In this issue of Blood, Brammer et al present a phase 1/2 trial that demonstrates in patients with T-large granular lymphocyte leukemia (T-LGLL) that blocking interleukin-15 (IL-15) binding to CD132 using the pegylated peptide BNZ-1 results in the resolution of cytopenias in 20% of patients.1 This is the first trial that has the potential to advance our treatment options beyond the current approach of using either methotrexate, cyclophosphamide, or cyclosporine.
Large granular lymphocyte (LGL) leukemia is a chronic leukemia due to the clonal expansion of LGLs, which can be either cytotoxic T lymphocytes (CTLs) or natural killer (NK) cells.2 The World Health Organization classification differentiates T- from NK-cell LGL leukemia. Normal T-LGLs become activated through antigen recognition and undergo significant expansion with subsequent death by apoptosis upon antigen clearance. In LGLL, the LGLs persist and infiltrate the blood, marrow, and spleen.3,4 T-LGLL is a relatively unique condition in which, unlike most lymphoproliferative diseases, the indication for treatment is not disease bulk but rather the resultant cytopenias—particularly neutropenia and anemia and less commonly pure red cell aplasia, aplastic anemia, and thrombocytopenia.2
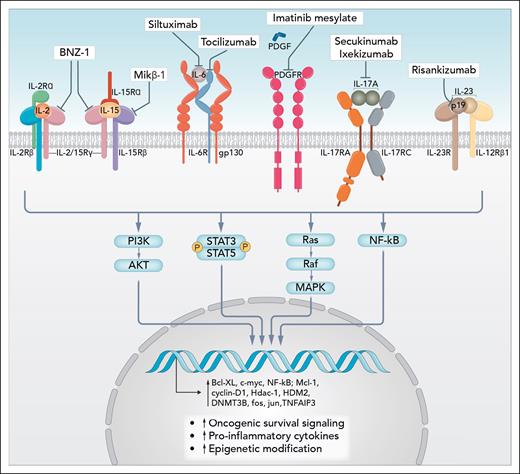
T-LGLL is thought to be triggered by chronic antigen stimulation that drives cytotoxic T cells to develop a terminal effector memory phenotype that transforms to a clonal proliferation.5 During disease development, T-LGLL cells may acquire the ability to sustain proliferative signaling by either producing growth factors and their cognate receptors themselves, resulting in chronic autocrine proliferative stimulations, or by responding to soluble growth factors present in a proinflammatory microenvironment. The most important cytokines driving this process are IL-15, platelet-derived growth factor (PDGF), IL-2, and IL-6. The interactions of these cytokines and the downstream oncogenic signaling drivers active in T-LGLL, that is, Jak/STAT, Ras-Raf-1-MEK1ERK/MAPK, Pi3k/Akt, nuclear-factor κB, Fas, and Fas ligand (FasL) signaling, and the sphingolipids sphingosine-1-phosphate and ceramide pathways, lead to increased transcription of oncogenic driver genes such as c-MYC, cyclin D1, and BCL-xL, culminating in increased malignant cell proliferation and survival (see figure).6
Cytokine pathways involved in survival of large granular lymphocytes and examples of mechanisms of inhibition. Professional illustration by Somersault18:24.
Cytokine pathways involved in survival of large granular lymphocytes and examples of mechanisms of inhibition. Professional illustration by Somersault18:24.
IL-15 signals through a heterotrimeric receptor that include (1) the IL-15-specific receptor subunit, IL-15Rα; (2) IL-2/IL-15Rβ (CD122), ie, shared with IL-2; and (3) the common gamma-chain (CD132) that is shared with the IL-2, IL-4, IL-7, IL-9, and IL-21 receptors. This in turn activates downstream signals including Jak/STAT and MAPK, which suppress proapoptotic factors in the Bcl2 family.7 In vitro and mouse models targeting IL-15 demonstrated that blocking IL-15 signaling was able to suppress LGL leukemia.7 Constitutive activation of STAT3 has been demonstrated as a unifying feature in T-LGLL, promoting LGL survival through regulation of antiapoptotic proteins.8 Moreover, about 30% to 40% of patients with NK and T-LGL have somatic activating mutations in the STAT3 gene, and a few percent have STAT5b mutations.9
This trial in patients with T-LGLL with a terminal effector memory phenotype and significant cytopenias is the first to demonstrate the potential of targeting IL-15, using BNZ-1 to target the IL-15 receptor common gamma chain (CD132). The authors found that 87% of evaluable patients showed apoptotic response to BNZ-1 treatment, and 20% of patients had objective clinical responses in their cytopenias including transfusion independence and resolution of neutropenia. These responses occurred rapidly and were durable, with one lasting beyond 13 months. A prior study of a humanized antibody to IL-15, Hu-Mikβ1, demonstrated no therapeutic efficacy.10 Unlike BNZ-1, the Hu-Mikβ1 antibody was directed at the shared IL2/IL-15Rβ subunit (CD122) and blocked the trans presentation of IL-15 to CD122 on T cells but did not block IL-15 action in cells that expressed the heterotrimeric IL-15 receptor in cis.
The apoptosis studies in the Brammer et al trial clearly demonstrate that T-LGLL cells are dependent on IL-15 in vivo. Moreover, in those responding patients, apoptosis was maintained throughout the treatment period, suggesting that these cells remain dependent on IL-15, and thus are sensitive to ongoing BNZ-1 therapy. The lack of any difference of response due to STAT3 mutation status is likely explained by STAT3 mutations not rendering the cells IL-15 cytokine independent, but rather more sensitive to IL-15. The studies also demonstrate that in some patients, the LGLs lose their dependence on IL-15, likely due to other signaling pathways predominating, either driven by other cytokines or being cytokine independent.
The evaluation of response was not based on bulk reduction of the T-LGLL but on resolution of cytopenias, and response criteria were based on the large Eastern Cooperative Oncology Group E5998 trial. However, the exact mechanism by which the cytopenias resolved with BNZ-1 therapy has not been determined. The authors did not explore the Fas/FasL signaling pathway, which is known to be important in T-cell apoptosis and the cytopenias associated with T-LGLL. This clearly requires further investigation.
BNZ-1 is a rationally developed drug that has demonstrated efficacy despite the failure of a previous attempt at targeting the receptor for IL-15. Like in Castleman disease, in which we know an addiction to the IL-6 pathway is critical to prosurvival of the lymphoid cells, it has been proven here for the first time that a proportion of patients with T-LGLL are dependent on IL-15. However, given the modest response rate with BNZ-1 alone, it is clearly not the whole answer to managing this disease. As outlined in the figure, there are other rational targets for treating T-LGLL, including inhibitors of the other cytokines such as PDGF, IL-6, IL-17, and IL-23 and critical pathways such as Jak/STAT. Combinations of BNZ-1 with drugs that target these clearly warrant investigation.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal