 13 | Ref | 13 | Ref | ||||
| Imatinib | 13 | −0.466 | 0.628 (0.223-1.766) | .377 | 13 | −0.434 | 0.648 (0.238-1.765) | .396 |
| Nilotinib | 14 | −0.678 | 0.508 (0.17-1.516) | .224 | 14 | −0.533 | 0.587 (0.214-1.610) | .301 |
| Halving time, d | 37 | 0.072 | 1.074 (0.991-1.165) | .084 | 37 | 0.083 | 1.086 (1.006-1.173) | .034 |
| Leukocytes, BCR::ABL1 pos∗ | 39 | 1.258 | 3.519 (1.025-12.08) | .046 | 39 | 1.407 | 4.084 (1.201-13.89) | .024 |
| Granulocytes, BCR::ABL1 pos∗ | 40 | 2.158 | 8.654 (3.241-23.11) | .00002 | 40 | 1.871 | 6.494 (2.61-16.16) | .00006 |
| Monocytes, BCR::ABL1 pos∗ | 37 | 0.299 | 1.349 (0.531-3.426) | .529 | 37 | 0.243 | 1.28 (0.524-3.104) | .592 |
| B cells, BCR::ABL1 pos∗ | 38 | 0.035 | 1.0353 (0.3-3.568) | .956 | 38 | 0.113 | 1.119 (0.328-3.82) | .857 |
| T cells, BCR::ABL1 pos∗ | 39 | 1.165 | 3.206 (1.068-9.621) | .038 | 39 | 1.458 | 4.297 (1.246-14.82) | .021 |
| NK cells, BCR::ABL1 pos∗ | 37 | 0.01 | 1.01 (0.396-2.576) | .984 | 37 | 0.06 | 1.061 (0.421-2.676) | .9 |
| Variable . | No. of patients . | Univariate analysis at 24 mo . | No. of patients . | Univariate analysis at 60 mo . | ||||
|---|---|---|---|---|---|---|---|---|
| β coefficient . | Hazard ratio (95% CI) . | P . | β coefficient . | Hazard ratio (95% CI) . | P . | |||
| Gender | 40 | 40 | ||||||
| Male | 19 | Ref | 19 | Ref | ||||
| Female | 21 | 0.111 | 1.117 (0.462-2.702) | .806 | 21 | 0.17 | 1.185 (0.519-2.707) | .687 |
| Age at TFR attempt, y | 40 | 0.026 | 1.027 (0.986-1.069) | .196 | 40 | 0.017 | 1.017 (0.981-1.055) | .36 |
| Sokalscore | 36 | 36 | ||||||
| High | 8 | Ref | 8 | Ref | ||||
| Intermediate | 14 | 0.94 | 2.56 (0.695-9.432) | .158 | 14 | 0.753 | 2.123 (1.264-0.206) | .206 |
| Low | 14 | −0.104 | 0.901 (0.215-3.784) | .887 | 14 | −0.164 | 0.849 (0.236-3.049) | .802 |
| EUTOS score | 36 | 36 | ||||||
| High | 4 | Ref | 4 | Ref | ||||
| Low | 32 | −0.08 | 0.923 (0.211-4.045) | .915 | 32 | −0.296 | 0.744 (1.214-2.588) | .642 |
| Transcript type | 40 | 40 | ||||||
| e13a2 | 16 | Ref | 16 | Ref | ||||
| e14a2 | 18 | −1.106 | 0.331 (0.117-0.980) | .046 | 18 | −0.86 | 0.423 (0.162-0 1.103) | .078 |
| Both e13a2 and e14a2 | 6 | 0.435 | 1.544 (0.525-4.542) | .43 | 6 | 0.398 | 1.49 (0.511-4.340) | .465 |
| Duration of TKI therapy | 40 | 0.019 | 1.02 (0.913-1.139) | .731 | 40 | 0.03 | 1.031 (0.927-1.146) | .579 |
| Duration of MR4, y | 40 | −0.027 | 0.973 (0.807-1.174) | .971 | 40 | −0.005 | 0.995 (0.837-1.183) | .955 |
| Duration of MR4.5, y | 40 | −0.11 | 0.896 (0.719-1.116) | .327 | 40 | −0.078 | 0.925 (0.756-1.131) | .448 |
| TKI ceased | 40 | 40 | ||||||
| Dasatinib | 13 | Ref | 13 | Ref | ||||
| Imatinib | 13 | −0.466 | 0.628 (0.223-1.766) | .377 | 13 | −0.434 | 0.648 (0.238-1.765) | .396 |
| Nilotinib | 14 | −0.678 | 0.508 (0.17-1.516) | .224 | 14 | −0.533 | 0.587 (0.214-1.610) | .301 |
| Halving time, d | 37 | 0.072 | 1.074 (0.991-1.165) | .084 | 37 | 0.083 | 1.086 (1.006-1.173) | .034 |
| Leukocytes, BCR::ABL1 pos∗ | 39 | 1.258 | 3.519 (1.025-12.08) | .046 | 39 | 1.407 | 4.084 (1.201-13.89) | .024 |
| Granulocytes, BCR::ABL1 pos∗ | 40 | 2.158 | 8.654 (3.241-23.11) | .00002 | 40 | 1.871 | 6.494 (2.61-16.16) | .00006 |
| Monocytes, BCR::ABL1 pos∗ | 37 | 0.299 | 1.349 (0.531-3.426) | .529 | 37 | 0.243 | 1.28 (0.524-3.104) | .592 |
| B cells, BCR::ABL1 pos∗ | 38 | 0.035 | 1.0353 (0.3-3.568) | .956 | 38 | 0.113 | 1.119 (0.328-3.82) | .857 |
| T cells, BCR::ABL1 pos∗ | 39 | 1.165 | 3.206 (1.068-9.621) | .038 | 39 | 1.458 | 4.297 (1.246-14.82) | .021 |
| NK cells, BCR::ABL1 pos∗ | 37 | 0.01 | 1.01 (0.396-2.576) | .984 | 37 | 0.06 | 1.061 (0.421-2.676) | .9 |
Significant P values are shown in bold.
CI, confidence interval; EUTOS, European Treatment and Outcome Study; pos, positive; ref, reference.
Binary variables.
Multivariate Cox regression analysis of variables investigated as potential predictors of TFR
| Variable . | No. of patients . | β coefficient . | Hazard ratio (95% CI) . | P . | Concordance index (standard error) . |
|---|---|---|---|---|---|
| Multivariate analysis at 24 mo | |||||
| Granulocytes, BCR::ABL1 pos∗ | 39 | 1.867 | 6.467 (2.332-17.930) | .0003 | 0.756 (0.061) |
| T cells, BCR::ABL1 pos∗ | 39 | 0.76 | 2.138 (0.672-6.805) | .147 | |
| Granulocytes, BCR::ABL1 pos∗ | 37 | 1.857 | 6.402 (2.345-17.481) | .0003 | 0.727 (0.069) |
| Halving time, d | 37 | 0.053 | 1.055 (0.98-1.135) | .159 | |
| Multivariate analysis at 60 mo | |||||
| Granulocytes, BCR::ABL1 pos∗ | 39 | 1.531 | 4.625 (1.787-11.97) | .002 | 0.752 (0.062) |
| T cells, BCR::ABL1 pos∗ | 39 | 0.93 | 2.535 (0.816-7.88) | .108 | |
| Granulocytes, BCR::ABL1 pos∗ | 37 | 1.721 | 5.594 (2.076-15.077) | .0006 | 0.728 (0.069) |
| Halving time, d | 37 | 0.062 | 1.063 (0.899-1.143) | .093 | |
| Variable . | No. of patients . | β coefficient . | Hazard ratio (95% CI) . | P . | Concordance index (standard error) . |
|---|---|---|---|---|---|
| Multivariate analysis at 24 mo | |||||
| Granulocytes, BCR::ABL1 pos∗ | 39 | 1.867 | 6.467 (2.332-17.930) | .0003 | 0.756 (0.061) |
| T cells, BCR::ABL1 pos∗ | 39 | 0.76 | 2.138 (0.672-6.805) | .147 | |
| Granulocytes, BCR::ABL1 pos∗ | 37 | 1.857 | 6.402 (2.345-17.481) | .0003 | 0.727 (0.069) |
| Halving time, d | 37 | 0.053 | 1.055 (0.98-1.135) | .159 | |
| Multivariate analysis at 60 mo | |||||
| Granulocytes, BCR::ABL1 pos∗ | 39 | 1.531 | 4.625 (1.787-11.97) | .002 | 0.752 (0.062) |
| T cells, BCR::ABL1 pos∗ | 39 | 0.93 | 2.535 (0.816-7.88) | .108 | |
| Granulocytes, BCR::ABL1 pos∗ | 37 | 1.721 | 5.594 (2.076-15.077) | .0006 | 0.728 (0.069) |
| Halving time, d | 37 | 0.062 | 1.063 (0.899-1.143) | .093 | |
Significant P values are shown in bold.
Binary variables.
Results and discussion
BCR::ABL1 DNA was measured in leukocytes and in a total of 240 cellular fractions from 48 patients before stopping TKI, and it was detectable in leukocytes (64%), granulocytes (25%), monocytes (36%), B cells (88%), T cells (57%), and NK cells (56%) (supplemental Table 1). Forty patients attempted to achieve TFR for the first time, of whom 22 (58%) relapsed at a median of 5 months (range, 2-60 months) and 18 maintained TFR, with a median follow-up of 34 months (range, 8-65 months). Eight patients attempted to achieve TFR for a second time, of whom 7 lost MMR after a median of 3 months (range, 1-5 months) and 1 patient remained in TFR at 12 months. Patient characteristics are summarized in supplemental Table 2. Comparing patients who subsequently relapsed with those who maintained TFR, BCR::ABL1 DNA was present at higher levels in leukocytes (P = .032), granulocytes (P = .003), and T cells (P = .009) but not in monocytes, B cells, or NK cells (Figure 1A).
Prediction model of TFR outcomes based on the lineage-specific assessment of residual disease.BCR::ABL1 DNA levels in total leukocytes and in sorted cell populations. Blue dots represent TFR, and red dots represent patients who relapsed. (A) Kaplan-Meier analysis of probability of relapse based on BCR::ABL1 DNA positivity/negativity in (B) total leukocytes (30%; 95% confidence interval [CI], 2-68; vs 70%; 95% CI, 54-81; hazard ratio log rank [HR], 3.648; 95% CI, 1.510-8.812), (C) granulocytes (49% [95% CI, 27-68] vs 100%; HR, 5.174 [95% CI, 1.417-18.89]), and (D) T cells (30% [95% CI, 4-65] vs 73% [95% CI, 59-83]; HR, 3.262 [95% CI, 1.376-7.730]). (E) Personalized yes/no binary prediction model of TFR outcomes based on BCR::ABL1 positivity/negativity in granulocytes and T cells. Gran, granulocytes; m, months; +, positive; –, negative.
Prediction model of TFR outcomes based on the lineage-specific assessment of residual disease.BCR::ABL1 DNA levels in total leukocytes and in sorted cell populations. Blue dots represent TFR, and red dots represent patients who relapsed. (A) Kaplan-Meier analysis of probability of relapse based on BCR::ABL1 DNA positivity/negativity in (B) total leukocytes (30%; 95% confidence interval [CI], 2-68; vs 70%; 95% CI, 54-81; hazard ratio log rank [HR], 3.648; 95% CI, 1.510-8.812), (C) granulocytes (49% [95% CI, 27-68] vs 100%; HR, 5.174 [95% CI, 1.417-18.89]), and (D) T cells (30% [95% CI, 4-65] vs 73% [95% CI, 59-83]; HR, 3.262 [95% CI, 1.376-7.730]). (E) Personalized yes/no binary prediction model of TFR outcomes based on BCR::ABL1 positivity/negativity in granulocytes and T cells. Gran, granulocytes; m, months; +, positive; –, negative.
Because of the small number of patients attempting to achieve TFR a second time, we confined subsequent predictive analyses to the 40 patients who attempted to achieve TFR for the first time. The detection of BCR::ABL1 DNA was associated with a higher cumulative incidence of relapse by 60 months in total leukocytes (70% vs 30%; P = .023; Figure 1B), granulocytes (100% vs 49%; P < .0001; Figure 1C), and T cells (73% vs 30%; P = .022; Figure 1D). For those patients without BCR::ABL1 detectable in granulocytes, BCR::ABL1 in T cells was used to define 2 groups with differing relapse risk so that we were able to define 3 groups with differing probability of relapse by 60 months: granulocyte-positive group (n = 9) with 100%; granulocyte-negative/T-cell–positive group (n = 16) with 67%; and granulocyte-negative/T-cell–negative group (n = 14) with 25% relapse (P < .0001; Figure 1E). The accuracy of this model was 77%. All the patients in the granulocyte-positive group relapsed after a median interval of 3 months (range, 2-60 months), whereas the patients in the granulocyte-negative/T-cell–positive group relapsed later after a median of 8.5 months (range, 3.6-36 months; P = .003).
Candidate prognostic variables were included in the Cox regression analysis as shown in Tables 1 and 2. In univariate analysis, halving time (continuous), total leukocyte positivity, granulocyte positivity, and T-cell positivity (binary) were associated with molecular relapse at 60 months. In multivariate analysis, the model with granulocytes and T cells was the strongest (concordance, 0.752). Granulocytes remained the only prognostic factor (P = .002 at 60 months), whereas T cells lost significance (P = .108), indicating that they were likely covariates, as would be expected if they originated from a common precursor population. In a second model combining granulocytes and halving time, granulocytes were, again, independent predictors of relapse (P = .0006 at 60 months), whereas halving time was not statistically significant (P = .093).
We have shown that the detection of BCR::ABL1 DNA before TKI cessation in granulocytes and T cells, but not in other lineages, is a more specific predictor of relapse than BCR::ABL1 measured in total leukocytes. The finding of BCR::ABL1 DNA in granulocytes is evidence of the persistence of a CML precursor population giving rise to short-lived, terminally differentiated cells. Perhaps unexpectedly, the detection of BCR::ABL1 in T cells was also associated with relapse and presumably reflects the presence of a multipotent CML precursor population in a fraction of those cases. If rare multipotent precursors give rise to detectable T cells without detectable granulocytes, it might simply be a stochastic phenomenon, or there could be biological differences leading to a greater abundance of clonal T cells. In contrast, BCR::ABL1 DNA in B cells is likely explained by long-lived B cells even in the absence of a persistent precursor population. Halving time was predictive of relapse in univariate analysis, but it was not independent of granulocyte BCR::ABL1, which may be explained by a more rapid decline of BCR::ABL1, leading to a deeper suppression of the leukemic clone. Conversely, a longer halving time may lead to a higher likelihood of a persistent clonal population, including granulocytes and T cells.
These results are clinically important because they show that improved accuracy of prediction can be achieved by lineage-specific assessment of residual disease. Detection of BCR::ABL1-positive granulocytes can be regarded as a “red light” that indicates the futility of a TFR attempt at that time and the need for an alternative therapeutic strategy (more potent or prolonged TKI therapy, targeting other pathways). Double negativity represents a “green light,” with a 75% probability of TFR at 5 years. Larger studies are required to assess the interaction between lineage-specific BCR::ABL1 and other predictors of TFR,19 and these will likely contribute to a more accurate, personalized prediction of the TFR outcome for patients with CML.
Acknowledgments
The authors thank the patients who kindly donated blood samples to make this research possible. The authors also thank the staff of the South Australian Cancer Research Biobank for assistance with access to samples; the Adelaide Health and BioMedical Precinct Cytometry Core Facility for the sorting of the patient samples; and the South Australian Genomics Centre for the BCR::ABL1-targeted sequencing.
This research project was supported by the Leukaemia Foundation of Australia (Strategic Ecosystem Research Partnership grant) (I.S.P.); Cancer Council SA, Beat Cancer Project on behalf of its donors and the State Government of South Australia, through the Department of Health and Wellbeing (I.S.P.); and the National Health and Medical Research Council of Australia project grant (APP1138935) (T.P.H., A.S.M.Y., S.B., and D.M.R.) and investigator grant (2007908) (T.P.H.).
Authorship
Contribution: I.S.P. conceived the project, developed the concept, designed and performed experiments, analyzed the data, and wrote the paper; N.S. performed the Cox regression analysis, contributed clinical data, and reviewed the manuscript; P.D., V.A.S., M.T., and J.J. performed experiments and reviewed the paper; R.G. performed flow sorting; C.H.K. performed the bioinformatic analysis of the BCR::ABL1 breakpoints; J.A.B. and H.K.A. performed the BCR::ABL1 mRNA molecular analysis; D.T.Y. and A.S.M.Y. contributed clinical data and reviewed the manuscript; S.B. supervised the BCR::ABL1 mRNA molecular analysis and reviewed the manuscript; and T.P.H. and D.M.R. conceived the project, contributed clinical data, and wrote the paper.
Conflict-of-interest disclosure: N.S. received honoraria from Novartis and Takeda. D.T.Y. received research funding from Novartis and Bristol Myers Squibb (BMS) and honoraria from Novartis, Takeda, Pfizer, and Amgen. S.B. is a member of advisory boards of Qiagen, Novartis, and Cepheid; received honoraria from Qiagen, Novartis, and Cepheid; and research funding from Novartis and Cepheid. A.S.M.Y. is a member of an advisory board for Novartis; received research funding from Novartis and BMS/Celgene; and received honoraria from Novartis and BMS. T.P.H. is a member of advisory boards for Novartis, Takeda, Enliven, and Terns and received research funding from Novartis and BMS. D.M.R. has received research funding from Novartis and BMS/Celgene; served as a member of advisory boards for Novartis, BMS, and Menarini; and received honoraria from Novartis, BMS/Celgene, and Keros. The remaining authors declare no competing financial interests.
Correspondence: Ilaria S. Pagani, Precision Cancer Medicine Theme, Blood Cancer Program, Chronic Myeloid Leukaemia Research Group, South Australian Health & Medical Research Institute, North Terrace, Adelaide, SA 5000, Australia; email: ilaria.pagani@sahmri.com.
References
Author notes
All data are available on request from the corresponding author, Ilaria S. Pagani (ilaria.pagani@sahmri.com).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

![Prediction model of TFR outcomes based on the lineage-specific assessment of residual disease.BCR::ABL1 DNA levels in total leukocytes and in sorted cell populations. Blue dots represent TFR, and red dots represent patients who relapsed. (A) Kaplan-Meier analysis of probability of relapse based on BCR::ABL1 DNA positivity/negativity in (B) total leukocytes (30%; 95% confidence interval [CI], 2-68; vs 70%; 95% CI, 54-81; hazard ratio log rank [HR], 3.648; 95% CI, 1.510-8.812), (C) granulocytes (49% [95% CI, 27-68] vs 100%; HR, 5.174 [95% CI, 1.417-18.89]), and (D) T cells (30% [95% CI, 4-65] vs 73% [95% CI, 59-83]; HR, 3.262 [95% CI, 1.376-7.730]). (E) Personalized yes/no binary prediction model of TFR outcomes based on BCR::ABL1 positivity/negativity in granulocytes and T cells. Gran, granulocytes; m, months; +, positive; –, negative.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/142/25/10.1182_blood.2023021119/2/m_blood_bld-2023-021119-gr1.jpeg?Expires=1769090555&Signature=ywnasLByOv162MBgDemCosSFYMnZonA3JvOINRgG~nyIwfy~T8yJwfaaW1m3EKGWfc~eNHXgARzbkirhtJquipDENJwYgd0ocNUA0GoTj56djuYvP~YsrTxJ1rN~mBWckTbAeIk5HJHEjVo34G9emRScy3IaSdIOzowv0IW0Mih1x82mPWKJoq7qLj9W5pMO3i3PK2mPN5ncwLUVglELXDB0Hk9X7IhYZghNhHk2TbYwWzZSmbSxWyjFYlI3p5NohAOG98Rbz58brfOSEuy2FVJVwxnDW-0-oJkptDmdiruCb5HydulUt1u~E6Cx8lTlcfZp0zC-A-bE2jdnMoVrJQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Comments
Response to Machova Polakova et al.
1,2
In a prior work Machova Polakova and colleagues used paired analysis of BCR::ABL1 DNA and RNA and showed that patients with undetectable BCR::ABL1 using both measurements had the lowest relapse risk and those double-positive had the highest relapse risk3. They pose the question whether the two studies are reporting the same phenomenon: i.e. does the detection of BCR::ABL1 DNA without RNA reflect the presence of BCR::ABL1-positive T lymphocytes with absent RNA expression, and therefore an intermediate risk of relapse.
At diagnosis BCR::ABL1 is expressed in both B and T cells.2 The median fraction of BCR::ABL1 DNA positive lymphocytes was around 1% and the median expression ratio was similar, suggesting that the relative expression per cell is similar to other lineages that were tested, including granulocytes.
An alternative explanation for the pattern reported by Machova Polakova is that sensitive RNA-based methods have a background false positive rate.4,5 Since the odds of having a false positive result in both RNA and DNA MRD tests is lower, the finding of concordant values may reveal a higher-level of MRD (double-positive) or a stringent lower-level of MRD (double-negative), with discordant values representing an intermediate level. Rare transcripts from B lymphocytes could represent a kind of biological false positive, i.e. a technical true positive, but not associated with relapse risk.
Although BCR::ABL1 DNA PCR on sorted cells is technically demanding, lineage-specific MRD provides greater biological insights than bulk MRD into the cell types responsible for relapse risk.
References
1. Pagani, I.S., Shanmuganathan, N., Dang, P., Saunders, V.A., Grose, R., Kok, C.H., James, J., Tolland, M., Braley, J.A., Altamura, H.K., et al. (2023). Lineage-specific detection of residual disease predicts relapse in patients with chronic myeloid leukemia stopping therapy. Blood 142, 2192-2197. 10.1182/blood.2023021119.
2. Pagani, I.S., Dang, P., Saunders, V.A., Grose, R., Shanmuganathan, N., Kok, C.H., Carne, L., Rwodzi, Z., Watts, S., McLean, J., et al. (2020). Lineage of measurable residual disease in patients with chronic myeloid leukemia in treatment-free remission. Leukemia 34, 1052-1061. 10.1038/s41375-019-0647-x.
3. Machova Polakova, K., Zizkova, H., Zuna, J., Motlova, E., Hovorkova, L., Gottschalk, A., Glauche, I., Koblihova, J., Pecherkova, P., Klamova, H., et al. (2020). Analysis of chronic myeloid leukaemia during deep molecular response by genomic PCR: a traffic light stratification model with impact on treatment-free remission. Leukemia 34, 2113-2124. 10.1038/s41375-020-0882-1.
4. Franke, G.N., Maier, J., Wildenberger, K., Cross, M., Giles, F.J., Muller, M.C., Hochhaus, A., Niederwieser, D., and Lange, T. (2020). Comparison of Real-Time Quantitative PCR and Digital Droplet PCR for BCR-ABL1 Monitoring in Patients with Chronic Myeloid Leukemia. J Mol Diagn 22, 81-89. 10.1016/j.jmoldx.2019.08.007.
5. Branford, S. (2020). Why is it critical to achieve a deep molecular response in chronic myeloid leukemia? Haematologica 105, 2730-2737. 10.3324/haematol.2019.240739.
DNA based MRD analysis and treatment free remission in CML
These findings closely align with our published data (Machova Polakova et al. Leukemia 2020), which explored molecular relapse-free survival (MRFS) based on the detection of BCR::ABL1 DNA and RNA in total leukocytes before TKI cessation. Using a very similar traffic light system to Pagani et al, we identified three distinct patient groups with significant differences in MRFS probability (P= 0.0005). The green group (n=11, RNA negative/DNA negative) exhibited 100% MRFS at 18 months, the yellow group (n=16, MRD RNA negative/DNA positive) showed 63% MRFS, and the red group (n=15, double positive) had 20% MRFS.
Earlier work by Pagani et al. (Leukemia 2020) indicated that in patients in TFR with undetectable BCR::ABL1 transcripts, BCR::ABL1 DNA positivity was confined to the lymphoid compartment. Combining these findings, it appears that T cells may be BCR::ABL1 positive without expressing BCR::ABL1, and this likely explains the similarities in the TFR stratification models between both studies. We suggest that the approach taken in our study is easier to implement in routine practice since it does not require analysis of cell fractions.
References:
1. Pagani IS, Shanmuganathan N, Dang P, et al. Lineage-specific detection of residual disease predicts relapse in patients with chronic myeloid leukemia stopping therapy. Blood 2023; 142(25): 2192-2197
2. Machova Polakova K, Zizkova H, Zuna J, et al. Analysis of chronic myeloid leukaemia during deep molecular response by genomic PCR: a traffic light stratification model with impact on treatment-free remission. Leukemia 2020; 34:2113-2124.
3. Pagani IS, Dang P, Saunders VA, et al. Lineage of measurable residual disease in patients with chronic myeloid leukemia in treatment-free remission. Leukemia 2020; 34:1052-1061