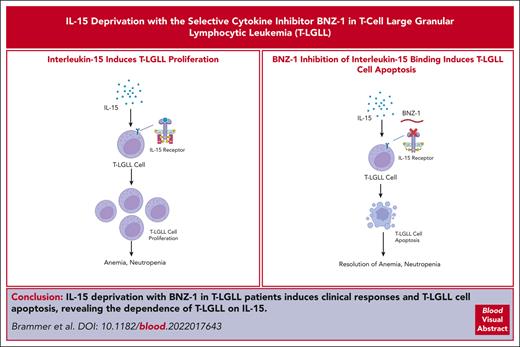
Key Points
In a phase 1/2 trial we show that BNZ-1, a selective cytokine inhibitor, is safe and induces clinical responses in patients with T-LGLL.
In vivo T-LGLL cells treated with BNZ-1 have increased apoptosis in response to BNZ-1, proving the critical role of IL-15 in T-LGLL.
Abstract
T-cell large granular lymphocytic leukemia (T-LGLL) is a clonal proliferation of cytotoxic T lymphocytes that can result in severe neutropenia, anemia, and bone marrow failure. Strong evidence from patients and mouse models demonstrate the critical role of interleukin-15 (IL-15) in T-LGLL pathogenesis. BNZ-1 is a pegylated peptide that selectively inhibits the binding of IL-15 and other γc cytokines to their cellular receptor complex, which has demonstrated efficacy in ex vivo T-LGLL cells and transgenic mice in preclinical studies. We conducted a phase 1/2 trial of BNZ-1 in patients with T-LGLL who had hematocytopenias (anemia or neutropenia) and required therapy. Clinical responses were assessed using hematologic parameters (improvement in hematocytopenias) based on response criteria from the Eastern Cooperative Oncology Group 5998 T-LGLL trial. BNZ-1 demonstrated clinical partial responses in 20% of patients with T-LGLL with minimal toxicity and the maximum tolerated dose was not reached. Furthermore, T-LGL leukemic cells showed significantly increased apoptosis in response to BNZ-1 treatment as early as day 2, including in clinical nonresponders, with changes that remained statistically different from baseline throughout treatment (P < .005). We report first-in-human proof that T-LGL leukemic cells are dependent on IL-15 and that intervention with IL-15 inhibition with BNZ-1 in patients with T-LGLL shows therapeutic effects, which carries important implications for the understanding of the pathogenesis of this disease. This trial was registered at www.clinicaltrials.gov as #NCT03239392.
Introduction
T-cell large granular lymphocytic leukemia (T-LGLL) is a rare clonal proliferation of cytotoxic T lymphocytes (CTLs), with a terminal effector memory phenotype (CD3+/CD8+/CD5dim/CD16+/CD57+/CD45RA+/CD45RO−/CCR7−CD62L−).1 T-LGLL is associated with autoimmune diseases such as rheumatoid arthritis and is thought to be triggered by long-term antigen stimulation that drives CTLs to develop a terminal effector memory phenotype that ultimately leads to clonal proliferation of T-LGLL cells.1-5 In patients, this leads to the development of neutropenia, anemia, or both and can result in recurrent infections, transfusion dependence, and significant impairment in quality of life and lifespan.1 In its more severe forms, T-LGLL can induce pure red cell aplasia or pancytopenia, which can appear similar to myelodysplasia or aplastic anemia.1,2
Currently, there are limited therapies available to treat T-LGLL. The current therapeutic approach is with immune suppression, using agents such as oral methotrexate, cyclophosphamide, and cyclosporine.2,6,7 However, these agents produce a modest clinical effect. In the only major prospective trial to be completed in T-LGLL, the Eastern Cooperative Oncology Group (ECOG) 5998 (E5998) trial, the overall response rate (ORR) of frontline methotrexate was 38%, with only 2% complete responses (CRs), which is similar with other agents.2,6,8 These poor results highlight the need for prospective biology-driven rational targeted therapies in T-LGLL.
Interleukin-15 (IL-15) is a common γ chain (γc) cytokine critical to the development, growth, and survival of various lineages of lymphocytes, including CTLs. IL-15 mediates its effect through multiple signaling pathways, including the Janus kinase and signal transducer and activator of transcription 3 (STAT3) and 5 (STAT5) axes.9,10 By computational modeling using network theory, IL-15 was identified as a crucial switch that regulates the survival of T-LGLL.11 Furthermore, transgenic mice with overexpression of IL-15 spontaneously develop a CD8 T-cell–type lymphocytic leukemia, analogous to T-LGLL in humans.12-16 In humans, increased IL-15 levels lead to activation of the STAT3/5 pathway in T-LGLL cells, whose downstream effects lead to dysregulation of cellular apoptosis by upregulating antiapoptotic Fas/FasL and the prosurvival MCL-1 pathway.17 This ultimately leads to the clonal proliferation of leukemic cells in T-LGLL and development of hematocytopenias.18,19
BNZ-1 is a pegylated 19-mer γc–binding peptide that selectively inhibits IL-2, IL-9, and IL-15 by blocking the binding of these cytokines to their cellular receptor complex involving γc.20,21 Although IL-2 shares signaling components and functions with IL-15, neither IL-2 nor IL-9 have been implicated in the pathogenesis of T-LGLL, and IL-2 reportedly restores apoptotic Fas signaling in T-LGLL cells.17,19 In preliminary in vitro studies using T-LGLL cells and ex vivo patient samples, BNZ-1 inhibited exogenous IL-15– and IL-2–mediated phosphorylation with a resultant increase in apoptosis, demonstrating its ability to block these cytokines.20-22 BNZ-1 also effectively blocked the in vivo development of CD8 T-cell leukemia in IL-15 transgenic mice and protected them from leukemia-induced death.20,22 Furthermore, in a first-in-human phase 1 study, no dose-limiting toxicities, or serious/severe treatment-emergent adverse events (AEs) were observed across a range of doses with BNZ-1.23 Based upon these promising data, we initiated a multicenter, prospective clinical trial to evaluate the effect of BNZ-1 in patients with T-LGLL and evaluate the effect of IL-15 deprivation on T-LGLL cells in patients with this rare leukemia.
Methods
Patients
Patients who were eligible had a diagnosis of T-LGLL, as defined by >400/mm3 CD3+CD57+ cells, or >650/mm3 CD3+CD8+ cells via flow cytometry, with a presence of a clonal T-cell receptor rearrangement detected via polymerase chain reaction or flow cytometry for T-cell receptor Vβ (>10%). Treatment criteria included absolute neutrophil count (ANC) < 500 cells per mm3; and ANC < 1500 cells per mm3 with recurrent infections, symptomatic anemia, or transfusion-dependent anemia. Patients had to be aged ≥18 years, without prior T-LGLL–directed therapy within 30 days, no concurrent malignancy, and no active infection (hepatitis B or C or HIV detected via serology), with adequate organ function. Pregnant women were excluded.
Study design
This study was a phase 1/2, open label, dose-ranging study to evaluate the safety, pharmacokinetics (PKs), pharmacodynamics, and preliminary efficacy of weekly dosing of BNZ-1. This study (NCT03239392) consisted of 2 parallel arms in which patients with T-LGLL and cutaneous T-cell lymphoma were included. This report describes the T-LGLL cohort only. This study included a phase 1 dose-escalation phase, followed by a phase 2 dose-expansion phase of the most efficacious, tolerated dose of BNZ-1. The study was divided into 3 treatment periods: a 4-week initial treatment period (safety assessment), followed by the optional 3-month extension period at the same weekly dose, and patients who were responding were permitted to stay on BNZ-1 in the long-term extension (LTE) period, until progression. Given the results of early phase 1 data, no infectious prophylaxis or premedication before BNZ-1 was used. All patients were observed for 6 weeks without BNZ-1. Patients were assigned to 1 of 4 doses (0.5 mg/kg, 1 mg/kg, 2 mg/kg, or 4 mg/kg), administered via infusion on days 1, 8, 15, and 22 of a 4-week cycle. For patients in the phase 1 portion of the trial, a standard 3 + 3 design was used to determine the maximum tolerated dose (MTD).
This multicenter phase 1/2 study was conducted at 4 centers, including The Ohio State University, The University of Virginia Cancer Center, Moffit Cancer Center, and City of Hope, and was approved by the institutional review boards at all sites. This study was sponsored by Bioniz Therapeutics, which has since been acquired by Equillium Pharmaceuticals as of February 2022. All patients provided informed consent, and this study was conducted in accordance with the principles of the Declaration of Helsinki.
End points and response assessments
The primary end point was to determine the MTD of BNZ-1 in patients with T-LGLL. Secondary end points included: ORR, CR rate, partial response (PR), and characterization of the PKs and pharmacodynamics of BNZ-1 in patients with T-LGLL. T-LGLL count and clinical laboratory tests (complete blood count and complete metabolic panel) were drawn at screening and on days 1, 8, 15, and 22 of cycle 1, then day 1 and day 15 of each subsequent cycle, and end of treatment. Cytokine blood samples were drawn on days 1, 2, and 8 in week 1 and day 15, then in week 5, and weeks 7, 9, 11, 13, 15, and 17. Toxicities were assessed using Common Terminology Criteria for Adverse Events version 4. Disease response was assessed based on a modified criteria similar to that used in the ECOG 5998 trial, in which improvement in the primary hematocytopenia (anemia or neutropenia) was assessed after treatment (supplemental Table 1; supplemental Methods, available on the Blood website). Partial response was defined by improvement in ANC to >500 cells per mm3 (or a 50% increase in ANC) for patients with neutropenia, whereas in patients with anemia, it was defined by a 50% decrease in transfusion requirements (or increase in hemoglobin by ≥1 g/dL). Complete response was defined by normalization of blood counts and absence of T-LGLL via flow cytometry. Assessment of response was determined locally and confirmed by an independent data review board.
Correlative analyses
To analyze the baseline status and changes of each blood subset in a patient with T-LGLL in response to BNZ-1 treatment, 3 panels of polychromatic flow cytometry were conducted. The first panel (lineage analysis) focused on enumerating the percentages of each major blood cell subset (ie, CD4 T cells, regulatory T cells, LGLL cells, normal CD8 T cells, B cells, natural killer [NK] cells, and CD56brightNK cells; 3 subsets [classical, nonclassical, and double positive] of monocytes [FITC-CD16 clone, BioLegend, San Diego, CA]; and phycoerythrin (PE)-CD16). The second panel (T-cell panel) analyzed the naive/memory breakdown of leukemic and nonleukemic T cells as well as perforin/granzyme B expression associated with each subset of T cells. Finally, to analyze the apoptotic response in each subset of peripheral blood mononuclear cells (PBMCs), a third panel (apoptosis panel) was used (FITC–annexin V). Details of specific antibodies used are shown in supplemental Table 2; which also includes additional methodology details.
STAT3 analysis
STAT3 analysis was performed as previously described.24 Briefly, DNA from PBMCs from T-LGLL cells was extracted, amplified using premade primers, and submitted for Sanger sequencing.
PKs
PK samples were drawn on days 1, 2, 8, 15, and 22 of cycle 1; days 1 and 15 of each subsequent cycle; and at end of treatment. PK samples were drawn at the end of infusion, at 0.5, 1, 2, and 4 hours after the start of the infusion. PK parameters were calculated after the first and fourth doses (steady state), including maximum plasma concentration (Cmax), area under the concentration–time curve (AUC) from time zero to time τ(C0-T), and elimination half-life (t½) after the fourth dose and were calculated using noncompartmental methods.
Statistical considerations
The data cutoff for this report was 1 August 2019, when the study was officially closed to accrual. Efficacy and safety data were performed for all enrolled patients who received at least 1 dose of BNZ-1. Descriptive statistics including medians, ranges, and frequencies were calculated. The time to maximum response was measured from the time of start of BNZ-1 until PR or CR, with patients who failed to respond censored at the end of their BNZ-1 therapy. For evaluating the difference of averages between measured time points shown in a few figures/tables, Student t test or analysis of variance was used, and P values < .05 were considered statistically significant.
Cytokine analysis with SOMAscan assay
We evaluated circulating cytokine levels after BNZ-1 across 14 patients, including 4 responders and 10 nonresponders at 7 different time points. Details of the SOMAscan methodology and analysis are included in the supplemental Methods. A full list of cytokines studied are shown in supplemental Tables 6-10 and 11-15.
Results
Patient characteristics and disposition
In total, 20 patients, with a median age of 62 years (range, 41-76 years) were enrolled in the trial; 14 were enrolled in the dose-finding cohort and 6 in the dose-expansion cohort. All patients had T-LGLL with a terminal effector memory phenotype. Among the 20 enrolled patients, 19 had a TCRα/β phenotype, and 1 had a γδ-type T-LGLL. Three patients (15%) had concomitant autoimmune disease, with only 1 patient (5%) having a diagnosis of rheumatoid arthritis. STAT3 mutations were observed in 35% (n = 7) of patients, with Y640F being the most common mutation (15% of all patients), followed by N641I (10%; n = 2), and D661V and D661Y (5% each; n = 1). Seven (35%) patients had untreated T-LGLL, whereas 13 (65%) patients had relapsed/refractory T-LGLL. The median number of prior therapies in patients who were treated was 2 (range, 1-6). The most common treatment indication was for neutropenia (50%; n = 10), followed by transfusion-dependent/symptomatic anemia (30%; n = 6), and both anemia/neutropenia (20%; n = 4). A summary of patient characteristics is shown in Table 1.
Demographic and baseline characteristics
| Characteristic . | N = 20 (%) . |
|---|---|
| Median age (range), y | 62 (41-76) |
| Sex | |
| Male | 12 (60) |
| Female | 8 (40) |
| Race | |
| White | 18 (90) |
| Black | 2 (10) |
| T-LGLL–associated hematocytopenia (or indication for treatment) | |
| Neutropenia | 10 (50) |
| Transfusion-dependent anemia | 4 (20) |
| Symptomatic anemia | 2 (10) |
| Both | 4 (20) |
| STAT3 mutation∗ | |
| No | 13 (65) |
| Yes | 7 (35) |
| D661Y | 1 (5) |
| D661V | 1 (5) |
| Y640F | 3 (15) |
| N641I | 2 (10) |
| Concomitant autoimmune disease | |
| Rheumatoid arthritis | 1 (5) |
| Other† | 2 (10) |
| None | 17 (85) |
| Prior therapy (median, 2; range, 1-6) | |
| Methotrexate | 12 (60) |
| Cyclophosphamide | 7 (35) |
| Cyclosporine | 7 (35) |
| Alemtuzumab | 2 (10) |
| Pentostatin | 1 (5) |
| Tofacitinib | 1 (5) |
| No prior therapy | 7 (35) |
| Characteristic . | N = 20 (%) . |
|---|---|
| Median age (range), y | 62 (41-76) |
| Sex | |
| Male | 12 (60) |
| Female | 8 (40) |
| Race | |
| White | 18 (90) |
| Black | 2 (10) |
| T-LGLL–associated hematocytopenia (or indication for treatment) | |
| Neutropenia | 10 (50) |
| Transfusion-dependent anemia | 4 (20) |
| Symptomatic anemia | 2 (10) |
| Both | 4 (20) |
| STAT3 mutation∗ | |
| No | 13 (65) |
| Yes | 7 (35) |
| D661Y | 1 (5) |
| D661V | 1 (5) |
| Y640F | 3 (15) |
| N641I | 2 (10) |
| Concomitant autoimmune disease | |
| Rheumatoid arthritis | 1 (5) |
| Other† | 2 (10) |
| None | 17 (85) |
| Prior therapy (median, 2; range, 1-6) | |
| Methotrexate | 12 (60) |
| Cyclophosphamide | 7 (35) |
| Cyclosporine | 7 (35) |
| Alemtuzumab | 2 (10) |
| Pentostatin | 1 (5) |
| Tofacitinib | 1 (5) |
| No prior therapy | 7 (35) |
No patients had STAT5b mutations.
One patient each with eosinophilic fasciitis and ulcerative colitis, respectively.
Clinical efficacy of BNZ-1 in T-LGLL
Fourteen patients were included in the dose-finding cohort, including n = 3 at the 0.5 mg/kg level, n = 4 at the 1 mg/kg level, n = 5 at the 2 mg/kg level, and n = 8 at the 4 mg/kg level. An additional 6 patients were included in the dose-expansion cohort at 2 mg/kg (n = 1) and 4 mg/kg (n = 5) to determine the potential for clinical responses based upon initial responses observed in this group, with preclinical20 and phase 123 data suggesting that 2 or 4 mg/kg may be the most efficacious dose. All patients completed the initial 4-week treatment period, and 17 (85%) entered the 3-month extension period. Of these patients, 16 completed the 3-month extension, and 4 patients (20% of the overall group) entered the LTE. No dose-limiting toxicities were observed in any patients, and the MTD was not reached.
Using E5998 criteria, the ORR to BNZ-1 was 20% (4 of 20). Four PRs were observed, including 1 with treatment indication of neutropenia with infection and 3 with transfusion-dependent anemia (1 of them who also had mild neutropenia but was not treated for that indication; Table 2). Among responders, the median time to response was 8 weeks (range, 4-9 weeks), and the median follow-up time in patients who were responding was 26 weeks (range, 21-52 weeks). The median duration of response was 8 months (range, 5-13 months). PR was observed in the patient with neutropenia at the 1 mg/kg dose; defined by resolution of neutropenia (but with persistent elevation in T-LGLL cells). Partial responses were observed in the 3 patients with transfusion-dependent anemia (defined as transfusion-independence for a minimum of 2 months); 2 at the 2 mg/kg dose, and 1 at the 4 mg/kg dose of BNZ-1, and these 3 patients remained on BNZ-1 as part of the LTE. These patients received 5, 24, and 25 weekly doses of BNZ-1, respectively, and all patients remained transfusion independent while receiving BNZ-1. Of these 3 patients, 1 completed the LTE, 1 discontinued the drug because of progressive T-LGLL (worsening neutropenia) although hemoglobin was stable, and 1 patient was taken off study after 5 weeks on the LTE because the study closed. Only the patient with neutropenia completed the 3-month extension period, and neutropenia has not recurred >4 years later. Notably, 1 of these responders with transfusion-dependent anemia also had pure red cell aplasia that had failed numerous prior therapies including alemtuzumab (UVA-004; Table 3). Detailed data on the 4 patients who responded are shown in Table 3, whereas a detailed description of characteristics of all patients are shown in supplemental Table 3.
Clinical efficacy of BNZ-1 in patients with T-LGLL
| Objective response . | N = 20 (%) . |
|---|---|
| Partial response | 4 (20) |
| Transfusion-dependent anemia | 3 (75) |
| Neutropenia | 1 (25) |
| Progressive disease | 16 (80) |
| Overall response rate | 4 (20) |
| Objective response . | N = 20 (%) . |
|---|---|
| Partial response | 4 (20) |
| Transfusion-dependent anemia | 3 (75) |
| Neutropenia | 1 (25) |
| Progressive disease | 16 (80) |
| Overall response rate | 4 (20) |
Clinical features of patients who were responding to BNZ-1
| Patient . | Dose (mg/kg) . | STAT3 mutation . | Indication . | Prior treatment . | Baseline ANC . | Baseline HgB (g/dL) . | ANC best response . | HgB best response . | Overall response . | LTE (Y/N) . |
|---|---|---|---|---|---|---|---|---|---|---|
| L-OSU-004 | 1 | STAT3; p.Y640F, c.1919 A>T | Neutropenia, Infection | None (for LGL); abatacept for RA | 490 | 11.5 | 2230 (D28) | 12.1 | PR | N |
| L-OSU-006 | 2 | WT | Transfusion-dependent anemia, neutropenia | MTX | 580 | 9.2 | 3850 (D28) | 8.4 (stable) | PR | Y |
| L-UVA-004 | 2 | WT | Transfusion-dependent anemia | MTX, Cytoxan+pred, CsA, alemtuzumab | 430 | 7.7 | 880 (LTE WK 22) | 10.5 (LTE WK 9) | PR | Y |
| L-UVA-007 | 4 | D661Y | Transfusion-dependent anemia | MTX | 1140 | 10.4 | 2810 (WK 10) | 10.9 (WK 8) | PR | Y |
| Patient . | Dose (mg/kg) . | STAT3 mutation . | Indication . | Prior treatment . | Baseline ANC . | Baseline HgB (g/dL) . | ANC best response . | HgB best response . | Overall response . | LTE (Y/N) . |
|---|---|---|---|---|---|---|---|---|---|---|
| L-OSU-004 | 1 | STAT3; p.Y640F, c.1919 A>T | Neutropenia, Infection | None (for LGL); abatacept for RA | 490 | 11.5 | 2230 (D28) | 12.1 | PR | N |
| L-OSU-006 | 2 | WT | Transfusion-dependent anemia, neutropenia | MTX | 580 | 9.2 | 3850 (D28) | 8.4 (stable) | PR | Y |
| L-UVA-004 | 2 | WT | Transfusion-dependent anemia | MTX, Cytoxan+pred, CsA, alemtuzumab | 430 | 7.7 | 880 (LTE WK 22) | 10.5 (LTE WK 9) | PR | Y |
| L-UVA-007 | 4 | D661Y | Transfusion-dependent anemia | MTX | 1140 | 10.4 | 2810 (WK 10) | 10.9 (WK 8) | PR | Y |
CsA, cyclosporine; EO; end of treatment; HgB, hemoglobin; MTX, methotrexate; pred, prednisone; RA, rheumatoid arthritis; WT, wild-type.
Response of leukemic cells to BNZ-1 in the peripheral blood of patients with T-LGLL
To evaluate the effect of BNZ-1 on target T-LGLL cells, we performed an apoptosis analysis using flow cytometry on T-LGLL cells (defined by a CD3+CD8+CD5dimCD16+ phenotype by flow cytometry). As previously shown in vitro, rapid apoptosis was seen in the T-LGLL cells after BNZ-1 treatment but not in CD4+ T cells. This suggests that CD4+ T cells do not need IL-15 to maintain homeostasis in vivo. Representative results from 1 patient are shown in Figure 1A-C. These results strongly suggest that the increased annexin V staining observed with leukemic cells represents the apoptotic response of T-LGLL cells. Next, we aimed to determine whether BNZ-1 increased apoptosis in ex vivo T-LGLL cells. Here, we observed that 5 of 20 enrolled patients with T-LGLL showed constitutive high apoptosis of leukemic cells before BNZ-1 treatment (>42% annexin V+ leukemic cells at predose; supplemental Figure 1), likely intrinsic to these patients with proliferative T-LGLL, making it difficult to assess apoptotic response in these patients. Of the remaining 15 patients, 2 showed no major apoptotic response to BNZ-1 at any time during treatment. As shown in Figure 1D, the response to BNZ-1 treatment of CD4+ T cells in 15 patients with T-LGLL was nonexistent, with no statistically significant change in apoptotic response after BNZ-1 treatment (Table 4). In contrast, T-LGLL cells showed significant increase in apoptosis in response to BNZ-1 treatment as early as day 2, compared with baseline (predose, D1) with changes that remained statistically different from baseline through the treatment period (P < .005; Figure 4E; Table 4). Those responses reverted to the basal level during the washout (follow-up) period. Thus, we obtained in vivo proof-of-concept that cytokine inhibition by treatment with BNZ-1 induced a strong apoptotic response in T-LGLL cells, in support of our initial hypothesis that T-LGLL cells are dependent on IL-15.
Analysis of apoptotic response to BNZ-1 of CD4+ T cells and T-LGLL cells. (A) Flow cytometric analysis of CD4+ T cells and T-LGLL cells to BNZ-1. A representative flow pattern (annexin-V-FITC, BioLegend) of apoptotic cells in the CD4 T-cell subset (defined by CD45+CD3+CD4+) and T-LGLL cells (defined by CD45+CD3+CD8+CD5dimCD57+CD45ROloCCR7lo) before and on day 2 of the BNZ-1 treatment (patient ID: UVA-001; dose cohort 1, 0.5 mg/kg) is shown. PBMCs were purified from blood using Ficoll-Paque, and 2 million cells were stained as described in the text. After staining with fluorochrome-conjugated antibodies (BioLegend; see “Methods” for details), each target cell was gated using the markers (top), and their annexin V staining is shown as histograms. Staining of fluorescein isothiocyanate (FITC)–annexin V in phosphate-buffered saline was used as the negative control to set the photomultiplier tubes (PMT) of the flow machine (BD FACSAria II) and gating windows with the FlowJo analysis. (B.) JC-1 (mitochondrial membrane potential) assessment of apoptotic response of the T-LGLL cells (a representative staining pattern). PBMCs from a patient (UVA-001; before and on day 2 of BNZ-1 treatment at 0.5 mg/kg) were purified using Ficoll-Paque, and 2 million PBMCs were stained with JC-1 dye at 37°C for 10 minutes per the manufacturer’s instructions, followed by staining with fluorochrome-conjugated antibodies including allophycocyanin (APC) annexin V. T-LGLL cells were defined by CD3+CD8+CD5dimCD57+CD45ROloCCR7lo, and the JC-1 (excited by 488 nm laser, signal collected in the 575/25 channel) and annexin V staining of gated T-LGLL cells are shown. The majority of annexin V–positive cells (those which have lost the polarity of the plasma membrane) showed lowered mitochondrial membrane potential. (C) Cleavage of caspase 9 in CD4 and T-LGLL cells after BNZ-1 treatment (a representative result). PBMCs were collected from a patient with LGLL (UVA-001) before and on day 2 of BNZ-1 treatment. CD4 T cells and T-LGLL cells were sorted using the aforementioned marker definition using BD FACSAria II and lysed radioimmunoprecipitation assay (RIPA) buffer. Cellular lysates were then resolved in a sodium dodecyl sulfate gel electrophoresis, transferred to a polyvinylidene difluoride membrane, and probed with anticaspase-9 antibody (Cell Signaling Technologies); cleavage of caspase 9 was only seen in T-LGLL cells after BNZ-1 treatment. The images were visualized using a standard electrochemiluminescence (ECL) (Femto-substrate, Thermo Fisher) and collected using a Gel Doc system (BioRad). (D) Longitudinal analysis (kinetics) of the apoptosis of CD4 T cells from 15 patients with T-LGLL. Of the 20 enrolled patients, 5 were excluded because their T-LGLL cells showed extremely high apoptosis before BNZ-1 treatment. The annexin V staining of the CD4 T cells (target cells were defined by surface markers as shown earlier) in PBMCs from the remaining 15 patients with T-LGLL are shown. Samples were collected at indicated time points (day 1, 2, 8, 15, and 29; weeks 9, 13, and 17) and 3 washout points (2, 4, and 6 weeks after the termination of the treatment: Z2, Z4, and Z6, respectively). The average and 95% confidence interval are shown. There was no statistical difference in annexin V positivity between day 1 (pretreatment) and any other time point; D01/day 1 is “predose.” (E) Longitudinal analysis (kinetics) of the apoptosis of T-LGLL cells (target cells were defined by the surface markers shown above) from 15 patients with T-LGLL. Of the 20 enrolled patients, 5 were excluded because their T-LGLL cells showed extremely high apoptosis before BNZ-1 treatment. Annexin V staining of the CD4 T cells in PBMCs from the remaining 15 patients with T-LGLL are shown. Samples were collected at indicated time points (days 1 (D01), D02, D08, D015, and D029; weeks 9 [W09], 13 [W13], and 17 [W17]), and 3 washout points (Z2, Z4, and Z6). D01/day 1 is predose. The average and 95% confidence interval are shown. P values from the comparison of the average (%) from ∼D2-D29 in comparison with that of D1 were all < .05. memb, membrane.
Analysis of apoptotic response to BNZ-1 of CD4+ T cells and T-LGLL cells. (A) Flow cytometric analysis of CD4+ T cells and T-LGLL cells to BNZ-1. A representative flow pattern (annexin-V-FITC, BioLegend) of apoptotic cells in the CD4 T-cell subset (defined by CD45+CD3+CD4+) and T-LGLL cells (defined by CD45+CD3+CD8+CD5dimCD57+CD45ROloCCR7lo) before and on day 2 of the BNZ-1 treatment (patient ID: UVA-001; dose cohort 1, 0.5 mg/kg) is shown. PBMCs were purified from blood using Ficoll-Paque, and 2 million cells were stained as described in the text. After staining with fluorochrome-conjugated antibodies (BioLegend; see “Methods” for details), each target cell was gated using the markers (top), and their annexin V staining is shown as histograms. Staining of fluorescein isothiocyanate (FITC)–annexin V in phosphate-buffered saline was used as the negative control to set the photomultiplier tubes (PMT) of the flow machine (BD FACSAria II) and gating windows with the FlowJo analysis. (B.) JC-1 (mitochondrial membrane potential) assessment of apoptotic response of the T-LGLL cells (a representative staining pattern). PBMCs from a patient (UVA-001; before and on day 2 of BNZ-1 treatment at 0.5 mg/kg) were purified using Ficoll-Paque, and 2 million PBMCs were stained with JC-1 dye at 37°C for 10 minutes per the manufacturer’s instructions, followed by staining with fluorochrome-conjugated antibodies including allophycocyanin (APC) annexin V. T-LGLL cells were defined by CD3+CD8+CD5dimCD57+CD45ROloCCR7lo, and the JC-1 (excited by 488 nm laser, signal collected in the 575/25 channel) and annexin V staining of gated T-LGLL cells are shown. The majority of annexin V–positive cells (those which have lost the polarity of the plasma membrane) showed lowered mitochondrial membrane potential. (C) Cleavage of caspase 9 in CD4 and T-LGLL cells after BNZ-1 treatment (a representative result). PBMCs were collected from a patient with LGLL (UVA-001) before and on day 2 of BNZ-1 treatment. CD4 T cells and T-LGLL cells were sorted using the aforementioned marker definition using BD FACSAria II and lysed radioimmunoprecipitation assay (RIPA) buffer. Cellular lysates were then resolved in a sodium dodecyl sulfate gel electrophoresis, transferred to a polyvinylidene difluoride membrane, and probed with anticaspase-9 antibody (Cell Signaling Technologies); cleavage of caspase 9 was only seen in T-LGLL cells after BNZ-1 treatment. The images were visualized using a standard electrochemiluminescence (ECL) (Femto-substrate, Thermo Fisher) and collected using a Gel Doc system (BioRad). (D) Longitudinal analysis (kinetics) of the apoptosis of CD4 T cells from 15 patients with T-LGLL. Of the 20 enrolled patients, 5 were excluded because their T-LGLL cells showed extremely high apoptosis before BNZ-1 treatment. The annexin V staining of the CD4 T cells (target cells were defined by surface markers as shown earlier) in PBMCs from the remaining 15 patients with T-LGLL are shown. Samples were collected at indicated time points (day 1, 2, 8, 15, and 29; weeks 9, 13, and 17) and 3 washout points (2, 4, and 6 weeks after the termination of the treatment: Z2, Z4, and Z6, respectively). The average and 95% confidence interval are shown. There was no statistical difference in annexin V positivity between day 1 (pretreatment) and any other time point; D01/day 1 is “predose.” (E) Longitudinal analysis (kinetics) of the apoptosis of T-LGLL cells (target cells were defined by the surface markers shown above) from 15 patients with T-LGLL. Of the 20 enrolled patients, 5 were excluded because their T-LGLL cells showed extremely high apoptosis before BNZ-1 treatment. Annexin V staining of the CD4 T cells in PBMCs from the remaining 15 patients with T-LGLL are shown. Samples were collected at indicated time points (days 1 (D01), D02, D08, D015, and D029; weeks 9 [W09], 13 [W13], and 17 [W17]), and 3 washout points (Z2, Z4, and Z6). D01/day 1 is predose. The average and 95% confidence interval are shown. P values from the comparison of the average (%) from ∼D2-D29 in comparison with that of D1 were all < .05. memb, membrane.
Statistical analysis of CD4 and T-LGLL apoptotic response to BNZ-1
| . | 1-month treatment . | Extended treatment . | Washout . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Predose . | D 2 . | D 8 . | D 15 . | D 29 . | Wk 9 . | Wk 13 . | Wk 17 . | Follow-up wk 2 . | Follow-up wk 4 . | Follow-up wk 6 . |
| CD4 T-cells | |||||||||||
| Mean ± SEM (%) | 11.59 ± 0.86 | 14.08 ± 1.68 | 14.24 ± 1.40 | 14.49 ± 1.63 | 12.70 ± 0.77 | 15.30 ± 1.38 | 11.70 ± 0.73 | 13.50 ± 1.80 | 12.30 ± 2.17 | 10.48 ± 0.99 | 10.48 ± 0.99 |
| P value (compared with predose) | .199 | .114 | .127 | .349 | .025 | .927 | .288 | .739 | .404 | .433 | |
| N = 132 | 15 | 15 | 14 | 15 | 14 | 11 | 12 | 8 | 11 | 9 | 8 |
| T-LGLL cells | |||||||||||
| Mean ± SEM (%) | 14.46 ± 2.06 | 48.36 ± 4.04 | 32.79 ± 5.67 | 35.92 ± 5.99 | 40.55 ± 5.38 | 37.25 ± 7.30 | 33.20 ± 5.98 | 30.50 ± 5.15 | 29.65 ± 4.11 | 15.02 ± 4.37 | 17.80 ± 3.75 |
| P value (compared with predose) | < .001 | .004 | .002 | < .001 | .002 | .003 | .002 | .002 | .697 | .403 | |
| N = 132 | 15 | 15 | 14 | 15 | 14 | 11 | 12 | 8 | 11 | 9 | 8 |
| . | 1-month treatment . | Extended treatment . | Washout . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Predose . | D 2 . | D 8 . | D 15 . | D 29 . | Wk 9 . | Wk 13 . | Wk 17 . | Follow-up wk 2 . | Follow-up wk 4 . | Follow-up wk 6 . |
| CD4 T-cells | |||||||||||
| Mean ± SEM (%) | 11.59 ± 0.86 | 14.08 ± 1.68 | 14.24 ± 1.40 | 14.49 ± 1.63 | 12.70 ± 0.77 | 15.30 ± 1.38 | 11.70 ± 0.73 | 13.50 ± 1.80 | 12.30 ± 2.17 | 10.48 ± 0.99 | 10.48 ± 0.99 |
| P value (compared with predose) | .199 | .114 | .127 | .349 | .025 | .927 | .288 | .739 | .404 | .433 | |
| N = 132 | 15 | 15 | 14 | 15 | 14 | 11 | 12 | 8 | 11 | 9 | 8 |
| T-LGLL cells | |||||||||||
| Mean ± SEM (%) | 14.46 ± 2.06 | 48.36 ± 4.04 | 32.79 ± 5.67 | 35.92 ± 5.99 | 40.55 ± 5.38 | 37.25 ± 7.30 | 33.20 ± 5.98 | 30.50 ± 5.15 | 29.65 ± 4.11 | 15.02 ± 4.37 | 17.80 ± 3.75 |
| P value (compared with predose) | < .001 | .004 | .002 | < .001 | .002 | .003 | .002 | .002 | .697 | .403 | |
| N = 132 | 15 | 15 | 14 | 15 | 14 | 11 | 12 | 8 | 11 | 9 | 8 |
Averages and standard deviations of the percentages of apoptotic cells within each subset (CD4 T cells or T-LGLL cells) were calculated. Average (of the % apoptotic cells within each subset) at given day of the treatment was compared with that of day 1 (pretreatment) and Student t test was performed to determine the P values to see whether the difference was significant (P < .05, shown in italic).
Numbers given in the table are the P values of each comparison.
Apoptotic percentages in the T-LGLL subset showed a significant difference from that at pretreatment at all times during treatment, whereas apoptotic death of CD4 T cells showed no significant deviation from that at pretreatment.
During the washout period, apoptotic death of T-LGLL subsided to the baseline level at pretreatment.
SEM, standard error of the mean.
Correlation of apoptosis to STAT3 mutation status, BNZ-1 dosage, and clinical features
We then examined whether the apoptosis of T-LGLL cells showed dose-dependent responses to BNZ-1. As shown in supplemental Figure 2, no dose-dependency to BNZ-1 treatment was observed in the apoptotic response of T-LGLL cells. In addition, we examined whether STAT3 mutation status affected the apoptotic response of T-LGLL cells. As shown in supplemental Figure 3, there was no apparent difference in apoptotic response to BNZ-1 treatment between leukemic cells in patients with wild-type STAT3 and mutated STAT3.
Finally, we evaluated whether there was a difference in apoptotic response in clinical responders vs nonresponders. Four responders and 16 nonresponders were compared (Table 5). The results show that the clinical responders represent cases in which the apoptotic response of T-LGLL cells persists during the first month of treatment into the extended 3-month period. Comparatively, nonresponders showed specific apoptotic response at day 2 (compared with predose day 1) that subsequently declined and became statistically not significant. Furthermore, it was not the degree of apoptotic response at day 2 but rather the persistence of this response by day 29 that was predictive of response to BNZ-1. These findings suggest that the mechanisms underlying the clinical improvements of T-LGLL disease symptoms (such as anemia and neutropenia), at least partly, include the continued removal of circulating leukemic cells via BNZ-1 therapy.
Statistical analysis of apoptotic response to BNZ-1 between responders and nonresponders
| . | 1-month treatment . | Extended treatment . | Washout . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Predose . | Day 2 . | Day 8 . | Day 15 . | Day 29 . | Week 9 . | Week 13 . | Week 17 . | Follow-up week 2 . | Follow-up week 4 . | Follow-up week 6 . | |
| Responders | |||||||||||
| Mean ± SEM (%) | 9.65 ± 3.12 | 56.95 ± 6.18 | 32.28 ± 4.91 | 42.75 ± 4.05 | 51.13 ± 2.73 | 19.00 ± 6.44 | 34.32 ± 8.77 | 19.20 ± 6.86 | 36.50 ± 5.74 | 23.15 ± 7.92 | 18.20 ± 2.81 |
| P value (compared with predose) | < .001 | .008 | < .001 | < .001 | .253 | .038 | .273 | .01 | .164 | .067 | |
| n | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Nonresponders | |||||||||||
| Mean ± SEM (%) | 29.78 ± 5.82 | 49.80 ± 4.87 | 35.10 ± 5.52 | 36.95 ± 5.79 | 41.50 ± 5.93 | 43.51 ± 5.74 | 37.20 ± 5.53 | 31.32 ± 7.46 | 30.30 ± 5.63 | 16.2 ± 3.10 | 26.70 ± 7.19 |
| P value (compared with predose) | .013 | .514 | .389 | .168 | .113 | .396 | .872 | .951 | .152 | .762 | |
| n | 16 | 16 | 15 | 16 | 16 | 12 | 10 | 10 | 12 | 7 | 7 |
| . | 1-month treatment . | Extended treatment . | Washout . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Predose . | Day 2 . | Day 8 . | Day 15 . | Day 29 . | Week 9 . | Week 13 . | Week 17 . | Follow-up week 2 . | Follow-up week 4 . | Follow-up week 6 . | |
| Responders | |||||||||||
| Mean ± SEM (%) | 9.65 ± 3.12 | 56.95 ± 6.18 | 32.28 ± 4.91 | 42.75 ± 4.05 | 51.13 ± 2.73 | 19.00 ± 6.44 | 34.32 ± 8.77 | 19.20 ± 6.86 | 36.50 ± 5.74 | 23.15 ± 7.92 | 18.20 ± 2.81 |
| P value (compared with predose) | < .001 | .008 | < .001 | < .001 | .253 | .038 | .273 | .01 | .164 | .067 | |
| n | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Nonresponders | |||||||||||
| Mean ± SEM (%) | 29.78 ± 5.82 | 49.80 ± 4.87 | 35.10 ± 5.52 | 36.95 ± 5.79 | 41.50 ± 5.93 | 43.51 ± 5.74 | 37.20 ± 5.53 | 31.32 ± 7.46 | 30.30 ± 5.63 | 16.2 ± 3.10 | 26.70 ± 7.19 |
| P value (compared with predose) | .013 | .514 | .389 | .168 | .113 | .396 | .872 | .951 | .152 | .762 | |
| n | 16 | 16 | 15 | 16 | 16 | 12 | 10 | 10 | 12 | 7 | 7 |
Averages and standard deviations of the percentages of apoptotic cells within each subset (CD4 T cells or T-LGLL cells) were calculated.
Average (percentage of the apoptotic cells within each subset) on the given day of the treatment was compared with that of day 1 (pretreatment), and Student t test was performed to determine the P values to check whether the difference was significant (P < .05 shown in italic).
Numbers given in the table are the P values of each comparison.
Note that apoptotic percentages in responders showed significant difference from that at pretreatment at all times during treatment and returned to baseline during the washout, whereas that in nonresponders was significantly elevated from that at pretreatment then subsided quickly, suggesting that the sustained apoptotic response of T-LGLL cells may differentiate responders from nonresponders.
Safety
Table 6 demonstrates all treatment-emergent AEs in this trial. In total, 33 AEs of any grade were observed during the trial, with the most common AEs (any grade) observed as hematologic, including neutropenia (24%; n = 8), anemia (24%; n = 8), and white blood count decrease (12%; n = 4). Of these, grade 3/4 AEs were observed in 7 of 8 (88%) patients with neutropenia, and 4 of 8 (50%) patients with anemia. Most of these hematologic AEs were deemed unrelated to BNZ-1, because hematocytopenias are a disease manifestation of T-LGLL. Only 7 of 20 (35%) of the hematologic AEs were deemed by the investigators to possibly be due to BNZ-1, and only 1 AE (anemia) was deemed probably associated with BNZ-1, although likely due to an underlying disease. Aside from hematologic toxicities, treatment-emergent AEs were rare, with only alanine aminotransferase increase (n = 3, any grade; n = 1, grade 3/4) and hypertension (n = 4, any grade; n = 1, grade 3/4) occurring more than once. Of these AEs, only 1 AE for hypertension and alanine aminotransferase increase, respectively, were deemed possibly related to BNZ-1. (Table 6) Three patients were treated on the LTE, and all AEs were thought unrelated to BNZ-1, or due to underlying disease (supplemental Table 4).
Treatment-emergent AEs with BNZ-1 in patients with T-LGLL
| AE, n (%) . | Any grade . | Grade 3/4 . | Relation to BNZ-1 . |
|---|---|---|---|
| Neutropenia | 8 | 7 | 3 of 8, possibly |
| Decreased WBC | 4 | 3 | 1, possibly |
| Anemia | 8 | 4 | 3 possible and 1 probable |
| Alanine transaminase increase | 3 | 1 | 1 possible |
| Atrial fibrillation | 1 | 1 | Unrelated |
| Bilirubin increase | 1 | 1 | Possibly |
| Fever | 1 | 1 | Unrelated |
| Hypertension | 4 | 1 | 1 possible |
| Hypoglycemia | 1 | 1 | Possible |
| Joint swelling | 1 | 1 | Unrelated |
| Pneumonia | 1 | 1 | Unrelated |
| Total | 33 | 22 |
| AE, n (%) . | Any grade . | Grade 3/4 . | Relation to BNZ-1 . |
|---|---|---|---|
| Neutropenia | 8 | 7 | 3 of 8, possibly |
| Decreased WBC | 4 | 3 | 1, possibly |
| Anemia | 8 | 4 | 3 possible and 1 probable |
| Alanine transaminase increase | 3 | 1 | 1 possible |
| Atrial fibrillation | 1 | 1 | Unrelated |
| Bilirubin increase | 1 | 1 | Possibly |
| Fever | 1 | 1 | Unrelated |
| Hypertension | 4 | 1 | 1 possible |
| Hypoglycemia | 1 | 1 | Possible |
| Joint swelling | 1 | 1 | Unrelated |
| Pneumonia | 1 | 1 | Unrelated |
| Total | 33 | 22 |
WBC, white blood cell.
Cytokine analysis
There were no changes across time in BNZ-1 target cytokines including IL-15, IL-2, IL-2β, and IL-9 (supplemental Tables 6-7; supplemental Figure 4) or across inflammatory cytokines (supplemental Tables 8-10; supplemental Figure 5). When stratified based on response, there was no definitive, directional correlation in either target or inflammatory cytokine levels with BNZ-1 treatment (supplemental Figures 11-15; supplemental Table 7A-D).
PKs
There was a dose-related increase in the mean BNZ-1 plasma concentrations on both day 1 (first dose) and day 22 (fourth dose) (supplemental Figure 8). For the total PK population, on day 1 the geometric mean AUCτ, essentially AUC0-168, increased from 1319 hour × μg/mL for the 0.5 mg/kg dose to 14 381 hour × μg/mL for the 4 mg/kg dose, and the geometric mean Cmax increased from 18.2 μg/mL for the 0.5 mg/kg dose to 148 μg/mL for the 4 mg/kg dose. Similar dose-related increases were observed on day 22. For the total PK population, the power model exponents for day 1 were 1.063 and 1.1913 for Cmax and AUC0-168, respectively; and for day 22 were 1.2637 and 1.3094. For the total PK population, the median ratios for Cmax ranged from 1.13 to 1.52 and those for AUC0-168 ranged from 1.16 to 1.73; neither appeared to vary consistently with dose, suggesting some accumulation with once-weekly dosing. Assuming an overall median of 1.5, this would suggest a t½ of ∼17 days.
Discussion
Based on previous data obtained by us and others, we hypothesized that in vivo leukemic cells in patients with T-LGLL are dependent on the cytokine IL-15 for their proliferation and homeostasis, which led us to conduct this phase 1/2 clinical trial targeting patients with T-LGLL by blocking the action of IL-15 using a selective γc cytokine–binding peptide, BNZ-1. In this study, we report first-in-human proof that T-LGLL cells are indeed dependent on IL-15 and that the intervention with IL-15 inhibition using BNZ-1 in patients with T-LGLL shows therapeutic effects.
This is the largest prospective trial in T-LGLL reported since the ECOG 5998 trial and the first trial to successfully target the underlying disease pathogenesis by targeting IL-15. Of patients who were evaluable, 87% showed apoptotic response to BNZ-1 treatment, and 20% of patients had objective clinical responses. Given stringent clinical response criteria, the responses were classified as PR, but this belies the clinical impact of BNZ-1 on patients with T-LGLL. CR required complete eradication of the T-cell clone, which has only been rarely documented in the literature.25 Of the 4 PRs, 3 had transfusion-dependent anemia that became transfusion independent with BNZ-1, and 1 patient had severe neutropenia who had resolution of neutropenia. There was no correlation of response with BNZ-1 dose, with responses occurring at the 1, 2, and 4 mg/kg dosage of BNZ-1. These responses occurred rapidly, with a median time to maximum response of 8 weeks. Importantly, these responses were durable, with a median duration of response of 8 months (range, 5-13 months), with 3 patients remaining on treatment into the LTE (of which only 1 progressed). The lack of any difference of response with STAT3 mutation status is not surprising, because previous studies have demonstrated that STAT3 gain-of-function mutations do not render T-LGLL cells IL-15 cytokine-independent but rather more sensitive to cytokine (IL-15) stimulation.22 Thus, there was no clear difference in response based on STAT3 mutation status. BNZ-1 was well tolerated, and few AEs were observed. The majority (61%) of AEs were hematologic AEs, which were unrelated to the study drug, and nearly universally due to the underlying T-LGLL, with no dose-limiting toxicities. These results demonstrate that BNZ-1 is safe, with efficacy in T-LGLL, and warrants future investigation in T-LGLL.
We also observed that BNZ-1 induced apoptosis of T-LGLL cells in the majority (87%; 13 of 15) of patients who were evaluable when compared with nonleukemic CD4+ T cells from each patient, 48 hours after BNZ-1 administration (P < .001). These responses continued with subsequent evaluations, reversed during the washout (follow-up) period, and were statistically different throughout the extended treatment period (Table 5). These data offer first-in-human proof that T-LGLL cells are dependent on IL-15 in vivo and that inhibition of IL-15–dependent T-LGLL cells with BNZ-1 induces apoptosis. It is important to note, that although the majority of patients experienced T-LGLL apoptosis immediately after BNZ-1 administration, only 4 of 20 (20%) patients developed a clinical response. Although all patients who were evaluable had statistically significant apoptosis (when compared with nonreactive CD4+ T cells) on day 2 after treatment, clinical nonresponders universally had decreased apoptosis, starting on day 8, without persistence of significant apoptosis, and continuing throughout the study period, whereas responders maintained statistically significant apoptosis throughout the first month of treatment, with decreased apoptosis thereafter (Table 5). Our data suggest that a sustained apoptotic response beyond the initial 24 hours after BNZ-1 infusion is necessary to sustain a clinical response. These findings suggest that there may be intrinsic changes in leukemia cells that may alter responses to BNZ-1 over time, thus further providing insights into the distinct role of IL-15 in the initial pathogenesis and homeostasis of T-LGLL cells. Although persistent apoptosis of T-LGLL cells was associated with clinical response, the exact mechanism by which hematocytopenias resolve in T-LGLL is not entirely clear, particularly because response criteria were assessed primarily based on hematologic parameters (improvement in hematocytopenias). Based on these data, it is likely that the clearance of T-LGLL cells, which also is a prerequisite for determining clinical response based on E5998 criteria, is the primary driver of improvement in hematocytopenias. However, the exact mechanisms of this response and the impact of BNZ-1 therapy on other sites of disease (eg, the bone marrow and spleen) should be investigated in future clinical trials.
Although our data demonstrate that there is near universal in vivo dependence of T-LGLL cells on IL-15 initially, as evidenced by apoptosis on day 2, not all patients responded clinically, and in many patients, apoptosis returned to baseline after an initial increase on day 2. Furthermore, in responders, apoptosis is maintained throughout the treatment period, suggesting that these cells remain dependent on IL-15 and, thus, are sensitive to ongoing BNZ-1 therapy. However, in patients who did not have persistent robust apoptosis (nonresponders), it is likely that these cells are no longer IL-15 dependent. There are several potential explanations for this observation. Stimulation of CTLs with IL-15 alone is sufficient to induce T-LGLL and induces LGL in transgenic mice.12,16 Furthermore, in patients with somatic STAT3 mutations (40% of T-LGLL), IL-15 amplifies aberrant signaling pathways.26 This suggests that IL-15 is critical to the initial proliferation of T-LGLL, but after downstream pathways are upregulated, its role is no longer crucial. Evidence from a phase 1 trial of the IL-2/IL-15–inhibiting antibody Hu-MikB1 in T-LGLL suggested that T-LGLL cells may become independent of cytokine signaling.27 In this study, it was observed that, ex vivo, patient T-LGLL cells did not produce or require IL-2 or IL-15 for their survival, suggesting that some T-LGLL cells can develop cytokine independence. This would explain the lack of response in a number of patients who might have required IL-15 for the initial proliferation and homeostasis of T-LGLL but were able to activate other pathways for cell growth upon deprivation of IL-15. Numerous alternative pathways, including the PI3K pathway, MAPK, and NF-κB pathways, have been implicated in the pathogenesis of T-LGLL, and upregulation of these pathways may explain the 20% response rate observed with BNZ-1.16,19,28,29 In our cytokine analysis, we observed no significant changes in target cytokine (IL-2, -9, and -15) levels over time, and no significant differences were observed in cytokine levels between responders and nonresponders over time (supplemental Tables 6-10 and 11-15), suggesting that BNZ-1–induced cytokine alterations are not responsible for the effects of BNZ-1. These results are not surprising, because BNZ-1 inhibits the binding of common γ chain (γc) cytokines and, thus, would not affect the levels of these cytokines. Genomic transcriptomic analysis will be needed to evaluate the changes in gene expression before and after BNZ-1. The reason certain cells may be more cytokine dependent than others is unknown, and additional studies are needed to uncover the mechanism of BNZ-1 escape to develop potential combinatorial strategies with BNZ-1.
An important limitation of this study is the fact that BNZ-1 inhibits IL-2, IL-9, and IL-15 γc cytokines and is not only specific for IL-15. In our study, we did not observe significant decreases in regulatory T cells, which are dependent on IL-2, in comparison with IL-15–dependent NK cells, in which we saw a marked decrease in NK-cell populations. This suggests that competition for IL-15 between T-LGLL cells and non-LGLL cells (eg, NK cells and memory CD8 cells) is much greater for IL-15 than for IL-2, and that IL-2 plays a limited role in the pathogenesis of this disease. Furthermore, there is no evidence that IL-9 induces the development of T-LGLL, so its inhibition is not likely to contribute to the effects seen in this study.17 Despite this limitation, the findings of this study provide crucial in vivo evidence that IL-15 inhibition alone induces apoptosis in patients with T-LGLL in vivo.
BNZ-1 is a potential new therapeutic agent for the treatment of T-LGLL and represents the first rationally developed drug based on disease pathogenesis to have demonstrated efficacy in T-LGLL. The use of BNZ-1 in combination with other novel, or standard-of-care agents, may enhance the clinical efficacy of BNZ-1 by limiting the ability of T-LGLL cells to develop resistance to IL-15 cytokine blockade and should be explored. The results of this study, with substantial apoptosis in the vast majority of patients with T-LGLL treated with IL-15 deprivation, for the first time provide proof of the IL-15 dependency of T-LGLL in humans and provide hope for future therapeutic interventions targeting this cytokine in this rare disease.
Acknowledgments
The authors thank the University of Virginia LGL Leukemia Registry personnel, especially Bryna Shemo, Andrea Hines, and Matt Schmachtenberg, for their support of this study.
This work was supported by National Center for Advancing Translational Sciences, National Institutes of Health grant KL2TR002734 (J.E.B.). T.L. is supported by the National Cancer Institute. National Institutes of Health under award number R01CA178393. Additional support (J.E.B.) was provided by a generous anonymous donor in Columbus, OH, and further support (T.L.) was provided by the Bess Family Charitable Fund, the LGL Leukemia Foundation, Charles and Katharine Hutton Tweedy, William J. Branch, Szabolcs Szentpetery, and 2 generous anonymous donors (T.L.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: J.E.B. designed the study, led the study as overall principal investigator, analyzed the data, and wrote the manuscript; K.B., L.S., C.Q., and R.N., were site principal investigators on the study, analyzed the data, and revised and approved the final manuscript; A.M. assisted in the design of correlatives for the study, analyzed the data, and revised and approved the final manuscript; E.M.M. performed statistical analyses, and approved the final manuscript; D.F. performed all STAT3-related studies, analyzed the data, and revised and approved the final manuscript; T.A.W. designed BNZ-1 and the study and analyzed the data; N.A. created the BNZ-1 product, led all commercial aspects of the trial, analyzed the data, and revised and approved the final manuscript; Y.T. designed correlative studies, performed all correlative studies related to the data, and drafted, revised, and approved the manuscript; T.L. designed/wrote the protocol, analyzed the data, performed preclinical studies in his laboratory, and revised and approved the final manuscript.
Conflict-of-interest disclosure: J.E.B. serves on the scientific advisory board for Dren Bio and Kymera Therapeutics. T.L. served on the scientific advisory board and has stock options in Keystone Nano, Bioniz Therapeutics, and Dren Bio. T.L. and D.F. received honoraria from Kymera Therapeutics. D. F. received research funding from AstraZeneca. Y.T. served on the scientific advisory board and received research funding for the presented work. The remaining authors declare no competing financial interests.
Thomas A. Waldmann died on 25 September 2021.
Correspondence: Jonathan E. Brammer, The James Comprehensive Cancer Center, The Ohio State University, 2121 Kenny Drive, Room 7168, Columbus, OH 43210; e-mail: jonathan.brammer@osumc.edu.
References
Author notes
∗Y.T. and T.L. contributed equally to this study.
Data (except individual participant data) and protocols are available on request from the corresponding author, Jonathan E. Brammer (jonathan.brammer@osumc.edu).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

![Analysis of apoptotic response to BNZ-1 of CD4+ T cells and T-LGLL cells. (A) Flow cytometric analysis of CD4+ T cells and T-LGLL cells to BNZ-1. A representative flow pattern (annexin-V-FITC, BioLegend) of apoptotic cells in the CD4 T-cell subset (defined by CD45+CD3+CD4+) and T-LGLL cells (defined by CD45+CD3+CD8+CD5dimCD57+CD45ROloCCR7lo) before and on day 2 of the BNZ-1 treatment (patient ID: UVA-001; dose cohort 1, 0.5 mg/kg) is shown. PBMCs were purified from blood using Ficoll-Paque, and 2 million cells were stained as described in the text. After staining with fluorochrome-conjugated antibodies (BioLegend; see “Methods” for details), each target cell was gated using the markers (top), and their annexin V staining is shown as histograms. Staining of fluorescein isothiocyanate (FITC)–annexin V in phosphate-buffered saline was used as the negative control to set the photomultiplier tubes (PMT) of the flow machine (BD FACSAria II) and gating windows with the FlowJo analysis. (B.) JC-1 (mitochondrial membrane potential) assessment of apoptotic response of the T-LGLL cells (a representative staining pattern). PBMCs from a patient (UVA-001; before and on day 2 of BNZ-1 treatment at 0.5 mg/kg) were purified using Ficoll-Paque, and 2 million PBMCs were stained with JC-1 dye at 37°C for 10 minutes per the manufacturer’s instructions, followed by staining with fluorochrome-conjugated antibodies including allophycocyanin (APC) annexin V. T-LGLL cells were defined by CD3+CD8+CD5dimCD57+CD45ROloCCR7lo, and the JC-1 (excited by 488 nm laser, signal collected in the 575/25 channel) and annexin V staining of gated T-LGLL cells are shown. The majority of annexin V–positive cells (those which have lost the polarity of the plasma membrane) showed lowered mitochondrial membrane potential. (C) Cleavage of caspase 9 in CD4 and T-LGLL cells after BNZ-1 treatment (a representative result). PBMCs were collected from a patient with LGLL (UVA-001) before and on day 2 of BNZ-1 treatment. CD4 T cells and T-LGLL cells were sorted using the aforementioned marker definition using BD FACSAria II and lysed radioimmunoprecipitation assay (RIPA) buffer. Cellular lysates were then resolved in a sodium dodecyl sulfate gel electrophoresis, transferred to a polyvinylidene difluoride membrane, and probed with anticaspase-9 antibody (Cell Signaling Technologies); cleavage of caspase 9 was only seen in T-LGLL cells after BNZ-1 treatment. The images were visualized using a standard electrochemiluminescence (ECL) (Femto-substrate, Thermo Fisher) and collected using a Gel Doc system (BioRad). (D) Longitudinal analysis (kinetics) of the apoptosis of CD4 T cells from 15 patients with T-LGLL. Of the 20 enrolled patients, 5 were excluded because their T-LGLL cells showed extremely high apoptosis before BNZ-1 treatment. The annexin V staining of the CD4 T cells (target cells were defined by surface markers as shown earlier) in PBMCs from the remaining 15 patients with T-LGLL are shown. Samples were collected at indicated time points (day 1, 2, 8, 15, and 29; weeks 9, 13, and 17) and 3 washout points (2, 4, and 6 weeks after the termination of the treatment: Z2, Z4, and Z6, respectively). The average and 95% confidence interval are shown. There was no statistical difference in annexin V positivity between day 1 (pretreatment) and any other time point; D01/day 1 is “predose.” (E) Longitudinal analysis (kinetics) of the apoptosis of T-LGLL cells (target cells were defined by the surface markers shown above) from 15 patients with T-LGLL. Of the 20 enrolled patients, 5 were excluded because their T-LGLL cells showed extremely high apoptosis before BNZ-1 treatment. Annexin V staining of the CD4 T cells in PBMCs from the remaining 15 patients with T-LGLL are shown. Samples were collected at indicated time points (days 1 (D01), D02, D08, D015, and D029; weeks 9 [W09], 13 [W13], and 17 [W17]), and 3 washout points (Z2, Z4, and Z6). D01/day 1 is predose. The average and 95% confidence interval are shown. P values from the comparison of the average (%) from ∼D2-D29 in comparison with that of D1 were all < .05. memb, membrane.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/142/15/10.1182_blood.2022017643/3/m_blood_bld-2022-017643-gr1.jpeg?Expires=1764958555&Signature=pWOoXNnmpIIG9KWrtTso3z1c1Fbx7GF4pS3LZFXaYqWg5xjW9Fh9JOVxevaxH488A1Vb0nVlgrw47thgqAaQS8AeLm7FVxlqhovnSUgaxIpP~4uJwhph9-LrZHoYukOWlcIJ89vPsBDk520ydejmTHHRkhPo5VvSRHaLzW1fG3~pDySfUbvkLcCztR4lqJr5KbkQvcm7CjVV-v78n~fm2ACpol0K6Y1adK3Tr85b2Md-1Mxhr5ujeEggJbW7JZxtVuniAPeUGbewuhzA~-9R6Q~gx8Z2qNKvGUT~3NUhX~WkFMQFEIXx9ejGHIbSjYwUf5A69ja~L3Q2KzqiMftMhg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal