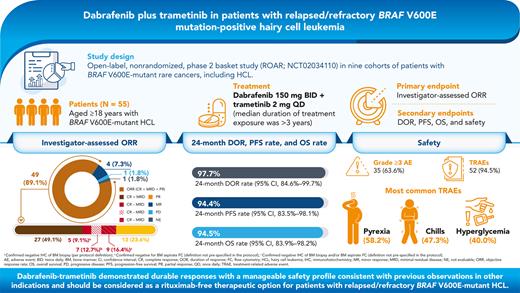
Key Points
Dabrafenib + trametinib showed durable responses with a manageable safety profile in patients with relapsed/refractory BRAF V600E–mutant HCL.
This combination should be considered a meaningful therapeutic option for patients with relapsed/refractory BRAF V600E–mutant HCL.
Abstract
BRAF V600E is the key oncogenic driver mutation in hairy cell leukemia (HCL). We report the efficacy and safety of dabrafenib plus trametinib in patients with relapsed/refractory BRAF V600E mutation–positive HCL. This open-label, phase 2 study enrolled patients with BRAF V600E mutation–positive HCL refractory to first-line treatment with a purine analog or relapsed after ≥2 prior lines of treatment. Patients received dabrafenib 150 mg twice daily plus trametinib 2 mg once daily until disease progression, unacceptable toxicity, or death. The primary endpoint was investigator-assessed objective response rate (ORR) per criteria adapted from National Comprehensive Cancer Network-Consensus Resolution guidelines. Secondary endpoints included duration of response (DOR), progression-free survival (PFS), overall survival (OS), and safety. Fifty-five patients with BRAF V600E mutation–positive HCL were enrolled. The investigator-assessed ORR was 89.0% (95% confidence interval, 77.8%-95.9%); 65.5% of patients had a complete response (without minimal residual disease [MRD]: 9.1% [negative immunohistochemistry of bone marrow {BM} biopsy], 12.7% [negative BM aspirate flow cytometry {FC}], 16.4% [negative immunohistochemistry and/or FC results]; with MRD, 49.1%), and 23.6% had a partial response. The 24-month DOR was 97.7% with 24-month PFS and OS rates of 94.4% and 94.5%, respectively. The most common treatment-related adverse events were pyrexia (58.2%), chills (47.3%), and hyperglycemia (40.0%). Dabrafenib plus trametinib demonstrated durable responses with a manageable safety profile consistent with previous observations in other indications and should be considered as a rituximab-free therapeutic option for patients with relapsed/refractory BRAF V600E mutation–positive HCL. This trial is registered at www.clinicaltrials.gov as #NCT02034110.
Introduction
Hairy cell leukemia (HCL) is a rare, indolent B-cell lymphoproliferative disease usually associated with pancytopenia and splenomegaly.1 Approximately 1100 new cases are reported in the United States annually.2,3 The recommended first-line treatment for patients with HCL is purine analogs such as cladribine or pentostatin,4-6 and these purine analogs were associated with complete response (CR) rates of 76% to 91%, with treatment-free intervals exceeding 10 years.7-10 Combining purine analogs with rituximab, an anti-CD20 monoclonal antibody, led to CR without detectable minimal residual disease (MRD) in 92% to 97% of patients,11,12 albeit with chemotherapy-associated toxicities.13-17
However, treatment options for patients progressing after first-line therapy with a purine analog and/or rituximab remain limited.18 The anti-CD22 immunotoxin moxetumomab pasudotox received the approval of the US Food and Drug Administration for use in patients with relapsed/refractory HCL who have failed at least 2 prior lines of therapy (including a purine analog). Moxetumomab pasudotox demonstrated an objective response rate (ORR) of 75% and a durable CR rate of 30% (CR with maintenance of hematologic remission for >180 days).19 The Bruton tyrosine kinase inhibitor ibrutinib has also been evaluated in relapsed/refractory HCL, demonstrating an ORR of 54% and a 36-month progression-free survival (PFS) rate of 73% after continuous treatment with ibrutinib.20 Despite these advances, additional treatments that increase the rate of durable CR are needed for patients with relapsed/refractory HCL.
Notably, oncogenic mutations in BRAF (primarily V600E), a key kinase in the MAPK pathway, are observed in 90% to 100% of patients with HCL. Mutant BRAF constitutively activates downstream MAPK signaling, promoting cell survival. BRAF V600E appears to be directly associated with key molecular and morphologic cell characteristics of HCL.1 Targeting mutant BRAF with vemurafenib administered for a fixed and short duration of 16 to 18 weeks in 2 studies conducted in Italy and the United States demonstrated ORRs of 96% and 100% and CR rates of 35% and 42%, respectively, in relapsed/refractory HCL. However, response duration was limited with a median relapse-free survival of 9 months.21 Upon relapse, reactivation of the MAPK pathway through various bypass mechanisms, including acquired RAS mutation and NF1/2 deletions, has been observed, potentially driving acquired resistance.21,22 Results from a separate study, wherein vemurafenib (in combination with rituximab) was administered for an even shorter duration of 8 weeks, revealed improved durability of responses, with a CR rate of 87% and no MRD in 65% of patients.23
Combining BRAF inhibition with inhibition of downstream MEK has been successful in several tumor types, including unresectable or metastatic melanoma, in which combination therapy prevented or delayed acquired resistance and led to improved clinical outcomes versus BRAF inhibitor monotherapy.24,25 Furthermore, addition of an MEK inhibitor attenuated BRAF inhibitor–mediated hyperproliferative skin toxicities, including cutaneous squamous cell carcinoma (cSCC) and keratoacanthoma.24 Combined BRAF/MEK inhibition is now the standard of care in BRAF V600E–mutated melanoma, non–small cell lung cancer, and anaplastic thyroid cancer,25-28 but data in patients with HCL are lacking. We conducted a multicenter, open-label, nonrandomized, phase 2 basket study of dabrafenib plus trametinib in patients with BRAF V600E mutation–positive rare cancers (ROAR; NCT02034110; supplemental Figure 1, available on the Blood website).27,29 Here, we report the efficacy and safety for the HCL cohort.
Methods
Patients
This study enrolled patients aged ≥18 years with histologically confirmed HCL according to the World Health Organization (2008)30 morphologic and immunophenotypic criteria who had experienced relapse following ≥2 prior lines of treatment or had refractory disease, defined as no response or disease progression in ≤1 year following first-line treatment with a purine analog. Enrolled patients had an Eastern Cooperative Oncology Group performance status of 0 to 2 and adequate organ function (supplemental Table 1). Enrollment based on local assessment of BRAF V600E mutation status was permitted (central confirmation was not required to be enrolled), and bone marrow (BM) aspirate and blood samples were collected for retrospective assessment by using the bioMérieux THxID BRAF kit at a central reference laboratory (Hematogenex, Tinley Park, IL). All patients had leukemic cells in the peripheral blood (PB) or BM aspirate along with any of the following: symptomatic splenomegaly, hemoglobin level <10 g/dL, platelet count <100 × 109/L, or absolute neutrophil count <1 × 109/L. If any patient had an opportunistic infection, the infection had to be adequately managed, and the patient had to be clinically stable.
The study was sponsored and designed by GlaxoSmithKline and Novartis Pharmaceuticals Corporation in collaboration with the investigators; dabrafenib and trametinib are assets of Novartis AG as of March 2, 2015. The study was approved by the institutional review board at each participating institution and was conducted in accordance with the Guidelines for Good Clinical Practice and ethical principles described in the Declaration of Helsinki. All patients provided written informed consent.
Study design
This was an open-label, nonrandomized phase 2 basket study (NCT02034110) in 9 cohorts of patients with BRAF V600E mutation–positive rare cancers, including HCL. Patients received oral dabrafenib (150 mg twice daily) and oral trametinib (2 mg once daily) until unacceptable toxicity, disease progression, or death (supplemental Figure 1). Patients underwent disease assessments by local investigators (no central assessments) every 4 weeks for the first 48 weeks of the study treatment and every 8 weeks thereafter, until disease progression. CR was confirmed by BM biopsy and computed tomography once blood counts were resolved for 4 weeks and disappearance of leukemic cells by routine stains of PB. BM biopsies were repeated after 6 months, 1 year, 2 years, and 3 years and then every 2 years. MRD was assessed using immunohistochemistry (IHC) and/or multiparameter flow cytometry (FC) in the PB and BM. Each response assessment was based on PB analysis and BM biopsy if available. For patients who discontinued study treatment, follow-up visits were conducted within 28 days after the last dose, every month for the first 6 months for dermatologic assessments, every 3 months for the first 6 months for secondary malignancies, and every 3 months thereafter for survival data.
The primary endpoint was investigator-assessed ORR using criteria adapted from the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology for HCL,31 the 1987 Consensus Resolution criteria,32 and definitions used in other HCL studies (supplemental Table 2). It is important to note that this trial predates the 2017 international consensus guidelines for HCL,4 and hence, the definitions of the response criteria differ. Secondary endpoints were duration of response (DOR), PFS, overall survival (OS), and safety. Adverse events (AEs) were graded according to the Common Terminology Criteria for Adverse Events v4.0.33
Statistical analysis
This study was designed with 9 cohorts of different tumor types. To address the small sample sizes per histologic cohort, an adaptive Bayesian hierarchical model design27 was used to increase the power by borrowing information across cohorts while controlling the type I error rate. The primary analysis cohort was to enroll a maximum of 25 patients per tumor type. Multiple interim analyses (every 12 weeks) were performed to monitor the safety and efficacy and to determine whether a cohort should discontinue enrollment early because of success or futility. If a cohort closed early for efficacy, a histology-specific expansion cohort could be opened to accommodate additional patient enrollment.
The primary endpoint of ORR was also analyzed using the frequentist methodology (point estimates and 95% confidence intervals [CIs]) including patients from the primary and expansion cohorts. Time-to-event secondary endpoints were right censored if the event was not observed during the study follow-up. Additional information about the exclusion criteria, secondary endpoints, and statistical analysis is provided in the supplemental Methods.
Results
Patient characteristics
From April 17, 2014, through July 25, 2018, 206 patients with BRAF V600E mutation–positive tumors were enrolled across 8 of the 9 cohorts (supplemental Figure 1), 55 of whom were included in the intent-to-treat (ITT) population of the HCL-specific cohort at the interim analysis data cutoff (September 14, 2020; primary analysis cohort, n = 24; expansion cohort, n = 31). The BRAF V600E evaluable set (centrally confirmed BRAF V600E mutation) included 50 patients (primary analysis cohort, n = 22; expansion cohort, n = 28). At data cutoff, 33 patients (60.0%) were continuing study treatment, 9 (16.4%) were in follow-up, and 13 (23.6%) had discontinued from the study (withdrawal of consent, n = 3 [5.5%]; lost to follow-up, n = 2 [3.6%]; investigator decision, n = 1 [1.8%]; death, n = 7 [12.7%]). The median patient follow-up was 43.2 months (range, 0.1-72.9 months).
Baseline characteristics of patients are presented in Table 1. Median age was 66 years (range, 40-89 years). Fifty-four patients were BRAF V600E mutation–positive per local testing, and 1 patient was enrolled by central testing. Seven patients had undergone prior splenectomy. All patients had received prior systemic therapies for HCL; most patients (n = 53 [96.4%]) received ≥2 prior regimens. All patients received prior cladribine and/or pentostatin. Eleven patients (20.0%) received moxetumomab pasudotox.
Baseline demographics and disease characteristics (intent-to-treat population)
| Characteristic . | HCL (N = 55) . |
|---|---|
| Age, median (range), y | 66.0 (40-89) |
| Male sex, n (%) | 47 (85.5) |
| Race, n (%) | |
| White–European/Caucasian | 48 (87.3) |
| White–Arabic or North African | 1 (1.8) |
| Missing | 6 (10.9) |
| ECOG PS, n (%) | |
| 0 | 28 (50.9) |
| 1 | 26 (47.3) |
| 2 | 1 (1.8) |
| Central BRAF V600E mutation status, n (%) | |
| Positive | 50 (90.9) |
| Negative | 4 (7.3) |
| Missing | 1 (1.8) |
| Time since diagnosis, median (range), y | 12.5 (0.2-33.2) |
| Number of prior treatment regimens, n (%) | |
| 1 | 2 (3.6) |
| 2 | 9 (16.4) |
| 3 | 16 (29.1) |
| ≥4 | 28 (50.9) |
| Prior treatment regimen, n (%)∗ | |
| Cladribine | 52 (94.5) |
| Rituximab | 35 (63.6) |
| Pentostatin | 13 (23.6) |
| Interferon | 12 (21.8) |
| Moxetumomab pasudotox | 11 (20.0) |
| Cladribine + rituximab | 6 (10.9) |
| Pentostatin + rituximab | 4 (7.3) |
| Investigational drug | 4 (7.3) |
| Bendamustine + rituximab | 3 (5.5) |
| Interferon alfa | 2 (3.6) |
| Peginterferon alfa-2a | 2 (3.6) |
| Dexamethasone + pentostatin | 1 (1.8) |
| Fludarabine + rituximab | 1 (1.8) |
| Rituximab + sargramostim | 1 (1.8) |
| Ibrutinib | 1 (1.8) |
| Ruxolitinib phosphate | 1 (1.8) |
| Ofatumumab | 1 (1.8) |
| Sargramostim | 1 (1.8) |
| Methotrexate | 1 (1.8) |
| Blood counts, median (range) | |
| Hemoglobin, g/L | 98.0 (57.0-175.0) |
| Neutrophils, × 109/L | 0.8 (0.1-4.6) |
| Platelets, × 109/L | 70.0 (5.0-179.0) |
| Spleen size, mm, median (interquartile range)† | |
| CT scan, n = 29 | 150.0 (130.0-203.0) |
| Direct physical examination, n = 21 | 150.0 (130.0-202.0) |
| Ultrasound, n = 4 | 153.0 (131.0-167.5) |
| MRI, n = 2 | 155.0 (150.0-160.0) |
| Characteristic . | HCL (N = 55) . |
|---|---|
| Age, median (range), y | 66.0 (40-89) |
| Male sex, n (%) | 47 (85.5) |
| Race, n (%) | |
| White–European/Caucasian | 48 (87.3) |
| White–Arabic or North African | 1 (1.8) |
| Missing | 6 (10.9) |
| ECOG PS, n (%) | |
| 0 | 28 (50.9) |
| 1 | 26 (47.3) |
| 2 | 1 (1.8) |
| Central BRAF V600E mutation status, n (%) | |
| Positive | 50 (90.9) |
| Negative | 4 (7.3) |
| Missing | 1 (1.8) |
| Time since diagnosis, median (range), y | 12.5 (0.2-33.2) |
| Number of prior treatment regimens, n (%) | |
| 1 | 2 (3.6) |
| 2 | 9 (16.4) |
| 3 | 16 (29.1) |
| ≥4 | 28 (50.9) |
| Prior treatment regimen, n (%)∗ | |
| Cladribine | 52 (94.5) |
| Rituximab | 35 (63.6) |
| Pentostatin | 13 (23.6) |
| Interferon | 12 (21.8) |
| Moxetumomab pasudotox | 11 (20.0) |
| Cladribine + rituximab | 6 (10.9) |
| Pentostatin + rituximab | 4 (7.3) |
| Investigational drug | 4 (7.3) |
| Bendamustine + rituximab | 3 (5.5) |
| Interferon alfa | 2 (3.6) |
| Peginterferon alfa-2a | 2 (3.6) |
| Dexamethasone + pentostatin | 1 (1.8) |
| Fludarabine + rituximab | 1 (1.8) |
| Rituximab + sargramostim | 1 (1.8) |
| Ibrutinib | 1 (1.8) |
| Ruxolitinib phosphate | 1 (1.8) |
| Ofatumumab | 1 (1.8) |
| Sargramostim | 1 (1.8) |
| Methotrexate | 1 (1.8) |
| Blood counts, median (range) | |
| Hemoglobin, g/L | 98.0 (57.0-175.0) |
| Neutrophils, × 109/L | 0.8 (0.1-4.6) |
| Platelets, × 109/L | 70.0 (5.0-179.0) |
| Spleen size, mm, median (interquartile range)† | |
| CT scan, n = 29 | 150.0 (130.0-203.0) |
| Direct physical examination, n = 21 | 150.0 (130.0-202.0) |
| Ultrasound, n = 4 | 153.0 (131.0-167.5) |
| MRI, n = 2 | 155.0 (150.0-160.0) |
CT, computed tomography; ECOG PS, Eastern Cooperative Oncology Group performance status; MRI, magnetic resonance imaging.
Patients may have had more than 1 prior therapy.
Patients may have had spleen size assessments by more than 1 method. Physical examination could estimate spleen size by palpation below and percussion above the left costal margin.
The median daily dose was 2 mg (range, 1-2 mg) for trametinib and 280.5 mg (range, 120-300 mg) for dabrafenib. The median dose intensity was 100% for trametinib (range, 50%-100%) and 93.5% (range, 40%-100%) for dabrafenib. The median duration of exposure was 38 months (range, 1-71 months) for dabrafenib and 37 months (range, 1-71 months) for trametinib. Forty-eight patients (87.3%) received study medications for >12 months.
Efficacy
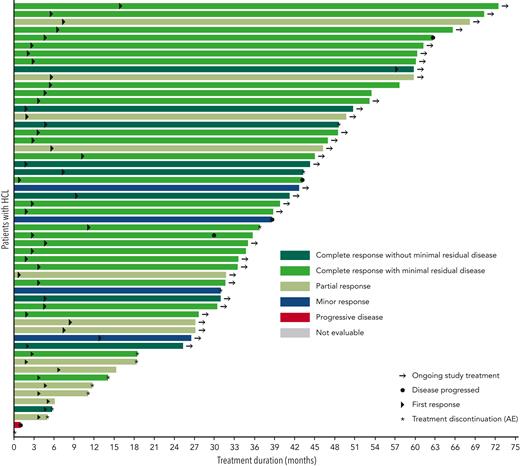
For the 55 patients in the HCL ITT population, the ORR (CR ± MRD + partial response [PR]) was 89.1% (49 of 55 patients; 95% CI, 77.8%-95.9%; Table 2). Overall, 36 patients (65.5%) achieved a CR, including 9 without MRD and 27 with MRD. Per protocol definition, the MRD-negativity rate was 9.1% (5 patients with confirmed negative IHC of BM biopsy). Per post hoc analyses (definitions of MRD-negativity not prespecified in the protocol), the MRD-negativity rate was 12.7% (7 patients negative for BM aspirate FC) and 16.4% (9 patients with negative IHC of BM biopsy and/or BM aspirate FC). Of the 9 patients without MRD, 5 were negative for both the tests. No tests of MRD were positive when the BM aspirate FC were negative. Two of these 9 patients had received prior moxetumomab pasudotox therapy. In addition, 13 patients (23.6%) achieved PR, 4 (7.3%) had a minor response, and 1 (1.8%) had progressive disease as the best overall response. All 13 patients with PR had resolution of platelets, neutrophils, and hemoglobin complying with the updated 2017 consensus definition of PR, which required these counts to be at CR levels and a minimum of 50% improvement in both organomegaly and BM biopsy infiltration with HCL.4 The median percentage of leukemic cells as a percentage of mononuclear cells in patients with CR + MRD ranged from 0% to 80% without any clear pattern with regards to duration of treatment. In the 4 patients with negative central BRAF V600E test result, the best response was CR without MRD (n = 2) and CR with MRD (n = 2). One patient with missing central result was nonevaluable and died of non-treatment-related pneumonia and sepsis before the first on-treatment disease assessment (Figure 1).
Investigator-assessed best overall response
| Investigator-assessed response . | Primary cohort (N = 24) . | ITT population (N = 55) . | BRAF V600E evaluable population (n = 50) . |
|---|---|---|---|
| Best response, n (%) | |||
| CR ± MRD | 18 (75.0) | 36 (65.5) | 32 (64.0) |
| CR without MRD | 4 (16.7)∗ | 5 (9.1)∗ | 4 (8.0)∗ |
| 5 (20.8)† | 7 (12.7)† | 6 (12.0)† | |
| CR with MRD | 5 (20.8)‡ | 9 (16.4)‡ | 7 (14.0)‡ |
| 13 (54.2) | 27 (49.1) | 25 (50.0) | |
| PR | 4 (16.7) | 13 (23.6) | 13 (26.0) |
| Minor response | 1 (4.2) | 4 (7.3) | 4 (8.0) |
| Stable disease | 0 | 0 | 0 |
| Progressive disease | 1 (4.2) | 1 (1.8) | 1 (2.0) |
| Not evaluable | 0 | 1 (1.8) | 0 |
| ORR (CR ± MRD + PR), n (%) | 22 (91.7) | 49 (89.1) | 45 (90.0) |
| 95% CI§ | 73.0-99.0 | 77.8-95.9 | 78.2-96.7 |
| Investigator-assessed response . | Primary cohort (N = 24) . | ITT population (N = 55) . | BRAF V600E evaluable population (n = 50) . |
|---|---|---|---|
| Best response, n (%) | |||
| CR ± MRD | 18 (75.0) | 36 (65.5) | 32 (64.0) |
| CR without MRD | 4 (16.7)∗ | 5 (9.1)∗ | 4 (8.0)∗ |
| 5 (20.8)† | 7 (12.7)† | 6 (12.0)† | |
| CR with MRD | 5 (20.8)‡ | 9 (16.4)‡ | 7 (14.0)‡ |
| 13 (54.2) | 27 (49.1) | 25 (50.0) | |
| PR | 4 (16.7) | 13 (23.6) | 13 (26.0) |
| Minor response | 1 (4.2) | 4 (7.3) | 4 (8.0) |
| Stable disease | 0 | 0 | 0 |
| Progressive disease | 1 (4.2) | 1 (1.8) | 1 (2.0) |
| Not evaluable | 0 | 1 (1.8) | 0 |
| ORR (CR ± MRD + PR), n (%) | 22 (91.7) | 49 (89.1) | 45 (90.0) |
| 95% CI§ | 73.0-99.0 | 77.8-95.9 | 78.2-96.7 |
Patients with negative IHC of BM biopsy.
Patients negative for BM aspirate FC.
Patients had negative IHC and/or negative FC results in PB and BM specimens.
Exact 2-sided 95% CI based on the Clopper-Pearson method.
Treatment duration and best response (intent-to-treat population). A swimmer plot for individual patients’ treatment duration and time to events is shown. The color code shows investigator-assessed best response for each patient. Arrows designate patients with ongoing study treatment. Circles represent the time at which disease progressed. Triangles represent the time to first response. Asterisks represent treatment discontinuation owing to adverse events.
Treatment duration and best response (intent-to-treat population). A swimmer plot for individual patients’ treatment duration and time to events is shown. The color code shows investigator-assessed best response for each patient. Arrows designate patients with ongoing study treatment. Circles represent the time at which disease progressed. Triangles represent the time to first response. Asterisks represent treatment discontinuation owing to adverse events.
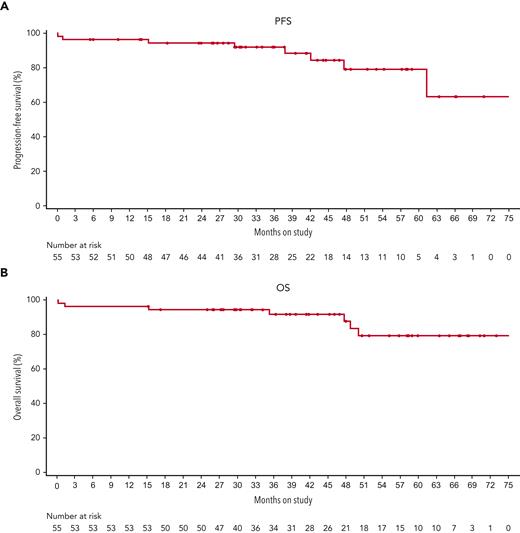
In the 49 patients with an investigator-confirmed response, the median DOR was not reached, with a 24-month DOR rate of 97.7% (95% CI, 84.6%-99.7%); 3 patients had disease progression, and 2 patients who had CRs died without prior disease progression (Table 3). Three responders whose disease had later progressed had best response of CR + MRD. The median time to first response was 3.7 months (range, 0.8-56.1 months), and 37 responding patients remained event-free for at least 24 months (Figure 1). The median time to first CR was 6.0 months (range, 1.8-34.0 months). The estimated proportion of patients maintaining a CR 6 months after first documentation of CR was 97.1% (95% CI, 81.4%-99.6%); 75.0% of patients with a CR (27 of 36) were still in follow-up with a continued hematologic response (having counts consistent with CR) at data cutoff. The median DOR was not estimable (1 event) for the 13 patients with PR; none had disease progression, and 1 death was reported. The median investigator-assessed PFS and OS were not reached (Figure 2). The 24-month PFS and OS rates were 94.4% (95% CI, 83.5%-98.1%) and 94.5% (95% CI, 83.9%-98.2%), respectively (Table 3).
Kaplan-Meier estimates of duration of response, progression-free survival, and overall survival of patients with HCL at different time points (intent-to-treat population)
| . | n . | 6 months . | 12 months . | 24 months . |
|---|---|---|---|---|
| DOR, % (95% CI) | 49 | 100.0 (100.0-100.0) | 97.7 (84.6-99.7) | 97.7 (84.6-99.7) |
| PFS, % (95% CI) | 55 | 96.4 (86.2-99.1) | 96.4 (86.2-99.1) | 94.4 (83.5-98.1) |
| OS, % (95% CI) | 55 | 96.4 (86.2-99.1) | 96.4 (86.2-99.1) | 94.5 (83.9-98.2) |
| . | n . | 6 months . | 12 months . | 24 months . |
|---|---|---|---|---|
| DOR, % (95% CI) | 49 | 100.0 (100.0-100.0) | 97.7 (84.6-99.7) | 97.7 (84.6-99.7) |
| PFS, % (95% CI) | 55 | 96.4 (86.2-99.1) | 96.4 (86.2-99.1) | 94.4 (83.5-98.1) |
| OS, % (95% CI) | 55 | 96.4 (86.2-99.1) | 96.4 (86.2-99.1) | 94.5 (83.9-98.2) |
PFS (A) and OS (B) for patients included in the intent-to-treat population treated with dabrafenib plus trametinib.
PFS (A) and OS (B) for patients included in the intent-to-treat population treated with dabrafenib plus trametinib.
A post hoc exploratory analysis conducted to assess the influence of baseline characteristics on the achievement of CR using univariate logistic regression models of CR against individual baseline variables suggested a slightly better probability of CR with higher baseline neutrophils (estimate, 1.1902; 95% CI, 0.0111-2.3692; P = .048) and lower baseline spleen size (estimate, −0.0184; 95% CI, −0.0371 to 0.0003; P = .054; supplemental Table 3). In addition, the relationship of prior splenectomy with achievement of CR was assessed. Of the 7 patients with prior splenectomy, 5 achieved CR, which did not provide strong evidence of the relationship between prior splenectomy and CR (2-sided Fisher exact test P = 1.0). However, given the post hoc nature of the analysis and multiple testing, the results should be interpreted with caution.
Median hemoglobin, platelet count, and absolute neutrophil count recovered to normal (as defined in supplemental Table 2) by week 8, week 4, and week 8, respectively (supplemental Figure 2). The median number of hairy cells as a percentage of circulating mononuclear cells decreased from 5.0% (range, 0.0%-95.0%) at baseline to 0.1% (range, 0.0%-7.4%) at week 12. Some patients who did not meet the criteria for a response did have significant clinical benefit, as evidenced by the recovery in their hematologic parameters.
Safety
All patients experienced ≥1 AEs, and 35 patients (63.6%) experienced a grade ≥3 event (supplemental Table 4). The most common grade ≥3 AEs were hyperglycemia (9.1%), pyrexia, neutropenia, and pneumonia (each 7.3%). The most common (≥5%) hematologic events of any grade were anemia (18.2%), neutropenia (10.9%), and thrombocytopenia (5.5%). Treatment-related AEs occurred in 52 patients (94.5%), most frequently pyrexia (58.2%), chills (47.3%), and hyperglycemia (40.0%; Table 4). AEs that led to dose reduction, dose interruption, or permanent discontinuation of either treatment were observed in 29 (52.7%), 38 (69.1%), and 12 (21.8%) patients, respectively (Table 4, supplemental Table 5, and supplemental Table 6). In 1 patient, autoimmune hemolytic anemia (grade 3 treatment-related serious AE) led to permanent discontinuation of the study treatment. The patient was transfused with packed red blood cells and treated with immunoglobulin IV and prednisone, following which the event resolved.
Treatment-related adverse events, serious adverse events, and adverse events leading to dose reductions and dose interruptions (all patients treated, N = 55)
| . | n (%) . |
|---|---|
| Total number of patients with treatment-related AEs | 52 (94.5) |
| Treatment-related AEs (>10% incidence) | |
| Pyrexia | 32 (58.2) |
| Chills | 26 (47.3) |
| Hyperglycemia | 22 (40.0) |
| Dermatitis acneiform | 21 (38.2) |
| Fatigue | 19 (34.5) |
| Myalgia | 18 (32.7) |
| Aspartate aminotransferase increased | 18 (32.7) |
| Peripheral edema | 17 (30.9) |
| Nausea | 17 (30.9) |
| Rash, maculopapular | 16 (29.1) |
| Dry skin | 15 (27.3) |
| Alanine aminotransferase increased | 14 (25.5) |
| Blood alkaline phosphatase increased | 14 (25.5) |
| Headache | 14 (25.5) |
| Arthralgia | 11 (20.0) |
| Pain in extremity | 8 (14.5) |
| Blurred vision | 8 (14.5) |
| Vomiting | 7 (12.7) |
| Dry mouth | 7 (12.7) |
| Basal cell carcinoma | 7 (12.7) |
| Rash | 6 (10.9) |
| Diarrhea | 6 (10.9) |
| Treatment-related serious AEs | 19 (34.5) |
| Pyrexia | 7 (12.7) |
| Basal cell carcinoma | 3 (5.5) |
| Squamous cell carcinoma | 3 (5.5) |
| Squamous cell carcinoma of skin | 3 (5.5) |
| Chills | 2 (3.6) |
| Neutrophil count decreased | 1 (1.8) |
| Autoimmune hemolytic anemia | 1 (1.8) |
| Chronic lymphocytic leukemia | 1 (1.8) |
| Fat necrosis | 1 (1.8) |
| Metastatic squamous cell carcinoma | 1 (1.8) |
| Pulmonary granuloma | 1 (1.8) |
| Upper respiratory tract infection | 1 (1.8) |
| Bladder neoplasm | 1 (1.8) |
| Guillain-Barré syndrome | 1 (1.8) |
| Myocarditis | 1 (1.8) |
| Urinary tract infection | 1 (1.8) |
| AEs leading to dose reductions | 29 (52.7) |
| Most common AEs (>5% incidence) leading to dose reductions | |
| Pyrexia | 18 (32.7) |
| Chills | 14 (25.5) |
| Fatigue | 6 (10.9) |
| Rash, maculopapular | 4 (7.3) |
| Vomiting | 3 (5.5) |
| Myalgia | 3 (5.5) |
| Nausea | 3 (5.5) |
| Peripheral edema | 3 (5.5) |
| AEs leading to dose interruptions | 38 (69.1) |
| Most common AEs (>5% incidence) leading to dose interruptions | |
| Pyrexia | 18 (32.7) |
| Chills | 13 (23.6) |
| Fatigue | 6 (10.9) |
| Nausea | 3 (5.5) |
| Vomiting | 3 (5.5) |
| Diarrhea | 3 (5.5) |
| Headache | 3 (5.5) |
| Rash, maculopapular | 3 (5.5) |
| Vision blurred | 3 (5.5) |
| Constipation | 3 (5.5) |
| Dermatitis acneiform | 3 (5.5) |
| . | n (%) . |
|---|---|
| Total number of patients with treatment-related AEs | 52 (94.5) |
| Treatment-related AEs (>10% incidence) | |
| Pyrexia | 32 (58.2) |
| Chills | 26 (47.3) |
| Hyperglycemia | 22 (40.0) |
| Dermatitis acneiform | 21 (38.2) |
| Fatigue | 19 (34.5) |
| Myalgia | 18 (32.7) |
| Aspartate aminotransferase increased | 18 (32.7) |
| Peripheral edema | 17 (30.9) |
| Nausea | 17 (30.9) |
| Rash, maculopapular | 16 (29.1) |
| Dry skin | 15 (27.3) |
| Alanine aminotransferase increased | 14 (25.5) |
| Blood alkaline phosphatase increased | 14 (25.5) |
| Headache | 14 (25.5) |
| Arthralgia | 11 (20.0) |
| Pain in extremity | 8 (14.5) |
| Blurred vision | 8 (14.5) |
| Vomiting | 7 (12.7) |
| Dry mouth | 7 (12.7) |
| Basal cell carcinoma | 7 (12.7) |
| Rash | 6 (10.9) |
| Diarrhea | 6 (10.9) |
| Treatment-related serious AEs | 19 (34.5) |
| Pyrexia | 7 (12.7) |
| Basal cell carcinoma | 3 (5.5) |
| Squamous cell carcinoma | 3 (5.5) |
| Squamous cell carcinoma of skin | 3 (5.5) |
| Chills | 2 (3.6) |
| Neutrophil count decreased | 1 (1.8) |
| Autoimmune hemolytic anemia | 1 (1.8) |
| Chronic lymphocytic leukemia | 1 (1.8) |
| Fat necrosis | 1 (1.8) |
| Metastatic squamous cell carcinoma | 1 (1.8) |
| Pulmonary granuloma | 1 (1.8) |
| Upper respiratory tract infection | 1 (1.8) |
| Bladder neoplasm | 1 (1.8) |
| Guillain-Barré syndrome | 1 (1.8) |
| Myocarditis | 1 (1.8) |
| Urinary tract infection | 1 (1.8) |
| AEs leading to dose reductions | 29 (52.7) |
| Most common AEs (>5% incidence) leading to dose reductions | |
| Pyrexia | 18 (32.7) |
| Chills | 14 (25.5) |
| Fatigue | 6 (10.9) |
| Rash, maculopapular | 4 (7.3) |
| Vomiting | 3 (5.5) |
| Myalgia | 3 (5.5) |
| Nausea | 3 (5.5) |
| Peripheral edema | 3 (5.5) |
| AEs leading to dose interruptions | 38 (69.1) |
| Most common AEs (>5% incidence) leading to dose interruptions | |
| Pyrexia | 18 (32.7) |
| Chills | 13 (23.6) |
| Fatigue | 6 (10.9) |
| Nausea | 3 (5.5) |
| Vomiting | 3 (5.5) |
| Diarrhea | 3 (5.5) |
| Headache | 3 (5.5) |
| Rash, maculopapular | 3 (5.5) |
| Vision blurred | 3 (5.5) |
| Constipation | 3 (5.5) |
| Dermatitis acneiform | 3 (5.5) |
New primary or secondary malignancies were also observed in this study. Nine patients (16.4%) had cSCC, and 13 patients (23.6%) had basal cell carcinoma (BCC; supplemental Table 7). In addition, few patients developed other secondary malignancies such as bladder neoplasm (3.6%), colon cancer, chronic lymphocytic leukemia, gastrointestinal stromal tumor (GIST), lymphoma, Hodgkin lymphoma, pancreatic adenocarcinoma, and prostate cancer (1.8% each).
There were 7 deaths during the study; none were related to the study treatment. Four patients died because of progressive disease; all had confirmed BRAF V600E mutation, a long disease history (9.6-32.8 years since initial diagnosis), and at least 4 prior treatments. One of these patients had a best response of CR + MRD, 2 had minor responses, and 1 had progressive disease. Two of these patients died more than 300 days and 2 patients less than 10 days after the last dose of the study treatment. One patient died because of serious AEs (sepsis and pneumonia, which occurred 2 days after the last treatment), 1 patient died because of influenza, and 1 patient died because of spontaneous cerebellar hematoma leading to cardiac arrest.
Discussion
The results from the HCL cohort of this study demonstrate that treatment with dabrafenib in combination with trametinib provides durable and clinically meaningful responses in adult patients with relapsed or refractory BRAF V600E mutation–positive HCL. The investigator-assessed ORR was 89%, with 65% of patients achieving a CR. The estimated proportion of patients maintaining any response at 24 months or a CR at 6 months was 98% and 97%, respectively, indicating durability of responses. PFS and OS estimates at 24 months were >90%.
Previous clinical studies in HCL using the BRAF inhibitor vemurafenib, conducted in Italy and the United States, showed CR rates of 35% and 42%, respectively.21 The Italian study reported a median relapse-free survival of 19 months for patients achieving a CR and 6 months for patients achieving a PR; no patients achieved MRD-negative CR.21 The results of the current study differ from those of the Italian study; these differences can be attributed to the increased inhibition of the MAPK pathway by using the combination of BRAF and MEK inhibitors and differences in treatment duration. In the Italian study, vemurafenib was administered for 8 to 20 weeks, depending on the response. In the current study, dabrafenib and trametinib were administered until unacceptable toxicity or progressive disease was observed. In a recent retrospective analysis of 27 patients treated with BRAF inhibitor monotherapy (vemurafenib and/or dabrafenib), the duration of treatment did not affect the CR rate; however, interpretation of the data was limited by the availability of BM biopsies.34 In another recently reported study, a short treatment (8-12 weeks) with dabrafenib monotherapy achieved CR rates of 30%.35 In our study, the MRD-negativity rate tested by IHC of BM biopsy was 9%, by BM aspirate FC was 13%, and by IHC of BM biopsy and/or BM aspirate FC was 16%. However, it should be noted that hemodilution of BM aspirate may underestimate the MRD.
Combination treatment has also been shown to be more effective than monotherapy in patients with HCL, with vemurafenib plus IV rituximab achieving a CR in 87% of patients, including 65% who were MRD negative, although care must be taken with cross-trial comparison.23 Additionally, this study predated the published modern 2017 international consensus guidelines for HCL,4 which is a potential limitation, as this restricts comparability across different studies. Also, we have not estimated relapse-free survival and PFS using the 2017 consensus definition of hematologic relapse, which limits the comparability across studies. Advancements for relapsed/refractory HCL include the recently approved anti-CD22 recombinant immunotoxin moxetumomab pasudotox, which demonstrated an ORR of 75.0% (95% CI, 64.1%-84.0%) based on blinded independent central review.19 A limitation of moxetumomab pasudotox is immunogenicity due to the bacterial toxin; approximately 75% of patients had neutralizing antibodies posttreatment. Moxetumomab pasudotox achieved 34% complete remissions without MRD and high response durability.19 In our study, 11 patients received treatment with dabrafenib plus trametinib after prior moxetumomab pasudotox, and all responded. In a recently published case report of 4 relapsed patients with HCL previously treated with moxetumomab pasudotox, 2 achieved CR without MRD after treatment with the vemurafenib (reduced to 240 mg twice daily) and rituximab combination for 16 weeks. These 2 patients who derived clinical benefit from retreatment with vemurafenib and rituximab regimen eventually relapsed after discontinuation of vemurafenib and rituximab retreatment and regained a hematologic remission after indefinite therapy with dabrafenib and trametinib.36 Concurrent use of moxetumomab pasudotox with targeted therapies such as dabrafenib and trametinib might be useful to determine whether MRD-free CR could be achieved earlier, prior to immunogenicity, and obviate long-term therapy.
The 24-month PFS and OS estimates in the current study were 94.4% and 94.5%, respectively, which is noteworthy for this patient population, heavily pretreated with a median of 4 prior anticancer therapies. These results are consistent with those observed in a study evaluating vemurafenib plus rituximab, in which PFS was 78% at a median follow-up of 37 months.23
Dabrafenib plus trametinib was well tolerated with acceptable incidences of dose modifications and discontinuations. AEs were manageable and similar to those observed in patients with other BRAF V600–mutated tumors, including unresectable or metastatic melanoma and adjuvant melanoma,24,26 non–small cell lung cancer,28 and anaplastic thyroid cancer.27 Pyrexia (58.2%), known to be associated with dabrafenib plus trametinib treatment,24,26 was the most frequently reported treatment-related AE in this study. It is generally manageable through standard measures, including treatment interruption, dose modification, and concomitant medications. Only 1 patient discontinued treatment because of pyrexia. Here, we have not reported the median time for onset of all pyrexia events, which is a limitation, as occurrence of pyrexia before resolution of neutropenia (median time to resolution of neutropenia was 8 weeks after initiating dabrafenib plus trametinib) would complicate patient management because of the differential diagnosis with febrile neutropenia. Furthermore, it was common for patients to report AEs early on during the treatment and then continue treatment without any AEs for a prolonged period. In this study, only 12 patients (21.8%) permanently discontinued either dabrafenib or trametinib treatment due to AEs.
Hyperproliferative skin lesions, including cSCC, keratoacanthoma, BCC, and risk of secondary malignancies, have been associated with paradoxical activation of the MAPK pathway in BRAF wild-type cells in patients treated with BRAF inhibitor monotherapy. The addition of downstream MEK inhibition can attenuate these toxicities, as has been observed in patients with melanoma treated with BRAF/MEK inhibitor combination therapy. In this study, the incidence of hyperproliferative events was somewhat higher than previously reported for dabrafenib plus trametinib in other tumor types.24,37 This finding could partially be explained by the fact that patients with HCL are inherently susceptible to secondary malignancies.38,39 Specifically, 3 of the 9 patients who developed cSCC had a history of SCC, and 6 of the 13 patients who developed BCC had a history of BCC. In most cases, cSCC and BCC could be managed by simple resection, but routine monitoring is recommended, both for secondary malignancies and for skin toxicities. Of the 2 patients with bladder neoplasm, 1 had a history of low-grade papillary bladder tumor, and the other had preexisting CD5+ monoclonal B-cell lymphocytosis at baseline and a history of colon adenoma and BCC. The patient diagnosed with GIST after enrollment had clear radiographic evidence of GIST before enrollment. The advanced age of patients (median, 66 years) would be expected to be associated with a significant rate of diagnosis of new malignancies, and patients with HCL are reported to be at an increased risk of secondary malignancies. It is unknown whether the relatively high rate of secondary malignancies is due to background risks in patients who are older and with HCL, because of their previous diagnoses of the same or similar malignancies, or due to prolonged treatment with dabrafenib and trametinib, particularly the former, which, like other BRAF inhibitors, can increase proliferation through BRAF on non-HCL cells.
It is important to acknowledge the difficulties in comparing indefinite treatment with dabrafenib plus trametinib with other therapies that have a shorter duration of treatment. With long-term treatment compared with short-course treatment, there was a higher discontinuation rate (22%) and higher rate of secondary malignancies, but these are expected when time of observation and treatment are prolonged. The advantages of long-term treatment and prolonged response must be balanced with need for treatment interruptions and dose modifications. The current study provides valuable clinical data with which decisions on durations of treatment can be applied to particular patients. However, the lack of data on incidence and frequency of recurrence of the same AE or distinct episodes of toxicity per patient is a limitation because a certain AE can occur multiple times in the same patient being treated for a median duration of >3 years. Future studies may benefit from inclusion of disease-specific quality-of-life assessments, which were not included in this basket trial with multiple tumor types. The absence of a matched control group of patients in this study warrants further patient quality-of-life data/analysis to validate the findings. During the final analysis, there is an opportunity to assess the quality of life for all the cohorts included in the basket trial, the outcomes of which may be published in due course.
The combination of dabrafenib plus trametinib is ideal for patients with relapsed HCL who prefer an oral regimen with a high chance of CR, including MRD-free CR, including patients who cannot tolerate additional rituximab. Moreover, dabrafenib plus trametinib may be a reasonable combination in the current scenario with COVID-19 and/or concerns of poor COVID-19 vaccine immune response in patients receiving rituximab.40,41 On the other hand, dabrafenib plus trametinib could also be tested in combination with rituximab for a shorter duration of time to increase its MRD-free CR rate.
It is important to note that infections must not be overlooked in this patient population. The dabrafenib plus trametinib combination also holds promise to improve hematologic parameters without causing prolonged myelosuppression and immunosuppression42,43 and can serve as a potential regimen for HCL patients with active infections. Like vemurafenib, dabrafenib plus trametinib could be tested in front line to delay definitive first-line chemotherapy of HCL when patients have cytopenias or infections that put them at excessive risk for chemotherapy.
In contrast to BRAF V600 mutation–positive solid tumors, such as melanoma and colorectal cancer, for which mechanisms of acquired resistance to BRAF inhibitors have been extensively studied,44 there is limited understanding about the mechanistic basis of resistance in HCL. Previous reports of vemurafenib in HCL noted persistent phosphorylation of extracellular signal-regulated kinase (ERK) in BM leukemic cells at treatment end, indicating reactivation of the MAPK pathway as seen with BRAF inhibitors in other cancers.21 However, the lack of on-treatment ERK phosphorylation status of residual leukemic cells in this study is a major limitation, restricting us from understanding the contribution of MEK inhibitor in this treatment regimen. Whole-genome and deep-targeted sequencing of a patient with HCL before vemurafenib treatment and again at relapse implicated reactivation of MEK-ERK signaling as the likely mechanism of resistance; indeed, subsequent treatment with vemurafenib plus cobimetinib led to resolution of symptoms and platelet count recovery.22 The deep and durable responses seen in our study are consistent with the hypothesis that combined treatment with BRAF and MEK inhibitors may be less susceptible to resistance mechanisms.
In conclusion, dabrafenib plus trametinib demonstrated a high rate of durable responses in patients with relapsed/refractory BRAF V600E–mutated HCL with a manageable safety profile. Dabrafenib plus trametinib should be considered a meaningful, rituximab-free therapeutic option for patients with relapsed or refractory BRAF V600E–mutated HCL.
Acknowledgments
The authors thank the patients participating in this clinical trial and their families, as well as the staff at each participating institution who assisted with the study. NIH patients were managed, in part, by several investigators, including Theresa Yu, Lacey James, and Dai Chihara. The authors also acknowledge Maurizio Voi (Novartis NPC) for contributions to this study.
Medical writing/editorial assistance was provided by Shruti Shah and Sharol Janice Rodrigues (Novartis Healthcare Pvt Ltd), as well as Michael Demars (ArticulateScience, LLC), and funded by Novartis Pharmaceuticals Corporation in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3). Support with the Bayesian study design and analysis was provided by Berry Consultants LLC. R.J.K. is supported in part by the Intramural Research Program of the US National Institutes of Health. V.S. is supported by National Institutes of Health (NIH), National Cancer Institute (NCI) grant R01CA242845, and University of Texas MD Anderson Cancer Center is supported by NIH, NCI support grant P30 CA016672. This study was supported by Novartis Pharmaceuticals Corporation.
Authorship
Contribution: The study was designed by the funder in collaboration with all the authors, and R.J.K. contributed to the conceptualization of the study; R.J.K., P.M., F.R., M.H., A.G., A.-S.M., Z.A.W., A.S., S.D., M.J.A.d.J., W.W., J.D.G, E.A., and V.S. evaluated patients on the study and contributed to data curation; P.B. performed statistical analysis; all authors had access to all the data reported in the study and contributed to data interpretation; R.J.K., P.I., E.G., and V.S. prepared the first draft of the manuscript, with editorial assistance from a medical writer paid for by the funder; and all authors reviewed and edited the manuscript and agreed to submit the manuscript for publication.
Conflict-of-interest disclosure: R.J.K. reports grants and nonfinancial support from Novartis during the conduct of the study; grants and nonfinancial support from AstraZeneca/MedImmune/Innate Pharma and Genentech; and nonfinancial support from Teva, outside of the submitted work. In addition, he is a co-inventor on the NIH patent for moxetumomab pasudotox and receives royalties from NIH. P.M. reports personal fees from Celgene, Janssen, Takeda, Amgen, and AbbVie outside the submitted work. F.R. reports personal fees from Novartis during the conduct of the study, personal fees from Novartis, Celgene, BMS, Astellas, Xencor, Agios, Amgen, Orsenix, AstraZeneca, and Jazz Research, and research funding from BMS, AbbVie, Amgen, Macrogenics, Xencor, Orsenix, and Astex outside of the submitted work. M.H. reports research funding from AbbVie, Celgene, Genentech, Genmab, Incyte, Novartis, Roche, Sanofi, and Takeda and personal fees from Genmab, Roche, and Takeda outside of the submitted work. Z.A.W. reports personal fees from Eli Lilly, Bayer, Merck, Daiichi, Novartis, Ipsen, Array BioPharma, and Five Prime Therapeutics outside of the submitted work. A.S. reports grants from Bristol Myers Squibb, Merck KgA, and Servier and personal fees from Bristol Myers Squibb, MSD, Merck KgA, Roche, Amgen, Sanofi, Servier, and Eli Lilly outside of the submitted work. P.I. is an employee of Novartis and reports stock ownership in Novartis. P.B. is an employee of Novartis and owns stock from Novartis and GlaxoSmithKline. E.G. is a former employee of Novartis and owns stock. V.S. reports grants and other support from LOXO Oncology/Eli Lilly, Novartis, PharmaMar, and MedImmune; grants from Roche/Genentech, Bayer, GlaxoSmithKline, Nanocarrier, Vegenics, Celgene, Northwest Biotherapeutics, Berghealth, Incyte, Fujifilm, D3, Pfizer, Multivir, Amgen, AbbVie, Alfa-sigma, Agensys, Boston Biomedical, Idera Pharma, Inhibrx, Exelixis, Blueprint Medicines, Altum, Dragonfly Therapeutics, Takeda, the National Comprehensive Cancer Network, NCI-CTEP and UT MD Anderson Cancer Center, Turning Point Therapeutics, and Boston Pharmaceuticals; and other support from Helsinn, R-Pharma US, INCYTE, QED Pharma, ASCO, ESMO, and Medscape during the conduct of the study. The remaining authors declare no competing financial interests.
The current affiliation for E.G. is Innovent Biologics (USA) Inc, Rockville, MD.
Correspondence: Robert J. Kreitman, Laboratory of Molecular Biology, National Institutes of Health, 9000 Rockville Pike, Building 10, Room 13N248A, Bethesda, MD 20892-4255; e-mail: kreitmar@mail.nih.gov.
References
Author notes
Novartis is committed to sharing with qualified external researchers access to patient-level data and supporting clinical documents from eligible studies. These requests are reviewed and approved by an independent review panel based on scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trial, in line with applicable laws and regulations. This trial data availability is according to the criteria and process described on www.clinicalstudydatarequest.com.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal