Key Points
We describe a novel pathway leading to histiocytosis in which defective nucleoside transport drives hyperactivation of TLR–MAPK signaling.
Patients with the histiocytosis-predisposing genetic disorder H syndrome may benefit from MAPK–directed targeted therapy.
Abstract
Histiocytoses are inflammatory myeloid neoplasms often driven by somatic activating mutations in mitogen-activated protein kinase (MAPK) cascade genes. H syndrome is an inflammatory genetic disorder caused by germ line loss-of-function mutations in SLC29A3, encoding the lysosomal equilibrative nucleoside transporter 3 (ENT3). Patients with H syndrome are predisposed to develop histiocytosis, yet the mechanism is unclear. Here, through phenotypic, molecular, and functional analysis of primary cells from a cohort of patients with H syndrome, we reveal the molecular pathway leading to histiocytosis and inflammation in this genetic disorder. We show that loss of function of ENT3 activates nucleoside-sensing toll-like receptors (TLR) and downstream MAPK signaling, inducing cytokine secretion and inflammation. Importantly, MEK inhibitor therapy led to resolution of histiocytosis and inflammation in a patient with H syndrome. These results demonstrate a yet-unrecognized link between a defect in a lysosomal transporter and pathological activation of MAPK signaling, establishing a novel pathway leading to histiocytosis and inflammation.
Introduction
Histiocytoses are a heterogeneous group of inflammatory myeloid neoplasms characterized by infiltration of histiocytes into various tissues, accompanied by inflammation leading to tissue damage.1 Histiocytes, now commonly referred to as mononuclear phagocytes (MNPs), are a family of phagocytic cells of myeloid origin, including monocytes, dendritic cells (DCs), and macrophages.2 Different types of histiocytoses are diagnosed based on histological markers, including Langerhans cell histiocytosis (LCH), Erdheim-Chester disease, Rosai-Dorfman disease (RDD), and juvenile xanthogranuloma.1 Activation of extracellular signal–regulated kinase (ERK) is considered a universal driver of histiocytosis, and somatic alterations in mitogen-activated protein kinase (MAPK) cascade genes (predominantly BRAF) are identified in most cases.3-8 However, the pathogenesis of histiocytosis has not fully been elucidated. Despite the universal role of ERK activation in histiocytosis, somatic mutations in MAPK cascade genes are not identified in all cases,9 suggesting the existence of additional alternative pathways for MAPK activation.
Germ line biallelic loss-of-function mutations in the nucleoside transporter SLC29A3 cause H syndrome10 (histiocytosis-lymphadenopathy plus syndrome, OMIM #602782), an inflammatory genetic disorder predisposing to histiocytosis by an unknown mechanism. The disorder has high phenotypic variability and clinical manifestations encompass different combinations of symptoms, including lymphadenopathy, hepatosplenomegaly, anemia, insulin–dependent diabetes mellitus, arthritis, and elevated biomarkers of inflammation.11 Cutaneous features are common and include indurated skin, hyperpigmentation, and hypertrichosis. These typical skin lesions contain infiltrating histiocytes with emperipolesis and a CD68+ S100+ CD1a− immunophenotype, similar to RDD-type histiocytosis.12,13 RDD–like histiocytic lesions have also been reported in subcutis,14 lymph nodes,15 and nasal mucosa.16 However, the mechanism leading to histiocytosis in H syndrome remains unclear, and, to our knowledge, the involvement of histiocytes in other disease manifestations has not been investigated.
The SLC29A3 gene encodes the protein equilibrative nucleoside transporter 3 (ENT3).17 ENT3 functions as a transporter of nucleosides from endolysosomes/lysosomes to the cytoplasm after degradation of nucleic acids.18 This function is critical for phagocytic cells, which engulf and degrade cellular debris and thus break down large quantities of nucleic acids. In these cells, loss of ENT3 function results in accumulation of nucleosides inside endolysosomes, leading to elevation of lysosomal pH and impaired lysosomal function.18
Here, we conducted a comprehensive analysis of multiple samples from patients with H syndrome, combining single-cell RNA sequencing, multiparameter flow cytometry, and functional assays to identify the molecular mechanisms leading to histiocytosis and inflammation in H syndrome. We show that loss of function of ENT3 activates nucleoside-sensing toll-like receptors (TLRs) and downstream MAPK signaling, inducing cytokine secretion and inflammation. Importantly, MEK inhibitor therapy generated a dramatic response in a patient with H syndrome, inducing complete regression of a histiocytic tumor accompanied by significant improvement of the systemic inflammatory manifestations. These results demonstrate a yet unrecognized link between defective lysosomal nucleoside transport and pathological activation of MAPK signaling, establishing a novel pathway leading to histiocytosis, and opening new treatment options for these patients.
Methods
Patients
Patients ≤21 years old with documented biallelic mutations in the SLC29A3 gene, treated at 2 tertiary medical centers in Israel, Schneider Children’s Medical Center and Soroka Medical Center, between January 2019 and June 2022 were eligible for inclusion in our study. The cohort included 9 patients from 3 kindreds, of Bedouin and Palestinian Arab ethnicity. Median age was 14.2 years (range, 1.9-18.5). Patient characteristics and treatment modalities are summarized in Table 1 and supplemental Table 1, available on the Blood website. A graphical representation of the different ENT3 mutations is presented in supplemental Figure 1. The study was conducted in accordance with the Declaration of Helsinki, under approval of the institutional review boards of Rabin Medical Center and Soroka Medical Center, and was carried out according to local guidelines and regulations. Trametinib and tocilizumab were administered to patients as off-label treatment. Further details are summarized in supplemental Methods.
Patient characteristics and treatment modalities
| Characteristics . | n (%) . |
|---|---|
| Total | 9 (100) |
| Age at time of study, y | |
| Median (range) | 14.2 (1.9-18.5) |
| Sex | |
| Male | 2 (22) |
| Female | 7 (78) |
| Ethnicity | |
| Palestinian Arab | 4 (44) |
| Bedouin | 5 (56) |
| Germ line SLC29A3 mutations | |
| Homozygous c.1045delC | 3 (33) |
| Compound heterozygous c.1157G>A/c.1309G>A | 1 (11) |
| Homozygous c.1279G>A | 5 (56) |
| Clinical manifestations | |
| Cutaneous induration w/wo hyperpigmentation w/wo hypertrichosis | 9 (100) |
| Elevated CRP and ESR | 8 (89) |
| Short stature | 8 (89) |
| Sensorineural hearing loss | 6 (67) |
| Anemia | 5 (56) |
| Exocrine pancreatic insufficiency | 3 (33) |
| Type 1 IDDM | 3 (33) |
| Thrombocytopenia | 2 (22) |
| RDD–like tumor mass | 1 (11) |
| Retroperitoneal fibrosis with hydronephrosis and renal failure | 1 (11) |
| Acute arthritis | 1 (11) |
| Glomerulonephritis | 1 (11) |
| Cardiomyopathy | 1 (11) |
| Delayed puberty | 1 (11) |
| Joint deformities | 1 (11) |
| Treatment modalities | |
| Systemic corticosteroids | 5 (56) |
| Methotrexate | 3 (33) |
| Tocilizumab (anti–IL-6R) | 3 (33) |
| Trametinib (MEK inhibitor) | 1 (11) |
| Characteristics . | n (%) . |
|---|---|
| Total | 9 (100) |
| Age at time of study, y | |
| Median (range) | 14.2 (1.9-18.5) |
| Sex | |
| Male | 2 (22) |
| Female | 7 (78) |
| Ethnicity | |
| Palestinian Arab | 4 (44) |
| Bedouin | 5 (56) |
| Germ line SLC29A3 mutations | |
| Homozygous c.1045delC | 3 (33) |
| Compound heterozygous c.1157G>A/c.1309G>A | 1 (11) |
| Homozygous c.1279G>A | 5 (56) |
| Clinical manifestations | |
| Cutaneous induration w/wo hyperpigmentation w/wo hypertrichosis | 9 (100) |
| Elevated CRP and ESR | 8 (89) |
| Short stature | 8 (89) |
| Sensorineural hearing loss | 6 (67) |
| Anemia | 5 (56) |
| Exocrine pancreatic insufficiency | 3 (33) |
| Type 1 IDDM | 3 (33) |
| Thrombocytopenia | 2 (22) |
| RDD–like tumor mass | 1 (11) |
| Retroperitoneal fibrosis with hydronephrosis and renal failure | 1 (11) |
| Acute arthritis | 1 (11) |
| Glomerulonephritis | 1 (11) |
| Cardiomyopathy | 1 (11) |
| Delayed puberty | 1 (11) |
| Joint deformities | 1 (11) |
| Treatment modalities | |
| Systemic corticosteroids | 5 (56) |
| Methotrexate | 3 (33) |
| Tocilizumab (anti–IL-6R) | 3 (33) |
| Trametinib (MEK inhibitor) | 1 (11) |
CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; IDDM, insulin–dependent diabetes mellitus.
Results
Loss-of-function mutations in SLC29A3 are sufficient to drive formation of histiocytic lesions containing phospho-ERK–positive histiocytes
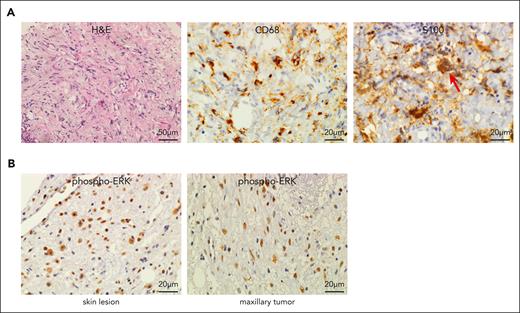
To gain a deeper understanding of the pathogenesis of histiocytosis in patients with H syndrome, we examined tissue biopsies from several patients. Histopathological analysis of a typical skin lesion (patient H1, supplemental Table 1) revealed the presence of infiltrating histiocytes, positive for CD68 and S100, and negative for CD1a, with emperipolesis, the histopathological hallmark of RDD19 (Figure 1A). This patient subsequently developed an extensive left maxillary sinus tumor mass with orbital compression. Histopathological examination of the tumor revealed similar findings, suggestive of an RDD-like tumor (supplemental Figure 2A). Because histiocytoses are generally driven by somatic-activating mutations in MAPK cascade genes, the infiltrating histiocytes in the lesions are generally positive for phosphorylated-ERK (phospho-ERK).3,4,20 However, activation of ERK in H syndrome–related histiocytosis, to our knowledge, has never been reported. Thus, we performed phospho-ERK staining of both skin and tumor biopsies. Remarkably, the infiltrating histiocytes in both biopsies were positive for phospho-ERK (Figure 1B). To examine whether a somatic alteration was the cause of ERK activation, exome sequencing was performed on tumor-derived DNA and whole-transcriptome sequencing was performed on tumor-derived RNA in search of gene fusions. No pathogenic somatic alterations were identified, suggesting that the germ line mutation in SLC29A3 itself is sufficient to drive the formation of RDD-like lesions containing phospho-ERK–positive histiocytes.
H syndrome tissue biopsies contain RDD–like infiltrating histiocytes positive for phospho-ERK. (A) FFPE sections of skin lesion (patient H1, supplemental Table 1). Left: H&E staining demonstrates diffuse infiltration of the dermis by histiocytes with a large pale cytoplasm, accompanied by scattered lymphocytes and plasma cells. Middle and right: infiltrating histiocytes are positive for CD68 and S100. Emperipolesis is marked by an arrow. (B) The infiltrating histiocytes in skin lesion (left panel) and maxillary sinus tumor (right panel) of patient H1 show strong positivity for phospho-ERK. H&E, hematoxylin and eosin.
H syndrome tissue biopsies contain RDD–like infiltrating histiocytes positive for phospho-ERK. (A) FFPE sections of skin lesion (patient H1, supplemental Table 1). Left: H&E staining demonstrates diffuse infiltration of the dermis by histiocytes with a large pale cytoplasm, accompanied by scattered lymphocytes and plasma cells. Middle and right: infiltrating histiocytes are positive for CD68 and S100. Emperipolesis is marked by an arrow. (B) The infiltrating histiocytes in skin lesion (left panel) and maxillary sinus tumor (right panel) of patient H1 show strong positivity for phospho-ERK. H&E, hematoxylin and eosin.
Although RDD–like cutaneous features are a common symptom of H syndrome, patients often present with other clinical manifestations. We examined biopsies of other affected tissues from patients with H syndrome. Histopathological examination of a biopsy of retroperitoneal tissue from a patient who presented with bilateral hydronephrosis and renal failure due to extensive retroperitoneal fibrosis (patient H4, supplemental Table 1) revealed infiltration by CD68+ S100+ CD1a− phospho-ERK+ foamy histiocytes (supplemental Figure 2B). A kidney biopsy from a patient with nephrotic syndrome (patient H9, supplemental Table 1), consistent with membranous nephropathy, revealed infiltrating phospho-ERK+ histiocytes with a similar immunophenotype (supplemental Figure 2C).
The presence of infiltrating histiocytes in tissue biopsies from patients with disease manifestations that were previously not associated with histiocytosis suggests that histiocytic infiltrations are a common feature in H syndrome, despite the heterogeneity in the clinical manifestations of this disease. The fact that the pathological histiocytes are positive for phospho-ERK proposes a central role of MAPK activation in H syndrome pathogenesis.
H syndrome monocytes display altered subset distribution and elevated expression of inflammatory genes
Peripheral blood MNP cells play a role in the pathogenesis of histiocytosis, both as precursors of the pathological lesion histiocytes21-23 and as aberrantly activated cells contributing to disease development from within the circulation.24,25 Thus, to explore the molecular pathogenesis of H syndrome–related histiocytosis and the mechanisms leading to ERK activation in the pathological histiocytes, we investigated circulating MNP cells from patients with H syndrome.
The human MNP compartment comprises several monocyte and DC subsets, which remain in equilibrium under healthy physiological conditions. Under certain pathological conditions, the equilibrium between the different subsets may be altered.26 First, we sought to characterize the peripheral blood MNP compartment in H syndrome, to identify pathologically affected MNP subsets. Peripheral blood mononuclear cells from patients with H syndrome and from age- and sex-matched healthy controls were analyzed by multiparameter flow cytometry using markers for multiple monocyte and DC subsets (gating strategy is depicted in supplemental Figure 3). This analysis revealed a significant anomaly in the monocytic compartment of H syndrome samples, with altered monocyte subset distribution (Figure 2A-B), implying that circulating monocytes are involved in H syndrome pathogenesis. No abnormalities were detected within the DC compartment. Serial patient complete blood cell counts demonstrated transient monocytosis in 2 patients, with no additional white blood cell subset abnormalities in the cohort (supplemental Table 2).
H syndrome monocytes display altered subset distribution and elevated expression of inflammatory genes. (A) Representative flow cytometry analyses of healthy control (left) and patient with H syndrome (right) classical (CD14high CD16−) and nonclassical (CD14dim CD16+) monocyte subset distribution (the depicted cells are HLA-DR+ lineage [Lin]− CD88+CD89+). Full gating strategy is depicted in supplemental Figure 3. (B) Dot plots depicting percentage of classical monocytes (left) and nonclassical monocytes (right) out of total monocytes, based on flow cytometry analysis (controls: n = 8; patients: n = 8). Statistical significance was determined using unpaired 2-tailed t tests; ∗∗∗P < .001. (C) Uniform manifold approximation and projection for dimension reduction (UMAP) visualization showing all cells included the single-cell RNA sequencing data sets, combining all healthy samples (n = 4, comprising a total of 13 028 single-cell transcriptome profiles) and all patient samples (n = 8, comprising a total of 34 375 single-cell transcriptome profiles). Monocyte fractions after enrichment were between 60% and 90% in different samples. (D) Principal component analysis (PCA) plot based on the top 1000 differentially expressed genes in monocytes (classical and nonclassical clusters); healthy controls (blue), H syndrome (red). (E) Volcano plots showing the differentially expressed genes in classical and nonclassical monocytes. Positive fold change (FC) represents genes that were upregulated in the H syndrome cohort and negative FC represents genes that were downregulated in the H syndrome cohort. FDR, false discovery rate; Not sig., not significant.
H syndrome monocytes display altered subset distribution and elevated expression of inflammatory genes. (A) Representative flow cytometry analyses of healthy control (left) and patient with H syndrome (right) classical (CD14high CD16−) and nonclassical (CD14dim CD16+) monocyte subset distribution (the depicted cells are HLA-DR+ lineage [Lin]− CD88+CD89+). Full gating strategy is depicted in supplemental Figure 3. (B) Dot plots depicting percentage of classical monocytes (left) and nonclassical monocytes (right) out of total monocytes, based on flow cytometry analysis (controls: n = 8; patients: n = 8). Statistical significance was determined using unpaired 2-tailed t tests; ∗∗∗P < .001. (C) Uniform manifold approximation and projection for dimension reduction (UMAP) visualization showing all cells included the single-cell RNA sequencing data sets, combining all healthy samples (n = 4, comprising a total of 13 028 single-cell transcriptome profiles) and all patient samples (n = 8, comprising a total of 34 375 single-cell transcriptome profiles). Monocyte fractions after enrichment were between 60% and 90% in different samples. (D) Principal component analysis (PCA) plot based on the top 1000 differentially expressed genes in monocytes (classical and nonclassical clusters); healthy controls (blue), H syndrome (red). (E) Volcano plots showing the differentially expressed genes in classical and nonclassical monocytes. Positive fold change (FC) represents genes that were upregulated in the H syndrome cohort and negative FC represents genes that were downregulated in the H syndrome cohort. FDR, false discovery rate; Not sig., not significant.
To elucidate the molecular pathways underlying the observed anomaly in circulating monocytes, we studied their transcriptomic profile at the single-cell level. Monocytes were enriched from peripheral blood mononuclear cells using a pan-monocyte negative selection method, and then single-cell RNA sequencing was performed. Unsupervised clustering analysis was applied, and cell clusters were annotated based on expression levels of a curated list of genes (Figure 2C). Then, principal component analysis based on differentially expressed genes in the monocyte clusters was performed. Although healthy control samples clustered together, H syndrome samples were clearly separated, indicative of an aberrant transcriptional pattern in H syndrome monocytes (Figure 2D). To characterize the transcriptional pattern of H syndrome monocytes, we performed differential expression analysis within each cluster, comparing the patient and healthy control groups. Remarkably, the list of upregulated genes in H syndrome monocytes contained many proinflammatory genes, with the top hits being inflammatory cytokines (CCL2, CCL3, CCL4, IL1B, and CXCL8; Figure 2E; supplemental Table 3). Notably, elevated levels of similar inflammatory cytokines were observed upon enforced expression of BRAFV600E in hematopoietic stem cells (HSCs).27
The analysis also revealed upregulation of several S100 genes (S100A8, S100A12, S100A11, S100A10, S100A6, S100A4, and S100A9. Figure 2E; supplemental Table 3). The S100 proteins are a family of calcium-binding proteins that play an important role in modulating inflammatory responses.28 When secreted, these proteins serve as alarmins and induce inflammatory cytokine secretion.29 Of note, a positive S100 stain is 1 of the histopathological characteristics of the infiltrating histiocytes in various subtypes of histiocytosis.12,19,30
Another noteworthy upregulated gene is BCL2A1 (Figure 2E; supplemental Table 3), a prosurvival member of the Bcl-2 family,31 which is frequently overexpressed in hematological malignancies.32 Specifically, BCL2A1 is upregulated in LCH-type histiocytic lesions and in monocyte-derived DCs from patients with LCH, and its expression level is associated with long-term survival of these cells.33
Intriguingly, several major histocompatibility complex (MHC) class II genes (HLA-DQA1, HLA-DQB1, HLA-DPA1, and HLA-DPB1) were conversely downregulated in H syndrome monocytes (Figure 2E; supplemental Table 3). The expression of MHC class II genes is positively regulated by the class II transactivator, which is known to be repressed by ERK at both the transcriptional and posttranslational levels.34,35 Indeed, class II transactivator expression was also moderately downregulated in H syndrome monocytes (supplemental Table 3). Thus, the observed downregulation of MHC class II genes is in line with enhanced MAPK signaling.
Taken together, these results indicate abnormalities in H syndrome monocytes and reveal an aberrant inflammatory transcriptional program. In addition, these results point out a resemblance between the consequences of loss-of-function mutations in ENT3 and activating mutations in MAPK cascade genes.
Loss of function of ENT3 leads to inflammatory cytokine secretion through activation of a signaling cascade involving TLR and MAPK
Next, we performed gene set enrichment analysis to identify the signaling pathways activated in H syndrome monocytes. In line with the histopathological results, MAPK signaling was enriched in H syndrome monocytes (Figure 3A). Additionally, TLR signaling was the top enriched signaling pathway in H syndrome monocytes (Figure 3A). TLRs are a family of pattern-recognition receptors that play a key role in the innate immune system. Several TLRs are localized in endolysosomes and are activated by endosomal short RNA fragments and nucleosides, after degradation of phagocytosed cells.36 Among these, human monocytes express TLR7 and TLR8.37,38 ENT3 deficiency is known to cause accumulation of nucleosides in macrophage lysosomes, damaging lysosomal function.18 Because elevated levels of nucleoside ligands enhance TLR signaling, we performed an in vitro lysosomal activity assay as an indication for nucleoside accumulation in H syndrome monocytes. Monocytes were incubated in vitro with a self-quenched substrate, which becomes fluorescent upon cleavage by lysosomal hydrolases. The fluorescence signal measured after incubation is proportional to the intracellular lysosomal activity. Notably, H syndrome monocytes showed markedly lower lysosomal activity than healthy donor monocytes (Figure 3B). These results suggest that H syndrome circulating monocytes are burdened by an accumulation of nucleosides, which is significant enough to cause lysosomal dysfunction, thus proposing that the observed enhanced TLR signaling is a result of elevated nucleoside ligand availability. Notably, a recent study focused on mouse models provides substantial support for these results by demonstrating that histiocytosis in Slc29a3−/− mice is TLR7-dependent, because Slc29a3−/−Tlr7−/− mice do not develop histiocytosis.39
Loss of function of ENT3 leads to inflammatory cytokine secretion through activation of a signaling cascade involving TLR and MAPK. (A) Gene set enrichment analysis using MSigDB KEGG data set. The top 5 enriched pathways ordered by normalized enrichment score (NES) in H syndrome classical (left) and nonclassical (right) monocytes are shown. A false discovery rate adjusted P value (q value) of <.25 was considered significant. (B) Bar graph depicting the mean fluorescence intensity (MFI; mean + standard error of the mean [SEM]) of monocytes after incubation with a self-quenched substrate (controls: n = 3; patients: n = 3). Statistical significance was determined using unpaired 2-tailed t test; ∗∗P < .01. Original flow cytometry histograms are shown in supplemental Figure 5A. (C) Bar graph depicting the mean + SEM of phospho-ERK–stained monocytes (from healthy donors, n = 3), untreated or after incubation for 1 hour with 5 μg/mL ssRNA40 alone or in combination with 5 μM Cu-CPT9a (TLR8 inhibitor). Values were collected from 3 separate experiments and are normalized to MFI of the control in each experiment. Statistical significance was determined using 1-way analysis of variance (ANOVA) with post hoc Tukey multiple comparisons test; ∗∗P < .01. Original flow cytometry histograms are shown in supplemental Figure 5B. (D) Bar graph depicting the MFI (mean + SEM) of phospho-ERK–stained monocytes (healthy controls: n = 5; patients with H syndrome: n = 6). Values were collected from 3 separate experiments and are normalized to MFI of the control(s) in each experiment. Statistical significance was determined using unpaired 2-tailed t test; ∗P < .05. Original flow cytometry histograms are shown in supplemental Figure 5C. (E) Dot plots depicting the concentrations of chemokine ligand 2 (CCL2), CCL4, chemokine (C-X-C motif) ligand 8 (CXCL8), and interleukin 6 (IL-6) (pg/mL) secreted in vitro by monocytes (healthy controls: n = 5, patients with H syndrome: n = 5). Statistical significance was determined using unpaired 2-tailed t tests; ∗P < .05. (F) Dot plots depicting the concentrations of CCL2, CCL4, CXCL8, and IL-6 (pg/mL) secreted in vitro by untreated monocytes (as depicted in panel E) and by monocytes incubated in the presence of 5 μM Cu-cpt9a (TLR8 inhibitor). (G) Dot plots depicting the concentrations of CCL2, CCL4, CXCL8, and IL-6 (pg/mL) secreted in vitro by untreated monocytes (as depicted in panel E) and by monocytes incubated in the presence of 25 μM PD98059 (MEK inhibitor). Statistical significance was determined by 2-way ANOVA with Šidák multiple comparisons test. ns, not significant.
Loss of function of ENT3 leads to inflammatory cytokine secretion through activation of a signaling cascade involving TLR and MAPK. (A) Gene set enrichment analysis using MSigDB KEGG data set. The top 5 enriched pathways ordered by normalized enrichment score (NES) in H syndrome classical (left) and nonclassical (right) monocytes are shown. A false discovery rate adjusted P value (q value) of <.25 was considered significant. (B) Bar graph depicting the mean fluorescence intensity (MFI; mean + standard error of the mean [SEM]) of monocytes after incubation with a self-quenched substrate (controls: n = 3; patients: n = 3). Statistical significance was determined using unpaired 2-tailed t test; ∗∗P < .01. Original flow cytometry histograms are shown in supplemental Figure 5A. (C) Bar graph depicting the mean + SEM of phospho-ERK–stained monocytes (from healthy donors, n = 3), untreated or after incubation for 1 hour with 5 μg/mL ssRNA40 alone or in combination with 5 μM Cu-CPT9a (TLR8 inhibitor). Values were collected from 3 separate experiments and are normalized to MFI of the control in each experiment. Statistical significance was determined using 1-way analysis of variance (ANOVA) with post hoc Tukey multiple comparisons test; ∗∗P < .01. Original flow cytometry histograms are shown in supplemental Figure 5B. (D) Bar graph depicting the MFI (mean + SEM) of phospho-ERK–stained monocytes (healthy controls: n = 5; patients with H syndrome: n = 6). Values were collected from 3 separate experiments and are normalized to MFI of the control(s) in each experiment. Statistical significance was determined using unpaired 2-tailed t test; ∗P < .05. Original flow cytometry histograms are shown in supplemental Figure 5C. (E) Dot plots depicting the concentrations of chemokine ligand 2 (CCL2), CCL4, chemokine (C-X-C motif) ligand 8 (CXCL8), and interleukin 6 (IL-6) (pg/mL) secreted in vitro by monocytes (healthy controls: n = 5, patients with H syndrome: n = 5). Statistical significance was determined using unpaired 2-tailed t tests; ∗P < .05. (F) Dot plots depicting the concentrations of CCL2, CCL4, CXCL8, and IL-6 (pg/mL) secreted in vitro by untreated monocytes (as depicted in panel E) and by monocytes incubated in the presence of 5 μM Cu-cpt9a (TLR8 inhibitor). (G) Dot plots depicting the concentrations of CCL2, CCL4, CXCL8, and IL-6 (pg/mL) secreted in vitro by untreated monocytes (as depicted in panel E) and by monocytes incubated in the presence of 25 μM PD98059 (MEK inhibitor). Statistical significance was determined by 2-way ANOVA with Šidák multiple comparisons test. ns, not significant.
Enhanced TLR signaling may account for the observed upregulation of MAPK signaling, because TLR7/8 can activate MEK-ERK through Myd88 and TRAF6.40 To test whether MAPK signaling is activated downstream of TLR7/8, healthy donor monocytes were incubated with the TLR7/8 activator R848, and levels of phospho-ERK were measured by flow cytometry. ERK phosphorylation increased upon treatment with R848 in a dose-dependent manner, indicating that activation of TLR7/8 drives downstream activation of MAPK signaling (supplemental Figure 4). To test specifically whether endosomal nucleosides stimulate ERK via TLR8, healthy donor monocytes were incubated with single-strand RNA 40 (ssRNA40) complexed with a cationic lipid that facilitates its endocytosis. ssRNA40 is endocytosed and degraded to nucleosides in endolysosomes, and its degradation products activate TLR7/8.41,42 Monocytes were incubated with ssRNA40 alone or with addition of the TLR8 inhibitor Cu-CPT9a, and phospho-ERK levels were measured. Incubation with ssRNA40 led to a significant elevation of phospho-ERK, which was abolished when combined with the TLR8 inhibitor (Figure 3C), indicating that endosomal nucleosides drive activation of MAPK signaling through TLR8. Taken together, these results suggest that loss-of-function mutation of ENT3 causes accumulation of nucleosides that activate TLR7/8 and downstream MAPK signaling.
To evaluate MAPK signaling in H syndrome monocytes, we measured phospho-ERK by flow cytometry in monocytes from healthy controls and patients with H syndrome. In line with the transcriptomic analysis, monocytes from patients with H syndrome exhibited higher levels of phospho-ERK, indicating enhanced activity of the MAPK signaling cascade (Figure 3D).
Because the transcriptomic data indicated that H syndrome monocytes express higher levels of inflammatory cytokines, we further hypothesized that enhanced TLR7/8-MAPK signaling in H syndrome monocytes induces inflammatory cytokine secretion. To test this hypothesis, monocytes from patients with H syndrome and from healthy controls were incubated in vitro for 8 hours, after which the concentration of CCL2, CCL4, CXCL8, and IL-6 was measured in the media. To test whether cytokine production was TLR8 and MEK dependent, the assay was simultaneously performed in the presence of either TLR8 or MEK inhibitors (Cu-CPT9a and PD98059, respectively). Of note, IL-6 was included in this assay although it was only moderately upregulated in the transcriptomic results (supplemental Table 3), because anti–IL-6 receptor (anti–IL-6R) therapy showed positive results in some patients with H syndrome.43,44 Remarkably, monocytes from patients with H syndrome secreted higher amounts of all cytokines and chemokines tested (Figure 3E), corroborating the gene expression data. Notably, although addition of either TLR8 or MEK inhibitors had no significant effect on healthy control monocytes, both inhibitors dramatically reduced the amount of cytokines secreted by H syndrome monocytes (Figure 3F-G). The reduction in cytokine secretion did not result from cytotoxicity of the inhibitors (supplemental Figure 6). These results indicate that enhanced cytokine secretion by H syndrome monocytes is driven by the TLR8–MEK–ERK signaling pathway.
MEK inhibitor therapy led to resolution of histiocytic lesions and inflammatory manifestations of a patient with H syndrome
Several immunomodulatory drugs have been administered to patients with H syndrome, with varying yet limited success.45,46 A patient in our cohort (patient H1; supplemental Table 1) experienced considerable improvement of debilitating RDD–like indurated skin lesions in response to administration of the anti–IL-6R antibody tocilizumab, in accordance with previous reports43,44 (Figure 4A). However, a concurrent extensive left maxillary sinus histiocytic tumor was not affected by tocilizumab therapy. After failure of systemic corticosteroids and methotrexate therapy, tumor infiltrating histiocytes were found to be phospho-ERK+ (Figure 1B) and treatment with the MEK inhibitor trametinib was initiated. Strikingly, MEK inhibition induced a slow yet persistent reduction in tumor volume, until complete tumor resolution (Figure 4B). Currently, the patient remains in remission from her maxillary tumor, with a 2-year follow-up since trametinib therapy cessation. Of note, despite discontinuation of tocilizumab administration during trametinib therapy, the patient’s skin lesions continued to improve (Figure 4C), suggesting that the molecular mechanisms driving the formation of skin lesions and tumor are similar, and that MEK and IL-6 belong to the same signaling pathway in H syndrome pathogenesis. In addition, systemic manifestations such as elevated C-reactive protein and erythrocyte sedimentation rate, anemia and hypoalbuminemia, which preceded tocilizumab therapy, remained in remission throughout the 2-year course of trametinib therapy (Figure 4D), suggesting that these manifestations of H syndrome are driven by excessive MAPK signaling as well. Thus, MEK inhibitors were effective for treating the patient’s various histiocytic infiltrates, as well as the systemic inflammatory manifestations.
Trametinib treatment resolved histiocytic lesions, and downregulated inflammatory gene expression. (A) Images of indurated skin lesions of patient H1. Top: before tocilizumab treatment; bottom: during tocilizumab treatment. (B) Magnetic resonance imaging (MRI) of left maxillary sinus tumor (patient H1). Top left: coronal T1-weighted fat suppressed with contrast material administration shows an enhancing soft tissue mass in the left maxillary sinus (arrow). Top right: axial T2-weighted images shows that the mass (arrow) is mostly hyperintense. Bottom left: a follow-up MRI performed after 28 months of trametinib treatment. Axial T2-weighted fat saturated image shows complete resolution of the finding (arrow). Bottom right: graph depicting tumor volume (cubic centimeters) as calculated according to MRIs. (C) Image of the skin of patient H1 during trametinib treatment. (D) Graphs depicting longitudinal measurements of inflammatory markers (C-reactive protein [CRP] and erythrocyte sedimentation rate [ESR]), hemoglobin (HB), and albumin (ALB) levels during clinical follow-up of patient H1. CRP (upper left panel; dotted red line delineates upper norm of 0.5 mg/dL), ESR (lower left panel; dotted red line delineates upper norm of 20 mm/h), HB (upper left panel; the dotted red line delineates lower norm of 11.4 g/dL), ALB (lower right panel; the dotted red line delineates lower norm of line 3.8 g/dL). Treatment periods are highlighted in red (methotrexate), green (tocilizumab), and blue (trametinib). (E) Flow cytometry analysis of classical (CD14high CD16−) and nonclassical (CD14dim CD16+) monocyte subset distribution of patient H1 during trametinib treatment. The dot plot depicts percentages of classical (top) and nonclassical (bottom) monocytes of total monocytes, based on flow cytometry analysis. Values of controls and patients with H syndrome as depicted in Figure 2B. (F) Volcano plots showing the differentially expressed genes in classical monocytes. Negative FC represents genes that were downregulated during therapy, and positive FC represents genes that were upregulated during therapy. FDR, false discovery rate.
Trametinib treatment resolved histiocytic lesions, and downregulated inflammatory gene expression. (A) Images of indurated skin lesions of patient H1. Top: before tocilizumab treatment; bottom: during tocilizumab treatment. (B) Magnetic resonance imaging (MRI) of left maxillary sinus tumor (patient H1). Top left: coronal T1-weighted fat suppressed with contrast material administration shows an enhancing soft tissue mass in the left maxillary sinus (arrow). Top right: axial T2-weighted images shows that the mass (arrow) is mostly hyperintense. Bottom left: a follow-up MRI performed after 28 months of trametinib treatment. Axial T2-weighted fat saturated image shows complete resolution of the finding (arrow). Bottom right: graph depicting tumor volume (cubic centimeters) as calculated according to MRIs. (C) Image of the skin of patient H1 during trametinib treatment. (D) Graphs depicting longitudinal measurements of inflammatory markers (C-reactive protein [CRP] and erythrocyte sedimentation rate [ESR]), hemoglobin (HB), and albumin (ALB) levels during clinical follow-up of patient H1. CRP (upper left panel; dotted red line delineates upper norm of 0.5 mg/dL), ESR (lower left panel; dotted red line delineates upper norm of 20 mm/h), HB (upper left panel; the dotted red line delineates lower norm of 11.4 g/dL), ALB (lower right panel; the dotted red line delineates lower norm of line 3.8 g/dL). Treatment periods are highlighted in red (methotrexate), green (tocilizumab), and blue (trametinib). (E) Flow cytometry analysis of classical (CD14high CD16−) and nonclassical (CD14dim CD16+) monocyte subset distribution of patient H1 during trametinib treatment. The dot plot depicts percentages of classical (top) and nonclassical (bottom) monocytes of total monocytes, based on flow cytometry analysis. Values of controls and patients with H syndrome as depicted in Figure 2B. (F) Volcano plots showing the differentially expressed genes in classical monocytes. Negative FC represents genes that were downregulated during therapy, and positive FC represents genes that were upregulated during therapy. FDR, false discovery rate.
To elucidate the effect of MEK inhibitor therapy on the patient’s circulating monocytes, we analyzed the patient’s cells by flow cytometry and single-cell RNA sequencing and compared the results with those of the H syndrome cohort. Notably, monocyte subset distribution in the patient’s blood was significantly different than observed in the H syndrome cohort, resembling that of healthy controls (Figure 4E). This suggests that the process that alters the proportions of classical and nonclassical monocytes in H syndrome may be driven by excessive MAPK signaling.
Finally, we performed differential expression analysis to identify relevant molecular pathways affected by therapy. Remarkably, MEK inhibitor therapy reverted the expression of the inflammatory cytokines and S100 family members, which were upregulated in the H syndrome cohort (Figures 2E and 4F; supplemental Table 4), indicating that MAPK signaling drives the inflammatory process in H syndrome. The prosurvival Bcl-2 family member BCL2A1 was also downregulated in response to MEK inhibitor therapy, as was recently described in response to BRAF inhibition in LCH.25 Conversely, MHC class II genes were upregulated during therapy, in line with multiple reports describing upregulation of MHC I/II genes in response to MAPK inhibition.47-52
Collectively, these results propose a central role for MAPK signaling in the pathogenesis of histiocytosis and inflammation in H syndrome and suggest a potential novel therapeutic approach.
Discussion
An association between germ line inactivating mutations in SLC29A3 and histiocytosis has long been recognized. However, the mechanism connecting the 2 has remained unclear. Through a detailed molecular and phenotypic analysis of samples from a cohort of patients with H syndrome, we identified the molecular mechanism leading to histiocytosis in H syndrome, revealing a yet-unrecognized link between loss of function of ENT3 and activation of MAPK signaling. We demonstrate that the germ line SLC29A3 mutation is sufficient to drive ERK activation and histiocytosis development, in the absence of additional somatic alterations. We further show that the MAPK signaling pathway is activated in H syndrome circulating monocytes, and that H syndrome monocytes overexpress inflammatory genes, resembling circulating monocytes and pathological LCH cells harboring the BRAFV600E mutation.24,25,53 We demonstrate that H syndrome circulating monocytes are burdened by accumulation of nucleosides, and that TLR signaling is activated, providing a mechanistic link between dysfunctional ENT3 and initiation of a signaling cascade leading to MAPK activation. Moreover, we show that H syndrome monocytes secrete elevated amounts of inflammatory cytokines in a TLR8- and MEK-dependent manner.
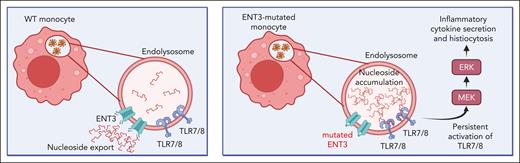
We thus propose a model in which phagocytosis leads to accumulation of nucleosides inside endolysosomes of monocytes, due to dysfunctional ENT3. Nucleoside accumulation induces persistent activation of TLR7/8, driving downstream activation of MAPK signaling. This deregulated signaling process results in constitutive activation of monocytes, enhanced cytokine secretion, and inflammation, and constitutes a novel pathway leading to histiocytosis (Figure 5).
Proposed model of the molecular mechanism driving histiocytosis and inflammation in H syndrome. In wild-type (WT) monocytes, nucleosides derived from phagocytosed cells, are exported from the endolysosome to the cytoplasm via ENT3. In H syndrome monocytes, lack of proper ENT3 function leads to accumulation of nucleosides inside endolysosomes, inducing persistent activation of TLR7/8 signaling. TLR7/8 drive downstream activation of MEK and ERK, inducing inflammatory cytokine secretion and histiocytosis. Created with BioRender.com.
Proposed model of the molecular mechanism driving histiocytosis and inflammation in H syndrome. In wild-type (WT) monocytes, nucleosides derived from phagocytosed cells, are exported from the endolysosome to the cytoplasm via ENT3. In H syndrome monocytes, lack of proper ENT3 function leads to accumulation of nucleosides inside endolysosomes, inducing persistent activation of TLR7/8 signaling. TLR7/8 drive downstream activation of MEK and ERK, inducing inflammatory cytokine secretion and histiocytosis. Created with BioRender.com.
Notably, a recent report supports our proposed model by demonstrating that histiocytosis in Slc29a3−/− mice is TLR7-dependent, because Slc29a3−/−Tlr7−/− mice do not develop histiocytosis.39 Interestingly, Slc29a3−/− mice did not exhibit signs of constitutive inflammation, which is a hallmark of the human disorder. This emphasizes the limitations of using mouse models for studying human disorders. Here, using patient-derived cells, we identified the downstream signaling pathway initiated by TLR7/8, highlighting MAPK signaling as a key component in the pathogenesis of H syndrome.
Our results indicate downregulation of MHC class II genes in H syndrome monocytes, which was reversed after MEK inhibitor treatment. Although our results describe these effects in circulating monocytes, an intriguing possibility is that a similar effect occurred in the patient tumor cells during MEK inhibitor therapy, contributing to tumor elimination by activation of a directed immune response. This possibility is supported by reports of the beneficial combination of MAPK inhibitors with immune checkpoint blockade for the treatment of different cancers.47,54-56 This hypothesis is potentially relevant to more common subtypes of histiocytoses, in line with a recent report describing a synergistic effect of an immune checkpoint and MEK inhibitor combination in a preclinical mouse model of LCH.57
Anemia, another common clinical manifestation of H syndrome, is often observed in patients with acute inflammation, occasionally associated with hemophagocytosis.58 Importantly, chronic activation of nucleoside-sensing TLRs has recently been shown to drive anemia via differentiation of specialized hemophagocytes.59 Thus, our model also provides a possible mechanism for anemia in H syndrome.
By describing the link between loss-of-function mutation of ENT3 and activation of MAPK signaling, we propose not only a putative mechanism for inflammation and histiocytosis in patients with H syndrome but also a readily available therapy. Indeed, our patient treated with the MEK inhibitor trametinib demonstrated encouraging results, with complete regression of the extensive paranasal sinus and cutaneous histiocytic infiltrates, accompanied by significant improvement of systemic inflammatory manifestations and anemia. Although data from additional patients are still required, our study suggests that MEK inhibitors are a promising new treatment for patients with H syndrome, for the treatment of both histiocytic infiltrations and systemic inflammatory manifestations. Although recurrent therapy with MEK inhibitors may be required during a patient’s lifetime, this therapeutic modality may be effective even when other therapies, such as anti–IL-6R antibodies, have failed.
We show that a significant elevation in the proportion of nonclassical monocytes is a prominent feature of H syndrome. Monocytes egress from the bone marrow and reside within the circulation as classical monocytes for ∼1 day. A small proportion of classical monocytes mature into intermediate, and then nonclassical monocytes, within the circulation.60 Although in healthy individuals, nonclassical monocytes comprise ∼10% of the circulating monocyte pool, nonclassical monocytes accounted for ∼30% of circulating monocytes in our H syndrome cohort. This significant elevation in the proportion of nonclassical monocytes could result from upregulation of the prosurvival member of the Bcl-2 family BCL2A1. In mice, enforced Bcl2 expression has been shown to prolong the life span of nonclassical monocytes.61 Its marked downregulation during MEK inhibitor therapy, in parallel with a significant reduction in nonclassical monocyte frequency, supports this hypothesis.
A previous study described elevated cell-surface expression of macrophage colony stimulating factor receptor (CSFR) in macrophages of Slc29a3−/− mice and suggested hyperactivation of CSFR signaling as a possible mechanism for histiocytosis development in these mice.18 We did not detect higher cell-surface levels of CSF1R, the human orthologue of macrophage CSFR, on monocytes of patients with H syndrome (supplemental Figure 7). It is possible that higher cell-surface expression of CSF1R due to loss-of-function mutation of ENT3 is restricted to macrophages and not found on monocytes. Additional explanations for this apparent discrepancy could be different consequences of complete knock out of Slc29a3 in mice and loss-of-function mutations in patients with H syndrome, or other biological differences between the mouse model and the human disorder.
Over recent years, several studies of Slc29a3−/− mice have been published, suggesting additional deregulated pathways. These include perturbation of T-cell homeostasis62 and defective lysosomal bile acid transport.63 However, these studies do not provide an explanation for the development of histiocytosis, which is 1 of the most common features of H syndrome. Another study described adult stem cell deficits, which, among other effects, skewed differentiation of HSCs toward the myeloid lineage in Slc29a3−/− mice.64 Although this effect may contribute to histiocytosis development, it still does not provide a sufficient mechanism. Interestingly, it has recently been shown that enforced expression of the BRAFV600E mutation in mouse HSCs similarly skewed differentiation toward the myeloid lineage, in a process involving a senescence-associated secretory phenotype.27 Whether a similar process occurs in H syndrome HSCs is an interesting subject for future studies. Nonetheless, this is yet another point of similarity between the consequences of loss-of-function mutations in SLC29A3 and activating mutations in MAPK cascade genes.
The histiocytic lesions in patients with H syndrome specifically resemble RDD-type histiocytosis.12,13 Notably, somatic alterations in MAPK genes have been reported in only ∼40% of patients with RDD,9 whereas the driving mutation in most cases remains unknown. A recent clinical study reported a significant response to MEK inhibitor therapy, even in patients with RDD who did not harbor somatic MAPK alterations,65 suggesting the presence of alternative pathways for pathological activation of MAPK. Our results provide an example of such a noncanonical pathway, demonstrating that mutations affecting nucleoside trafficking or TLR signaling can induce pathological MAPK activation. This described mechanism demonstrates that alteration of MAPK genes is not obligatory for pathological activation of the pathway, and that patients who do not harbor alterations in MAPK genes may still benefit from MAPK–directed targeted therapy.
Acknowledgments
The authors thank the patients and their families for participating in this study. The authors thank Eviatar Weizmann from the Mantoux Bioinformatics institute of the Nancy and Stephen Grand Israel National Center for Personalized Medicine, Weizmann Institute of Science, for the bioinformatic analysis of single-cell RNA sequencing data and Abed Nasereddin and Idit Shiff from the Core Research Facility of Hebrew University, Ein Karem Campus, for the preparation and sequencing of 10X libraries. They thank Jean-Francois Emile from Universite de Versailles Saint-Quentin, Paris, France, for the histopathological revision of their patient's tumor and Naomi Litichever and Dina Kugel, data managers at the Department of Pediatric Hematology and Oncology, Schneider Children's Medical Center of Israel. Furthermore, they thank Tamar Natanzon-Bracha, Rina Baslo, and all the nurses at the Schneider Medical Center Pediatric Hematology-Oncology Day Center for their assistance with blood sample collection and Oren Parnas, Abraham Zlotogorski, Vered Molho-Pessach, and Eli Pikarsky for useful discussions.
S.I. and S.E. are supported by the Israel Cancer Association, the Chaim Association, the Israel Childhood Cancer Fund (New York, NY), and the Israeli Ministry of Health. S.I. is an Israel Childhood Cancer Fund grant recipient and the Giorgio and Dora Shapiro Professor of Hematological Malignancies. Further support was obtained from the Israel Science Foundation Israel Precision Medicine Partnership grant, Waxman Cancer Research Foundation (New York, NY), the Noaber Foundation (Tel Aviv, Israel), and the Department of Systems Biology at City of Hope (Duarte, CA). S.Y. is supported by the Israel Science Foundation. S.E. is supported by the Davidoff Foundation. M.Z. and J.T. are supported by the National Institute for Cancer Research (Program EXCELES, ID project number LX22NPO5102), funded by the European Union (EU), Next Generation EU. R.L. is supported by an Ariane de Rothschild doctoral studentship.
Authorship
Contribution: R.S., S.I., and S.E. designed the study; R.S., S.Y., S.I., and S.E. analyzed and interpreted the data; R.S. performed most experiments; S.Y., R.L., and I.H. designed, performed, and analyzed Cytek flow cytometry experiments; O.D., G.A., Y. Birger, D.B., A.B., L.H., E.K., G.L., S.S., N.S.A., and S.E. were involved in patient care; O.D., E.K., and S.E. collected patient data and samples; E.O. and D.K. analyzed histopathology data; M.S.R. evaluated radiographic studies; M.Z. and J.T. performed and analyzed whole-transcriptome sequencing; S.C.-K. provided technical assistance; I.G. and Y. Borovitz critically reviewed the experiments and provided important advice; R.S., S.I., S.Y., and S.E. wrote the manuscript; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ruth Shiloh, The Rina Zaizov Division of Pediatric Hematology-Oncology, Schneider Children’s Medical Center, 14 Kaplan St, Petach Tikva 4920235, Israel; e-mail: ruthy.shiloh@gmail.com; and Sarah Elitzur, The Rina Zaizov Division of Pediatric Hematology-Oncology, Schneider Children’s Medical Center, 14 Kaplan St, Petach Tikva 4920235, Israel; e-mail: sarhae@clalit.org.il.
References
Author notes
Whole-exome sequencing and whole-transcriptome sequencing data have been deposited in Sequence Read Archive under accession number PRJNA924251. Single-cell RNA sequencing data have been deposited in Gene Expression Omnibus under accession number GSE221581.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

![H syndrome monocytes display altered subset distribution and elevated expression of inflammatory genes. (A) Representative flow cytometry analyses of healthy control (left) and patient with H syndrome (right) classical (CD14high CD16−) and nonclassical (CD14dim CD16+) monocyte subset distribution (the depicted cells are HLA-DR+ lineage [Lin]− CD88+CD89+). Full gating strategy is depicted in supplemental Figure 3. (B) Dot plots depicting percentage of classical monocytes (left) and nonclassical monocytes (right) out of total monocytes, based on flow cytometry analysis (controls: n = 8; patients: n = 8). Statistical significance was determined using unpaired 2-tailed t tests; ∗∗∗P < .001. (C) Uniform manifold approximation and projection for dimension reduction (UMAP) visualization showing all cells included the single-cell RNA sequencing data sets, combining all healthy samples (n = 4, comprising a total of 13 028 single-cell transcriptome profiles) and all patient samples (n = 8, comprising a total of 34 375 single-cell transcriptome profiles). Monocyte fractions after enrichment were between 60% and 90% in different samples. (D) Principal component analysis (PCA) plot based on the top 1000 differentially expressed genes in monocytes (classical and nonclassical clusters); healthy controls (blue), H syndrome (red). (E) Volcano plots showing the differentially expressed genes in classical and nonclassical monocytes. Positive fold change (FC) represents genes that were upregulated in the H syndrome cohort and negative FC represents genes that were downregulated in the H syndrome cohort. FDR, false discovery rate; Not sig., not significant.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/142/20/10.1182_blood.2023020714/2/m_blood_bld-2023-020714-gr2.jpeg?Expires=1765886956&Signature=F1vOVew3Xi~8juLLGsbB1VM1uwiDo8pLIjhsb1oYMmh0oUrs1w2fpGbEbzfOatPIGkrLd5XURA73p-kUTsDlUnf~ua~C7dTzX0SAGIai-E0J2nwWim7choFXYeiOvWAz~PVU2pnKwd1YygJdRGnVkQqrB8F6tJE~BVQzF~tHYd-SrDYjIiyvdwMah4Pl9klrCU-Bt4CcKYygGjT1IOM6IQfnP8v4uRLMiBOt~A8X367tNgxiwH-g4REJ4ZRpR-1ErRWYeuOfPX-PZ9wwwchBP48lMU2mP2L~07lv53-OAv2EwY4Xx3HaEe3uYW8W12BvWA9Bw5NQgnEfVhsJNFlKJg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Loss of function of ENT3 leads to inflammatory cytokine secretion through activation of a signaling cascade involving TLR and MAPK. (A) Gene set enrichment analysis using MSigDB KEGG data set. The top 5 enriched pathways ordered by normalized enrichment score (NES) in H syndrome classical (left) and nonclassical (right) monocytes are shown. A false discovery rate adjusted P value (q value) of <.25 was considered significant. (B) Bar graph depicting the mean fluorescence intensity (MFI; mean + standard error of the mean [SEM]) of monocytes after incubation with a self-quenched substrate (controls: n = 3; patients: n = 3). Statistical significance was determined using unpaired 2-tailed t test; ∗∗P < .01. Original flow cytometry histograms are shown in supplemental Figure 5A. (C) Bar graph depicting the mean + SEM of phospho-ERK–stained monocytes (from healthy donors, n = 3), untreated or after incubation for 1 hour with 5 μg/mL ssRNA40 alone or in combination with 5 μM Cu-CPT9a (TLR8 inhibitor). Values were collected from 3 separate experiments and are normalized to MFI of the control in each experiment. Statistical significance was determined using 1-way analysis of variance (ANOVA) with post hoc Tukey multiple comparisons test; ∗∗P < .01. Original flow cytometry histograms are shown in supplemental Figure 5B. (D) Bar graph depicting the MFI (mean + SEM) of phospho-ERK–stained monocytes (healthy controls: n = 5; patients with H syndrome: n = 6). Values were collected from 3 separate experiments and are normalized to MFI of the control(s) in each experiment. Statistical significance was determined using unpaired 2-tailed t test; ∗P < .05. Original flow cytometry histograms are shown in supplemental Figure 5C. (E) Dot plots depicting the concentrations of chemokine ligand 2 (CCL2), CCL4, chemokine (C-X-C motif) ligand 8 (CXCL8), and interleukin 6 (IL-6) (pg/mL) secreted in vitro by monocytes (healthy controls: n = 5, patients with H syndrome: n = 5). Statistical significance was determined using unpaired 2-tailed t tests; ∗P < .05. (F) Dot plots depicting the concentrations of CCL2, CCL4, CXCL8, and IL-6 (pg/mL) secreted in vitro by untreated monocytes (as depicted in panel E) and by monocytes incubated in the presence of 5 μM Cu-cpt9a (TLR8 inhibitor). (G) Dot plots depicting the concentrations of CCL2, CCL4, CXCL8, and IL-6 (pg/mL) secreted in vitro by untreated monocytes (as depicted in panel E) and by monocytes incubated in the presence of 25 μM PD98059 (MEK inhibitor). Statistical significance was determined by 2-way ANOVA with Šidák multiple comparisons test. ns, not significant.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/142/20/10.1182_blood.2023020714/2/m_blood_bld-2023-020714-gr3.jpeg?Expires=1765886956&Signature=02Yh4JazSwWKf~xKTfBngbdipJZ9YAowWuHyLgCK9kaZlEe8G7r-A4iQ4QQ~68LRe~Wc~AyG9UK531OehwQnV7tnVDOUdo6ym8dywYXdxQDGZAVYJslpWZydulcsMdyjQXLV5ZWu2PL46MwZdeuSj65pLkE6sbDX4I0hYFCx5qBzOi1Jby16QtnUUQIehRLe3T835l2IJi3YThhnJg466A-ipvmfMRJnnJCNqBRE-TrTN3tisKTVH4pTbVg7vHbgGyiajxidwX1HEff2AGaFRfairbHQFm7hdb06rKYeJ2IS6F~FP2uHST5f4viw4X4GYxzmdtGp5q1I7tXLff8MtQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Trametinib treatment resolved histiocytic lesions, and downregulated inflammatory gene expression. (A) Images of indurated skin lesions of patient H1. Top: before tocilizumab treatment; bottom: during tocilizumab treatment. (B) Magnetic resonance imaging (MRI) of left maxillary sinus tumor (patient H1). Top left: coronal T1-weighted fat suppressed with contrast material administration shows an enhancing soft tissue mass in the left maxillary sinus (arrow). Top right: axial T2-weighted images shows that the mass (arrow) is mostly hyperintense. Bottom left: a follow-up MRI performed after 28 months of trametinib treatment. Axial T2-weighted fat saturated image shows complete resolution of the finding (arrow). Bottom right: graph depicting tumor volume (cubic centimeters) as calculated according to MRIs. (C) Image of the skin of patient H1 during trametinib treatment. (D) Graphs depicting longitudinal measurements of inflammatory markers (C-reactive protein [CRP] and erythrocyte sedimentation rate [ESR]), hemoglobin (HB), and albumin (ALB) levels during clinical follow-up of patient H1. CRP (upper left panel; dotted red line delineates upper norm of 0.5 mg/dL), ESR (lower left panel; dotted red line delineates upper norm of 20 mm/h), HB (upper left panel; the dotted red line delineates lower norm of 11.4 g/dL), ALB (lower right panel; the dotted red line delineates lower norm of line 3.8 g/dL). Treatment periods are highlighted in red (methotrexate), green (tocilizumab), and blue (trametinib). (E) Flow cytometry analysis of classical (CD14high CD16−) and nonclassical (CD14dim CD16+) monocyte subset distribution of patient H1 during trametinib treatment. The dot plot depicts percentages of classical (top) and nonclassical (bottom) monocytes of total monocytes, based on flow cytometry analysis. Values of controls and patients with H syndrome as depicted in Figure 2B. (F) Volcano plots showing the differentially expressed genes in classical monocytes. Negative FC represents genes that were downregulated during therapy, and positive FC represents genes that were upregulated during therapy. FDR, false discovery rate.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/142/20/10.1182_blood.2023020714/2/m_blood_bld-2023-020714-gr4.jpeg?Expires=1765886956&Signature=HpaNMxPH0BbEA24rTvnkjzmxUny-dP0MTzSaz6vRQIGyel6wAy765NFX0q-fpGN8rCnjCIrzcdptXSOn~6ZJTPgbttMCsnFVrr~OHQ8zYMzQGTSzjbvqcx0F~M6pEHdrFt2J2KG5xoDLm5EWUumYBc10wyuM1kD~WTGdeAaMRajeqj6eZST4Nj-7SFYK8F51vKhVrF-uTekMnGL3mEHBaS2Ua61D8U~Sk~pR9pt5~x~V2MmApwu831O6EnA4EFLfZTYM1cUmGw29ttmg62RonKHZY2vFkpvMd5DanYpX7KJKoxBh~1M-bNr~sczPMb7Yh19iCNBvtJsVfLYxw5HOzw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal