Abstract
Our understanding and management of atypical hemolytic uremic syndrome (aHUS) have dramatically improved in the last decade. aHUS has been established as a prototypic disease resulting from a dysregulation of the complement alternative C3 convertase. Subsequently, prospective nonrandomized studies and retrospective series have shown the efficacy of C5 blockade in the treatment of this devastating disease. C5 blockade has become the cornerstone of the treatment of aHUS. This therapeutic breakthrough has been dulled by persistent difficulties in the positive diagnosis of aHUS, and the latter remains, to date, a diagnosis by exclusion. Furthermore, the precise spectrum of complement-mediated renal thrombotic microangiopathy is still a matter of debate. Nevertheless, long-term management of aHUS is increasingly individualized and lifelong C5 blockade is no longer a paradigm that applies to all patients with this disease. The potential benefit of complement blockade in other forms of HUS, notably secondary HUS, remains uncertain.
Introduction
Atypical hemolytic uremic syndrome (aHUS) is a devastating form of thrombotic microangiopathy (TMA) predominantly involving the kidney.1 Untreated, it leads to end-stage renal disease in up to 2 out of 3 affected patients, shortly after onset or following relapses.2-4 The unraveling of the pathogenic mechanisms underlying aHUS, namely an overactivation of the complement alternative pathway, helped in the design of a specific treatment. C5 blockade has transformed the prognosis of aHUS.5,6 An accurate, rapid diagnosis with a timely treatment have become crucial for the optimal management of patients with aHUS.
This therapeutic breakthrough has, to some extent, been dulled by the difficulty in making a reliable timely diagnosis of aHUS and by uncertainties around the spectrum of complement-mediated HUS. In the era of complement inhibitors, aHUS has become more a diagnostic and semantic issue than a therapeutic one.
Diagnosing aHUS: a question of semantics
A clear definition of aHUS is a prerequisite for an accurate diagnosis. To date, there is no universally accepted diagnostic criteria for aHUS. The diagnosis of HUS by itself relies on a classical triad of thrombocytopenia, microangiopathic hemolytic anemia, and acute kidney injury. However, this triad may be incomplete. Furthermore, it does not take into account the pathological features of the disorder, notably the incidental detection of TMA in kidney biopsy (mostly renal grafts, more rarely native kidneys) in the absence of peripheral hematological signs of TMA.
The term “aHUS” by itself is confusing and does not refer to an underlying pathogenic mechanism. More broadly, the exact spectrum of complement-mediated TMA is not clearly defined. The most important unresolved clinical question is determining whether aHUS and secondary HUS (associated to malignancies, drugs, infections, and autoimmune diseases) belong to the same spectrum of complement-mediated HUS, and thus, require a similar management. The answer to this question varies across countries and even institutions within the same country.
In the present discussion, we refer to aHUS as primary aHUS with no associated conditions/disorders. This approach is based on our previously reported experience7 showing that secondary HUS and aHUS have distinct presentations, outcomes, and genetic drivers. However, we acknowledge that concomitant conditions, mainly infections, may trigger primary aHUS, as recently illustrated by COVID-19,8 or rarely by Shiga toxin–producing Escherichia coli.9 Similarly, we do not rule out a transient activation of complement in some forms of secondary HUS.
In this discussion, we also use the term aHUS, in the absence of a widely accepted alternative. Nevertheless, we propose new diagnostic criteria for aHUS that integrate renal pathological findings (supplemental Table 1, available on the Blood website). We also propose the term “syndrome of renal TMA” as a potential replacement for HUS. Based on this nomenclature, aHUS is a predominantly complement-mediated and complement blockade–responsive syndrome of renal TMA, with a potential relapsing pattern.
Case 1
A 37-year-old female patient delivered a healthy infant at 36 gestation weeks of her third pregnancy. Delivery was preceded by moderate hypertension and proteinuria (0.9 g per day) and complicated by mild bleeding (blood loss ∼300 mL). Six days after discharge from hospital, she was readmitted for fatigue and dizziness. She reported having experienced diarrhea for 2 days. Her blood pressure was 165/90 mm Hg. Laboratory tests showed acute kidney injury (serum creatinine, 3.4 mg/dL; proteinuria, 1.6 g/L), mild thrombocytopenia (122 G/L), and microangiopathic hemolytic anemia (hemoglobin, 9.4 g/dL; lactate dehydrogenase [LDH], 2.7 times over the upper limit of normal [ULN]; undetectable haptoglobin; and presence of schistocytes [1%-2%] on blood smears). Due to the low probability of ADAMTS13 deficiency–associated thrombotic thrombocytopenic purpura (TTP) as estimated with the modified French score (score of 0), plasma exchanges were not started. Five days after admission, the diagnostic workup showed detectable ADAMTS13 activity (46%), a negative polymerase chain reaction for Shiga toxin in the stool, negative tests for anti–double-stranded DNA, extractable nuclear antigens and antiphospholipid antibodies, and a mildly increased plasma homocysteine level (29 μmol/L; normal, <15 μmol/L). Complement assays were normal. aHUS was diagnosed by exclusion. The patient received quadrivalent A, C, W, and Y conjugate and B meningococcal vaccines and was started on anti-C5 treatment (eculizumab). She also received prophylaxis with methylpenicillin throughout treatment. Platelet count normalized at day 5 of treatment, and serum creatinine declined at 2.2 mg/dL at day 14. Five weeks later, renal function had normalized. Four months after admission, complement genetic analysis showed the absence of pathogenic variants. Anti-C5 antibody was discontinued after 5 months of treatment. aHUS did not recur. Two years later, the patient had an uneventful fourth pregnancy.
Case 2
A 28-year-old female patient was admitted for blurred vision and headache. Her blood pressure was 210/105 mm Hg. She had no medical history and had not been taking any medication except for an oral contraceptive. Laboratory test showed acute kidney injury (serum creatinine, 6.2 mg/dL; proteinuria, 3.7 g/L), thrombocytopenia (96 G/L), and microangiopathic hemolytic anemia (hemoglobin, 8.2 g/dL; LDH, 3.4 times over the ULN; undetectable haptoglobin; and presence of schistocytes [2%-4%] on blood smears). Fundoscopy disclosed grade 4 hypertensive retinopathy. Renal Doppler ultrasound was unremarkable and cardiac echocardiography did not show left ventricular hypertrophy. Despite rapid blood pressure control, hemolysis, thrombocytopenia, and acute kidney injury persisted. The patient was started on plasma exchanges and on hemodialysis. Additional workup showed detectable ADAMTS13 activity (67%), negative tests for anti–double-stranded DNA and extractable nuclear antigens antibodies, and a mildly increased homocysteine plasma level at 42 mmol/L. Complement assays showed slightly decreased C3 and factor H plasma levels. Five days after admission, the patient was diagnosed with aHUS based on a suggestive clinical presentation and evolution under antihypertensive therapy. She was started on anti-C5 treatment (eculizumab) and received prophylaxis with methylpenicillin. Platelet count normalized and hemolysis resolved 12 days after the start of treatment. The patient remained dialysis dependent. Two months after admission, a kidney biopsy disclosed extensive glomerulosclerosis and interstitial fibrosis. C5 blockade was stopped. Complement genetics tests showed the presence of a rare pathogenic variant in complement factor H (CFH) gene. Nine months later, the patient underwent a renal transplantation with a living donor, her father who was not a carrier of the CFH variant, with prophylactic anti-C5 treatment. Three years later, she has a good function of her renal graft, is still receiving a complement blocker, and aHUS did not recur.
How I diagnose aHUS
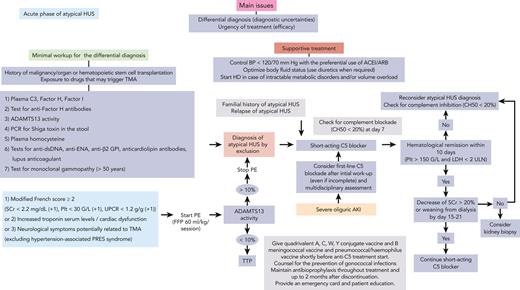
To date, aHUS remains a diagnosis by exclusion, as illustrated by these 2 cases. Unlike another prototypic complement-mediated disease, paroxysmal nocturnal hemoglobinuria (PNH), there is no positive diagnostic test for aHUS. The minimal workup required to rule out the differential diagnoses of aHUS is shown in Figure 1,10-18 and includes only tests that have direct implications for the patients’ clinical management. Additional tests require further assessment and are not yet fully validated for clinical practice (Table 1).
Proposed algorithm for the diagnosis and management of aHUS in the acute phase.aLow C3 and/or low factor H or I plasma levels are suggestive of complement-mediated aHUS. bPositivity at significant titers is diagnostic of autoimmune aHUS. cActivity of <10% is diagnostic of ADAMTS13 deficiency TTP. dPositivity is diagnostic of Shiga toxin–producing E. coli HUS. eLevel of >100 mmol/L is suggestive of cobalamin C deficiency HUS (as well as increased plasma and/or urine methylmalonic acid and low plasma methionine level). Confirmation by genetic testing (MMACHC gene). Cobalamin C deficiency HUS is increasingly diagnosed in young adults. fPositivity is suggestive of autoimmune disease–associated HUS. gMonoclonal gammopathy–associated TMA carries severe renal and potentially extrarenal (skin and central and peripheral nervous system) involvement, and frequent (up to 75% of cases) features of complement alternative pathway activation. ACEI/ARB, angiotensin converting enzyme inhibitors/angiotensin 2 receptor blocker; AKI, acute kidney injury; BP, blood pressure; dsDNA, double-stranded DNA; ENA, extractable nuclear antigen; FFP, fresh frozen plasma; GPI, glycoprotein I; HD, hemodialysis; PCR, polymerase chain reaction; PE, plasma exchange; Plt, platelet count; PRES, posterior reversible encephalopathy syndrome; SCr, serum creatinine; UPCR, urinary protein/creatinine ratio.
Proposed algorithm for the diagnosis and management of aHUS in the acute phase.aLow C3 and/or low factor H or I plasma levels are suggestive of complement-mediated aHUS. bPositivity at significant titers is diagnostic of autoimmune aHUS. cActivity of <10% is diagnostic of ADAMTS13 deficiency TTP. dPositivity is diagnostic of Shiga toxin–producing E. coli HUS. eLevel of >100 mmol/L is suggestive of cobalamin C deficiency HUS (as well as increased plasma and/or urine methylmalonic acid and low plasma methionine level). Confirmation by genetic testing (MMACHC gene). Cobalamin C deficiency HUS is increasingly diagnosed in young adults. fPositivity is suggestive of autoimmune disease–associated HUS. gMonoclonal gammopathy–associated TMA carries severe renal and potentially extrarenal (skin and central and peripheral nervous system) involvement, and frequent (up to 75% of cases) features of complement alternative pathway activation. ACEI/ARB, angiotensin converting enzyme inhibitors/angiotensin 2 receptor blocker; AKI, acute kidney injury; BP, blood pressure; dsDNA, double-stranded DNA; ENA, extractable nuclear antigen; FFP, fresh frozen plasma; GPI, glycoprotein I; HD, hemodialysis; PCR, polymerase chain reaction; PE, plasma exchange; Plt, platelet count; PRES, posterior reversible encephalopathy syndrome; SCr, serum creatinine; UPCR, urinary protein/creatinine ratio.
Potential tests for the positive diagnosis of complement-mediated HUS
| Available tests for the diagnosis of complement-mediated HUS . | Limitations . |
|---|---|
| Biomarkers of complement alternative pathway activation Low plasma C3 level/normal C4 level | Present in only 30% of cases of aHUS. Normal levels do not exclude the diagnosis of aHUS |
| Assessment of complement proteins plasma levels Factor H and factor I plasma levels | Low plasma levels provide evidence of complement dysregulation and of the pathogenicity of a potential rare variant identified in encoding gene. Nonspecific for aHUS |
| CD46 expression on neutrophils | Nonspecific decrease during the acute phase of several forms of TMA9 |
| Biomarkers of complement terminal pathway activation sC5b-9 plasma level | Overlap between several forms of TMA and healthy individuals |
| In vitro deposition of complement (C3c, C5b-9) at the surface of endothelial cells modified Ham test | Discrepant results between laboratories. Requires duplication and standardization19-21 |
| Available tests for the diagnosis of complement-mediated HUS . | Limitations . |
|---|---|
| Biomarkers of complement alternative pathway activation Low plasma C3 level/normal C4 level | Present in only 30% of cases of aHUS. Normal levels do not exclude the diagnosis of aHUS |
| Assessment of complement proteins plasma levels Factor H and factor I plasma levels | Low plasma levels provide evidence of complement dysregulation and of the pathogenicity of a potential rare variant identified in encoding gene. Nonspecific for aHUS |
| CD46 expression on neutrophils | Nonspecific decrease during the acute phase of several forms of TMA9 |
| Biomarkers of complement terminal pathway activation sC5b-9 plasma level | Overlap between several forms of TMA and healthy individuals |
| In vitro deposition of complement (C3c, C5b-9) at the surface of endothelial cells modified Ham test | Discrepant results between laboratories. Requires duplication and standardization19-21 |
These tests have not yet been fully validated and replicated, and their clinical relevance remains uncertain.
This minimal workup is not easily and rapidly available, even in several high-income countries. ADAMTS13 deficiency–related TTP is the most urgent cause of TMA to rule out (or in), due to its potential high morbidity and mortality22 and the need for specific treatments (plasma exchange/infusions, caplacizumab,23 and immunosuppressive drugs24,25). In the absence of rapidly available ADAMTS13 testing, clinicians can rely on scores such as the French or PLASMIC26 scores, to predict ADAMTS13 deficiency. Both scores are characterized by a high (>90%) negative predictive value for TTP. The recent addition of proteinuria-to-creatininuria ratio has further improved their performance.27,28 We use the modified French score (case 1) (Figure 1), while acknowledging its reduced sensitivity and specificity in the setting of pregnancy28 and potentially in patients aged >60 years.29 Moreover, in some instances, membrane cofactor protein (MCP)–related aHUS may mimic TTP.30
aHUS is diagnosed once all other causes of TMA have been excluded with a reasonable clinical probability. This implies that therapeutic decisions based on the diagnosis of aHUS, carry a risk of error, with potentially the subsequent recognition of a different cause of TMA.
There are 2 peculiar situations that add to the difficulty in the differential diagnosis of aHUS. The first setting is pregnancy (case 1), during which several disorders, far more frequent than aHUS, mainly preeclampsia and hemolysis, elevated liver enzymes, and low platelet count (HELLP), carry features of renal TMA syndrome. The differential diagnosis of TMA in pregnancy and post partum has been the subject of an international consensus paper recently published in Blood.31
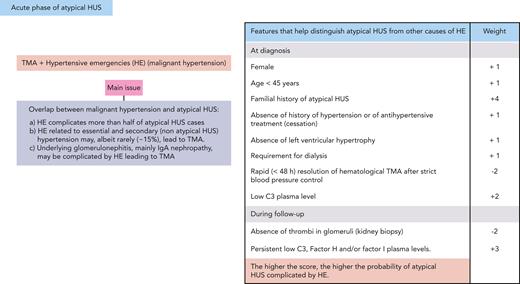
The second is the coexistence of renal TMA and malignant hypertension/hypertensive emergencies (HEs) (case 2). Indeed, aHUS and HE overlap (Figure 2).32-34 In our clinical practice, we rely on a set of clinical, biological, and pathological features of variable ponderation that we compiled from the available literature.32,34-40 These features are combined in a not yet validated score that helps us distinguish aHUS complicated with HE from HE due to other causes, leading to renal TMA (Figure 2). The higher the score, the higher the probability of aHUS.
Proposed features with variable weighting that help distinguish aHUS from other causes of HEs. These features may be used to calculate a score; the higher the score, the higher the probability of aHUS.
Proposed features with variable weighting that help distinguish aHUS from other causes of HEs. These features may be used to calculate a score; the higher the score, the higher the probability of aHUS.
Overall, the diagnosis of aHUS remains clinically challenging and a positive diagnostic test is crucially lacking.
How I interpret the results of complement genetic testing
Screening for complement gene variants should be part of the routine management of patients with aHUS. It provides important prognostic information that guides clinical decision making, as illustrated by cases 1 and 2. Complement genetics may provide the proof of a link between complement dysregulation and disease. It also helps in assessing aHUS severity, and, most importantly, in predicting the risk of recurrence after renal transplantation and of relapse after anti-C5 treatment discontinuation.
The diagnostic set of candidate genes in aHUS should include CFH, CD46, CFI, C3, CFB, and diacylglycerol kinase ε (DGKε) genes. We use a combination of next-generation sequencing panel and multiplex ligation–dependent probe amplification to allow for the identification of large deletions, hybrid genes, or other complex genomic rearrangements. The use of exome analysis still requires validation for the screening of hybrid genes. The potential relevance of rare variants identified in other complement or noncomplement genes remains debatable.
As a general rule, complement variants are classified as causal (or pathogenic) if they are rare in the healthy population (minor allele frequency < 0.1%) and carry a documented impact on the formation (C3 and FB gain-of-function variants) or the regulation (CFH, CFI, and MCP loss-of-function variants) of the alternative C3 convertase. A significant proportion of variants are missense variants without proven functional consequences and are therefore of uncertain biological or clinical relevance.41 Distinct lines of evidence are used to assess the probability that a variant may be relevant to the disease (reviewed).42 The fact that aHUS does not carry a Mendelian pattern of inheritance, and has an incomplete penetrance, adds to the complexity of the analysis of the disease genetics. Genetic predisposition for aHUS most probably results from the combination of rare and common complement gene variants, complement genes haplotypes; mainly homozygous CFH-H3 or/and CD46 GGAAC haplotypes (present in ∼5% of healthy individuals), and triggers (infections and pregnancy).
How I treat aHUS
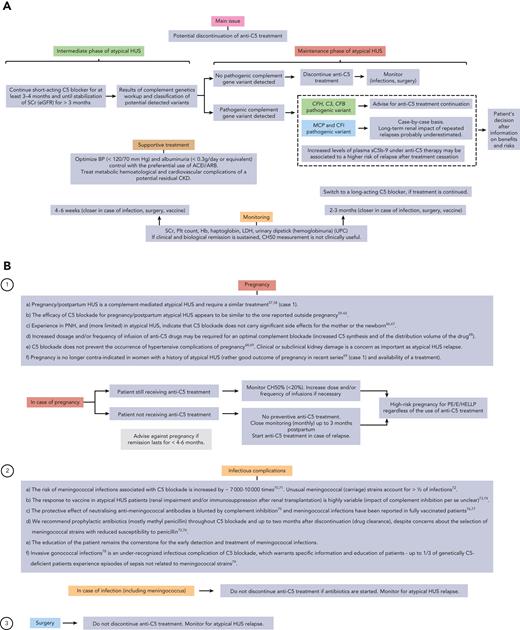
The clinical course of aHUS includes 2 distinct phases with specific clinical issues. In the acute phase (Figure 1), the most pressing aspects are the differential diagnosis of aHUS and the need for a rapid complement blockade. In the long-term remission phase (Figure 2), the main question is the feasibility of complement blockade discontinuation. Treatment of aHUS is not necessarily identical in these 2 phases of the disease. Furthermore, supportive treatment remains an important aspect of the management of patients with aHUS, both in the acute and remission phases (Figures 1 and 3).
Considerations regarding the long-term management of atypical HUS patients. (A) Proposed algorithm for the long-term management of aHUS. (B) Special considerations regarding (1) pregnancy-associated atypical HUS and pregnancy in women with a history of atypical HUS, (2) infectious complications of C5 blockade in atypical HUS patients, and (3) surgery in atypical HUS patients. CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; siRNA, silencing RNA.
Considerations regarding the long-term management of atypical HUS patients. (A) Proposed algorithm for the long-term management of aHUS. (B) Special considerations regarding (1) pregnancy-associated atypical HUS and pregnancy in women with a history of atypical HUS, (2) infectious complications of C5 blockade in atypical HUS patients, and (3) surgery in atypical HUS patients. CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; siRNA, silencing RNA.
Acute phase of aHUS
Since the approval of the first anti-C5 antibody in 2007, the gold standard treatment for aHUS is C5 blockade, when available. The evidence of the efficacy of this treatment is derived from prospective nonrandomized trials,5,6,43 the only possible design of a trial for an ultrarare disease with no proven efficacious treatment at the time of the assessment of the first C5 blocker, and from retrospective or observational series (comparison with historical controls).44,45 Based on these data, it is estimated that the risk of end-stage renal disease in patients with aHUS has decreased from 50% to 60% to ∼10% to 15%.1
There is no clear benefit of plasma exchanges on renal outcome in aHUS in particular2 and in TMA in general, with 2 main exceptions: ADAMTS13 deficiency–associated TTP and anti–factor H antibody–associated aHUS. Plasma exchanges are thus not a prerequisite for the treatment of aHUS (case 1), except where C5 blockers are not available or funded. Plasma exchanges are started during the initial workup, when there are uncertainties regarding a potential TTP. We suggest starting plasma exchanges only if the modified French score (or PLASMIC score) is highly suggestive (≥2) of ADAMTS13 deficiency and/or in the presence of severe extrarenal manifestations, mainly neurological and cardiac (Figure 1). Conversely, in the setting of severe oliguric acute kidney injury requiring dialysis, first-line treatment with eculizumab may be considered. In such a situation, the probability of TTP is extremely low (but not nil). Furthermore, early initiation of complement blockade will help improve renal outcome in case of aHUS, without excessive risks to the patient if an alternative diagnosis is finally made.
Once aHUS is diagnosed, plasma exchanges should be stopped, and a C5 blocker started. Currently, 2 types of C5 blockers are available in clinical practice: short-acting (eculizumab) and long-acting (ravulizumab). We advocate the use of a short-acting C5 blocker during the initial acute phase of aHUS, owing to the remaining uncertainties regarding the diagnosis of aHUS and to extensive experience, to date, with short-acting vs long-acting C5 blockers. A comparison of the 2 available types of C5 blockers suggests a slower renal function recovery with long-acting vs short-acting C5 blockers, with all the caveats of such a comparison (Table 2).
Comparison of eculizumab and ravulizumab in 3 prospective noncontrolled trials in adults with aHUS
| . | Eculizumab (n = 17) (Legendre et al)5 n, (%) . | Eculizumab (n = 41) (Fakhouri et al)6 n, (%) . | Ravulizumab (n = 58) (Rondeau et al)46 n, (%) . |
|---|---|---|---|
| Age, >65 y | Not available (range, 17-68) | 2 (5) | 8 (14.3) >60 |
| ≥1 variant in complement gene (or anti–factor H antibodies) | 13 (76) | 21 (51) | 8 (20.5) |
| Recurrence of aHUS | 10 (59) | 11 (26.8) | 3 (5.4) |
| Kidney transplant recipients | 7 (41) | 9 (22) | 8 (14.3) |
| Dialysis requirement at inclusion | 6 (35) | 24 (59) | 29 (51.8) |
| “Complete TMA response”∗ | 11 (6%) | 30 (73) | 30 (53.6) |
| “Modified TMA response”† | Not available | 23 (56) | 30 (53.6) |
| Patients requiring dialysis at inclusion and weaned from dialysis at 6 mo | 5/6 (83) | 20 (83) | 17 (58.6) |
| Patients requiring dialysis at 6 mo | 1 (6) | 6 (15) | 18 (32) |
| Patients requiring dialysis at last follow-up | 1 (6) | ||
| Death | 0 | 0 | 4 (7) |
| . | Eculizumab (n = 17) (Legendre et al)5 n, (%) . | Eculizumab (n = 41) (Fakhouri et al)6 n, (%) . | Ravulizumab (n = 58) (Rondeau et al)46 n, (%) . |
|---|---|---|---|
| Age, >65 y | Not available (range, 17-68) | 2 (5) | 8 (14.3) >60 |
| ≥1 variant in complement gene (or anti–factor H antibodies) | 13 (76) | 21 (51) | 8 (20.5) |
| Recurrence of aHUS | 10 (59) | 11 (26.8) | 3 (5.4) |
| Kidney transplant recipients | 7 (41) | 9 (22) | 8 (14.3) |
| Dialysis requirement at inclusion | 6 (35) | 24 (59) | 29 (51.8) |
| “Complete TMA response”∗ | 11 (6%) | 30 (73) | 30 (53.6) |
| “Modified TMA response”† | Not available | 23 (56) | 30 (53.6) |
| Patients requiring dialysis at inclusion and weaned from dialysis at 6 mo | 5/6 (83) | 20 (83) | 17 (58.6) |
| Patients requiring dialysis at 6 mo | 1 (6) | 6 (15) | 18 (32) |
| Patients requiring dialysis at last follow-up | 1 (6) | ||
| Death | 0 | 0 | 4 (7) |
A comparison of the 2 available types of C5 blockers is limited by the significant differences in the characteristics of patients included in 3 large studies with these 2 drugs. Indeed, patients included in the ravulizumab trial may not be fully representative of patients with aHUS (older age, low frequency of complement gene variants), an additional illustration of the importance of the definition of aHUS. Furthermore, end points also differ between studies.
Different definitions in the 3 trials: Legendre et al,5 platelet count and LDH level normalization sustained for at least 2 consecutive measurements over a period of ≥4 weeks; Fakhouri et al,6 platelet count and LDH level normalization, kidney function preservation (<25% increase in serum creatinine); Rondeau et al,46 platelet count and LDH level normalization, kidney function improvement (>25% decrease in serum creatinine).
In Fakhouri et al,6 defined as hematologic response (platelet and LDH as earlier), and kidney function improvement (>25% decrease in creatinine level).
In prospective studies, the median time for platelet count normalization after the initiation of C5 blockade is 10 days.5,6 We suggest evaluating the response of aHUS to C5 blockade at day 10 of treatment, pending that optimal complement blockade (total complement activity [CH50] < 20%) has been confirmed within 7 days of treatment initiation.
There is no universally accepted definition of aHUS response to anticomplement therapies. However, it is acknowledged that a hematological response is necessary but not sufficient and that an optimal response also requires some level of renal function improvement. We assess aHUS response to C5 blockade based on a platelet count normalization, LDH level decrease < 2 times over the ULN, and a decrease in serum creatinine of >20%, or weaning from dialysis at 10 days after treatment initiation.
We, however, acknowledge that renal function recovery may be slower (or even absent in a minority of cases), particularly in patients initially requiring dialysis. Nevertheless, the absence of response, as previously defined, at day 10 should prompt clinicians to check complement inhibition, reconsider aHUS diagnosis, and evaluate the feasibility and relevance of a kidney biopsy. A kidney biopsy would help exclude differential or superimposed diagnoses (mainly lupus nephritis, C3 glomerulopathy, cryoglobulinemic glomerulonephritis, or immunoglobulin A [IgA] nephropathy) and assess irreversible kidney fibrosis (case 2).
Intermediate and maintenance phase of aHUS
Anti-C5 treatment is usually maintained from at least 3 to 6 months after the acute phase of aHUS and until serum creatinine has reached a plateau (Figure 3). In clinical practice, renal function recovery occurs predominantly during the first 3 months of treatment.47 In patients who do not recover a dialysis-independent renal function after 3 to 4 months of anti-C5 treatment, we recommend stopping treatment, as an extrarenal (mostly hematological) relapse of aHUS has only rarely been reported.47,48 A kidney biopsy may also be helpful for the assessment of irreversible fibrotic changes in the kidney before treatment cessation (case 2).
Lifelong treatment with a C5 blocker is no longer a paradigm that applies to the management of all patients with aHUS (case 1) (Figure 3). First, there is no definite proof that complement activation occurs continuously in all patients with aHUS, including in carriers of rare variants in complement genes. Second, long-term C5 blockade exposes patients to infectious risks and is costly. Finally, retrospective series49-52 and a recent prospective multicentric trial53 indicate that C5 blocker discontinuation is safe in patients with no detected pathogenic rare complement variants. The risk of disease relapse in this subset of patients is estimated to be <5% (data reviewed in Fakhouri et al.53). A recurrence of aHUS after treatment discontinuation in these patients should prompt clinicians to reconsider the diagnosis of aHUS.53
In patients with detected pathogenic variants, the risk of relapse after C5 blocker discontinuation ranges from 23% (carriers of CFI gene variants) to 37% (carriers of MCP gene variants) and 64% (carriers of CFH gene variants).53 Hence, the decision to discontinue C5 blockade in these patients needs to be discussed on a case-by-case basis, the patient’s preference being paramount in the final decision (Figure 3).
In carriers of complement gene variants, significant residual chronic kidney disease (estimated glomerular filtration rate of <30 ml/min) urges for caution in the decision to discontinue C5 blockade, as a potential aHUS relapse may lead to irreversible additional renal damage.53 In all cases, any discontinuation of anti-C5 treatment implies a strict monitoring of blood and urine tests (including dipsticks mainly for the detection of hemoglobinuria)54 especially after infections, vaccines, and surgery (Figure 3) and the guarantee that C5 blocker can be easily funded and rapidly started in case of relapse.
Patients in whom C5 blockade discontinuation is not feasible or desired, a switch to a long-acting C5 blocker is an option to decrease the inconvenience of repeated infusions (Figure 3). In patients with clinical and biological remission, monitoring of complement blockade is not warranted, especially as the currently only available long-acting C5 blocker, ravuzilumab, does not allow for complement inhibition assessment using simple tests (CH50).
In countries where long-acting C5 blockers are not available, long-term treatment still relies on short-acting agents. Some clinicians reduce dosing or extend the interval between infusions of short-acting C5 blockers,55,56 to improve patients’ quality of life and to reduce costs. In this context, monitoring of residual trough levels of C5 blockers may be helpful. We aim for a trough level of eculizumab of >100 to 150 μg/L, to maintain optimal complement blockade in case of superimposed infections or other complement activation triggers.55 In case trough level measurement is not available, CH50 (<20%) can be used as a proxy for circulating eculizumab levels.56
Special considerations regarding the long-term treatment of aHUS
Some special considerations regarding several aspects of long-term treatment with a C5 blocker in a patient with aHUS are shown in Figure 3.
Pregnancy and postpartum HUS belong to the spectrum of complement-mediated aHUS and should be treated similarly (case 1).57,58 Anti-C5 antibodies seem to be efficacious and safe in this subtype of aHUS but require adjustment in dosage and/or infusion frequency.58-69 The availability of anticomplement therapies has also changed the perspectives of pregnancy in women with a history of aHUS. Pregnancy is no longer contraindicated in these women, in view of recent data underlining the rather good outcome of pregnancy in these patients69 (case 1) and the availability of a new efficacious treatment that can be used in pregnancy (see consensus document31). Prophylactic C5 blockade is not recommended in these women, as the risk of relapse of aHUS in pregnancy is highly unpredictable. However, close monitoring throughout pregnancy and up to 4 months post partum is mandatory to detect early and treat potential disease relapse.
The inherent infectious risks (mostly with encapsulated pathogens, particularly meningococcal strains) of C5 blockade are well recognized, based on observations in C5 genetically deficient patients. Antimeningococcal vaccines are not fully protective in patients treated with anti-C5 antibodies and we recommend prophylactic antibiotics (mostly methylpenicillin) throughout C5 blockade and up to 2 months after discontinuation (drug clearance) (cases 1 and 2).70-79 Noteworthy, in case of intercurrent infection (including meningococcal infection), we do not recommend eculizumab discontinuation, as infection per se may trigger complement activation and ultimately aHUS relapse.
Anti–factor H antibody–associated aHUS
Anti–factor H antibody–associated aHUS is an autoimmune form of HUS, affecting mainly adolescents. Anti–factor H antibodies are mostly IgG; IgM80 autoantibodies have also been reported, however, in patients with frequently coexistent diseases and/or rare complement gene variants. The occurrence of anti–factor H antibodies is associated with a homozygous deletion of the genes encoding CFH-related protein 1 and 3. This deletion is frequently encountered in healthy individuals (3%-33%),81 and its pathogenic link to the autoantibodies is not clear. In adults, monoclonal gammopathy with anti–factor H antibody activity has also rarely been reported.82 In patients with anti–factor H antibodies, there is no universally accepted therapeutic approach and management includes a variable combination of plasma exchanges, especially in cases of severe extrarenal manifestations (cardiac or neurological); immunosuppressive drugs (rituximab, cyclophosphamide, or mycophenolate mofetil); and C5 blockade. We recommend treatment discontinuation once the titer of anti–factor H antibodies has decreased below 1000 arbitrary units in the enzyme-linked immunosorbent assay test we use in routine83 (test positivity threshold, 100 arbitrary units). However, several anti–factor H antibody tests are used worldwide and require standardization to define a reliable threshold for the positive diagnosis of anti–factor H antibody–associated aHUS, and hence for treatment cessation.
aHUS and organ transplantation
Before the era of complement inhibitors, aHUS had a double negative impact on patients’ quality of life and survival. The disease not only led to end-stage kidney disease in a significant proportion of patients but also limited access to renal transplantation owing to the unacceptably high risk of aHUS recurrence in the renal allograft, especially in carriers of rare variants in CFH, C3, and FB genes. The availability of C5 blockers currently allows an individualized approach for renal transplantation in patients with aHUS, with the use of prophylactic C5 blockade in high-risk patients (case 2) (Table 3). Such strategy has greatly improved the outcome of renal transplantation in these patients.84 Nevertheless, in renal transplant recipients, as well as in solid organ transplants recipients in general, TMA may be related to several causes ranging from infection (in particular cytomegalovirus, parvovirus B19, and fungi), to the toxicity of immunosuppressive drugs (calcineurin and mTOR inhibitors), as well as severe rejection episodes.
Considerations regarding renal transplantation in patients with aHUS
| aHUS and organ transplantation |
| Posttransplant HUS is a rare but serious condition that can lead to poor patient and graft outcome |
| HUS can occur as a recurrence of aHUS after RT84 or as a de novo, potentially complement mediated, TMA in recipients of solid organ transplants85 as well as in recipients of hematopoietic stem cell transplantation86 |
| aHUS carries a risk of recurrence after RT. The risk is highest (up to 70%) in carriers of pathogenic variants in CFH, CFB, C3, or CFH/CFHR1 hybrid genes and in patients with a previous recurrence after RT.87 The lowest (<10%) risk is in patients with isolated MCP or DGKε variants and negative anti–factor H antibodies at the time of RT87 |
| The availability of the C5 blockers currently allows an individualized approach of RT in patients with aHUS. C5 blockade is frequently used for the prevention and/or early treatment of aHUS recurrence in patients at high risk. This strategy has greatly improved graft and patient outcome (<12% recurrence rate) and increased safe access to RT for patients with aHUS.84 An approach with living kidney donation (to reduce ischemia/reperfusion-triggered complement activation) without prophylactic C5 blockade has also been used with satisfactory results88 |
| Anti-C5 treatment is usually used lifelong after RT in patients with aHUS. However, case-by-case decisions can be made depending on the underlying genetic mutation |
| The difficulty remains in the differential diagnosis of aHUS, as in renal transplant recipients (and solid organ transplant recipients in general), TMA can be triggered by various events after transplantation: organ procurement (cold and warm ischemia/reperfusion injury), infection (in particular cytomegalovirus, influenza virus, parvovirus B19, and fungi), antiphospholipid antibodies, immunosuppressive drugs (calcineurin and mTOR inhibitors), and severe rejection episodes (in particular antibody-mediated rejection) |
| aHUS and organ transplantation |
| Posttransplant HUS is a rare but serious condition that can lead to poor patient and graft outcome |
| HUS can occur as a recurrence of aHUS after RT84 or as a de novo, potentially complement mediated, TMA in recipients of solid organ transplants85 as well as in recipients of hematopoietic stem cell transplantation86 |
| aHUS carries a risk of recurrence after RT. The risk is highest (up to 70%) in carriers of pathogenic variants in CFH, CFB, C3, or CFH/CFHR1 hybrid genes and in patients with a previous recurrence after RT.87 The lowest (<10%) risk is in patients with isolated MCP or DGKε variants and negative anti–factor H antibodies at the time of RT87 |
| The availability of the C5 blockers currently allows an individualized approach of RT in patients with aHUS. C5 blockade is frequently used for the prevention and/or early treatment of aHUS recurrence in patients at high risk. This strategy has greatly improved graft and patient outcome (<12% recurrence rate) and increased safe access to RT for patients with aHUS.84 An approach with living kidney donation (to reduce ischemia/reperfusion-triggered complement activation) without prophylactic C5 blockade has also been used with satisfactory results88 |
| Anti-C5 treatment is usually used lifelong after RT in patients with aHUS. However, case-by-case decisions can be made depending on the underlying genetic mutation |
| The difficulty remains in the differential diagnosis of aHUS, as in renal transplant recipients (and solid organ transplant recipients in general), TMA can be triggered by various events after transplantation: organ procurement (cold and warm ischemia/reperfusion injury), infection (in particular cytomegalovirus, influenza virus, parvovirus B19, and fungi), antiphospholipid antibodies, immunosuppressive drugs (calcineurin and mTOR inhibitors), and severe rejection episodes (in particular antibody-mediated rejection) |
RT, renal transplantation.
Perspectives in the treatment of aHUS
C5 blockade has become a cornerstone in the treatment of aHUS, and several C5 blockers are commercialized or in development for this indication. They differ in terms of their nature (antibody, peptide, and silencing RNA), route of administration, and half-life. Patients will ultimately choose the C5 blocker that best meets their preferences. Nevertheless, the cost of these drugs will certainly affect their funding by health authorities and insurance companies and their clinical use.
The therapeutic breakthrough provided by C5 blockers in aHUS should not preclude exploring alternative or combined treatment strategies. Selective C5a or C5a receptor blockade is a potential approach that carries the advantage of preserving C5b-dependent killing of encapsulated pathogens. However, in an animal model, genetic C5a receptor invalidation did not prevent aHUS.89
Alternative C3 convertase overactivation, and not C5 activation, is the initial driver of aHUS. Restoring a normal control of this enzyme with a targeted inhibitor (anti-C3, anti–factor B, and anti–factor D drugs), is the intuitive and most logical approach, currently explored in at least 1 prospective study. The assessment of C3 convertase inhibitors in aHUS is however limited in countries where C5 blockers are available, mainly due to ethical considerations.
Moreover, in PNH,90,91 only 1 out of 3 of patients completely normalize hemoglobin and hemolysis parameters under C5 blockade.92 A clinically pertinent additional effect of C3 convertase blockade on top of C5 blockade has recently been demonstrated in this setting. A similar additional effect of C3 convertase blockade would be difficult to demonstrate for aHUS. In the latter, the residual risk of the main outcome, end-stage kidney disease, has decreased to as low as 10% to 15% with C5 blockade alone.
Case 3
A 57-year-old male patient was admitted for bicytopenia and acute kidney injury. He had been treated for 9 months for a pancreatic adenocarcinoma and had completed 6 cycles of gemcitabine chemotherapy 1 month earlier. On admission, his blood pressure was 150/85 mm Hg. Laboratory tests showed acute kidney injury (serum creatinine, 1.8 mg/dL; proteinuria, 2 g/L), thrombocytopenia (82 G/L), and microangiopathic hemolytic anemia (hemoglobin, 7.2 g/dL; LDH, 3.7 times over the ULN; undetectable haptoglobin; and presence of schistocytes [1%] on repeated blood smears). ADAMTS13 activity was detectable (62%) and complement assays were normal. Five days later, serum creatinine increased to 2.8 mg/dL and thrombocytopenia and hemolysis (requiring transfusion) persisted. He underwent 5 sessions of plasma exchanges with no effect on hematological or renal parameters. The patient received 2 weekly infusions of a short-acting C5 blocker (eculizumab). Thrombocytopenia and hemolysis rapidly resolved, but severe renal impairment persisted. Further complement gene analysis did not reveal any pathogenic rare variant.
How I manage potentially complement-dependent TMA beyond aHUS
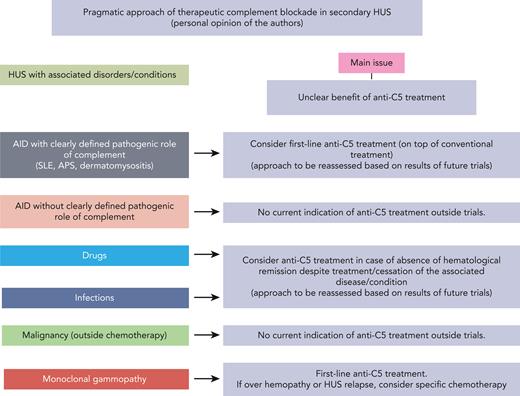
Since the availability of the first C5 blocker, the potential implication of complement activation, and its extent, in distinct forms of renal TMA, beyond aHUS, has become a debated subject. To assess the implication of complement in a given renal TMA, we propose a combination of clinical, biological, and genetic criteria (Table 4). These criteria do not fully apply, even to the prototypic complement-mediated renal TMA, aHUS; an additional illustration of the complexity of the role of complement in TMA. Nevertheless, as already discussed, these criteria help outline striking differences between aHUS and secondary HUS. Hence, it appears that complement overactivation is probably not the major driver of secondary HUS. We, however, propose a pragmatic approach to distinct forms of secondary HUS, based mainly, if not solely, on our experience and opinion (Figure 4). This approach is based on the assumption that complement activation is a potential “second-hit” that amplifies and prolongs TMA in some forms of secondary HUS. Planned or ongoing studies may help refine our approach to secondary HUS.
Proposed criteria for the assessment of complement implication in the pathogenesis of types of TMA
| Criteria . | aHUS (%) . | Secondary HUS (%) . | Remarks . |
|---|---|---|---|
| Enrichment in rare (pathogenic) variants in complement genes (vs healthy individuals) | 30-60 | <5 | Does not exclude transient complement (over)activation |
| Biomarkers of systemic complement activation | Not fully established | Not available | Reliability of complement biomarkers not validated, even in aHUS |
| Response to complement (C5) blockade | Shown in prospective nonrandomized trials | Variable | Difficulty in defining response to C5 blockade. Confounding effects of trigger withdrawal, treatment of underlying disease/condition |
| Relapse rate | <1 | ||
| Reflects transient vs sustained complement activation/dysregulation | 23-64 (carriers of complement gene variants) |
| Criteria . | aHUS (%) . | Secondary HUS (%) . | Remarks . |
|---|---|---|---|
| Enrichment in rare (pathogenic) variants in complement genes (vs healthy individuals) | 30-60 | <5 | Does not exclude transient complement (over)activation |
| Biomarkers of systemic complement activation | Not fully established | Not available | Reliability of complement biomarkers not validated, even in aHUS |
| Response to complement (C5) blockade | Shown in prospective nonrandomized trials | Variable | Difficulty in defining response to C5 blockade. Confounding effects of trigger withdrawal, treatment of underlying disease/condition |
| Relapse rate | <1 | ||
| Reflects transient vs sustained complement activation/dysregulation | 23-64 (carriers of complement gene variants) |
Pragmatic approach to the use of anti-C5 treatment in the management of HUS with coexistent conditions or diseases. AID, autoimmune disease; APS, antiphospholipid syndrome; SLE, systemic lupus erythematous.
Pragmatic approach to the use of anti-C5 treatment in the management of HUS with coexistent conditions or diseases. AID, autoimmune disease; APS, antiphospholipid syndrome; SLE, systemic lupus erythematous.
Conclusion
aHUS has been an example of a successful translational research that has changed the landscape of this rare but devastating form of TMA. aHUS, along with PNH, have also paved the way for the development of a new class of therapeutic complement modulators. Lifelong treatment with C5 blockers is no longer a paradigm for the management of all patients with aHUS, and complement genetics contribute to an individualized duration of treatment.
Challenges remain, however, in the management of aHUS. A positive rapid diagnostic test for aHUS is crucially lacking. Currently, complement genetics are not part of the initial diagnostic workup of HUS, mainly owing to delays in the sequencing and analysis of the results. An ultrarapid complement gene sequencing, as recently used in metabolic diseases,93 may help ensure the diagnosis of aHUS, at least in a substantial proportion of patients.
Acknowledgments
The authors thank Déla Golshayan and François Provôt for insightful discussions around the manuscript content.
Authorship
Contribution: All authors drafted and reviewed the manuscript and approved its final version.
Conflict-of-interest disclosure: F.F. has received consulting fees and honoraria from Alexion, Apellis, Roche, Novartis, and Vifor. V.F.-B. has received consulting fees and honoraria from Alexion, Apellis, Bioscrypt, Roche, and Novartis. N.S. declares no competing financial interests.
Correspondence: Fadi Fakhouri, Department of Medicine, Service of Nephrology and Hypertension, Lausanne University Hospital and Université de Lausanne, Rue du Bugnon, 1005 Lausanne, Switzerland; e-mail: fadi.fakhouri@unil.ch.
References
Author notes
Data are available on request from the corresponding author, Fadi Fakhouri (fadi.fakhouri@unil.ch).
The online version of this article contains a data supplement.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal