Key Points
Models adjusted for AML and patient-specific variables showed no benefit of allogeneic HCT in patients that are older or medically infirm.
Current practice of offering HCT to older and medically infirm patients with AML is not evidence based, which calls for randomized trials.
Abstract
We designed a prospective, observational study enrolling patients presenting for treatment of acute myeloid leukemia (AML) at 13 institutions to analyze associations between hematopoietic cell transplantation (HCT) and survival, quality of life (QOL), and function in: the entire cohort, those aged ≥65 years, those with high comorbidity burden, intermediate cytogenetic risk, adverse cytogenetic risk, and first complete remission with or without measurable residual disease. Patient were assessed 8 times over 2 years. Time-dependent regression models were used. Among 692 patients that were evaluable, 46% received HCT with a 2-year survival of 58%. In unadjusted models, HCT was associated with reduced risks of mortality most of the subgroups. However, after accounting for covariates associated with increased mortality (age, comorbidity burden, disease risks, frailty, impaired QOL, depression, and impaired function), the associations between HCT and longer survival disappeared in most subgroups. Although function, social life, performance status, and depressive symptoms were better for those selected for HCT, these health advantages were lost after receiving HCT. Recipients and nonrecipients of HCT similarly ranked and expected cure as main goal of therapy, whereas physicians had greater expectations for cure than the former. Accounting for health impairments negates survival benefits from HCT for AML, suggesting that the unadjusted observed benefit is mostly owing to selection of the healthier candidates. Considering patients’ overall expectations of cure but also the QOL burdens of HCT motivate the need for randomized trials to identify the best candidates for HCT. This trial was registered at www.clinicaltrials.gov as #NCT01929408.
Introduction
Acute myeloid leukemia (AML) accounts for the largest number of leukemia deaths in the United States.1 Allogeneic hematopoietic cell transplantation (HCT) is potentially curative, substantially reflecting a donor-derived graft-versus-leukemia effect.2 “Genetic randomization” studies, based largely on availability of HLA-matched sibling donors have shown benefit of allogeneic HCT for patients with AML aged <60 years with adverse and, to a lesser extent, intermediate-risk cytogenetics.2-4
However, most patients with AML are older than 65 years.5 Although studies suggest HCT improves overall survival (OS) in patients of older age,6-8 overall outcomes among most older patients with AML remain poor, with recent 5-year OS rates in the United States of only 6.8% to 9.3%, 1.1% to 1.5%, and 0% to 1.2% for patients aged 65 to 74, 75 to 84, and ≥85 years, respectively.9 Although the advent of reduced intensity conditioning regimens has increased the use of HCT and has been proposed to lengthen OS, population-based studies indicate that only 6% to 8% of patients aged 60 to 80 years receive HCT,10,11 raising the possibility of a selection bias. Furthermore, comorbidity burdens increase with increasing age,12 and these burdens increase the risk of mortality and morbidity after HCT.13-15 To what extent comorbidity and other health burdens among the overall AML population influence benefits from HCT compared with other therapies, is unknown.
Consequently, a comparison of HCT and non-HCT approaches in patients that are vulnerable (older and/or medically infirm) that considers potential differences in health burdens is an unmet need. Such a comparison should ideally account for geriatric health measures, which are often not reported despite strong recommendations to the contrary for patients with cancer aged ≥65 years.16-19 Observational studies of patients not enrolled into formal clinical trials, and thus, without overly restrictive eligibility criteria, are considered important for informing development of future studies and are more reflective of the patient populations that are actually treated.20 Hence, in preparation for a possible future randomized study, we designed a multicenter observational study to prospectively examine the effects of HCT on mortality, patient-reported outcomes, such as quality of life (QOL), function, frailty, depression, social support, cognition, and geriatric syndromes. We also examined patient preferences/goals of treatment and compared their perceptions of chances of cure with those of their physicians. These issues were examined in 6 cohorts: (1) all study participants, (2) those aged ≥65 years, (3) those with augmented HCT comorbidity index [HCT-CI] scores of ≥4,21 (4) those with intermediate risk according to the 2017 European LeukemiaNet (ELN) diagnostic guidelines,22 (5) those with ELN guideline–based adverse risk, (6) and those in first complete remission (CR1) or beyond CR1. The impact of minimal residual disease (MRD) was also studied.
Methods
Study design, setting, and participants
This was a multicenter, prospective, observational, nonpopulation-based longitudinal clinical trial (www.clinicaltrials.gov, #NCT01929408). Patients were enrolled between July 2013 and December 2017, after receiving AML therapy at 1 of 13 US centers (12 academic and 1 private practice). Assessments of QOL and other measures continued for 2 years after enrollment, whereas OS data were collected until April 2021. Inclusion criteria were (1) aged 18 to 80 years (to capture patients aged <65 years but also those who are <65 years but have significant comorbidity burdens); (2) newly diagnosed AML, relapsed/refractory AML, or high-risk myelodysplastic syndromes (MDSs) (10% to 19% blasts in bone marrow); (3) treated with AML-like therapy at either lower or higher intensity; (4) ability to speak and read English; and (5) provided informed consent. Exclusion criteria were (1) projected OS of <6 months owing to active second malignancy or other medical problem and (2) receipt of purely palliative/supportive care. All patients that met the eligibility criteria were approached, unless prevented for medical reasons. All sites followed similar steps to screen patients for study eligibility. The 2017 ELN classification was used to categorize patient risks including the use of molecular data.22
The trial was approved by the institutional review boards of each collaborating site, and participants provided written informed consent.
Timing of study evaluations, study time frame, data collection and sources, and bias management are described in supplemental Table 1, supplemental Figure 1, and in the supplemental Materials and methods, available on the Blood website.
Variables, data sources, and measurement
Study outcomes
The primary outcome was overall survival. Secondary outcomes included QOL, functional status, and frailty.
Exposure
Predictors
Factors used to predict outcomes, including AML risks, the comorbidity index, QOL, functional status, frailty, and other geriatric health problems are listed in Table 1 and in supplemental Materials and methods.
Baseline characteristics of study population
| . | All patients (N = 692), % . |
|---|---|
| Age, y | |
| 18-49 | 22 |
| 50-59 | 21 |
| 60-64 | 14 |
| 65-69 | 19 |
| 70-74 | 14 |
| 75-79 | 9 |
| ≥80 | 1 |
| Augmented HCT-CI | |
| 0-1 | 15 |
| 2-3 | 29 |
| 4-5 | 23 |
| ≥6 | 33 |
| 2017 ELN cytogenetic risk | |
| Favorable | 21 |
| Intermediate | 43 |
| Adverse | 36 |
| KPS | |
| >70 | 83 |
| ≤70 | 17 |
| AML composite model | |
| 0-3 | 13 |
| 4-6 | 32 |
| 7-9 | 32 |
| ≥10 | 23 |
| Diagnosis | |
| Newly diagnosed AML | 77 |
| Relapsed/refractory AML | 14 |
| High-risk MDS | 9 |
| Status posttreatment | |
| Never achieved CR | 44 |
| Achieved CR | 56 |
| . | All patients (N = 692), % . |
|---|---|
| Age, y | |
| 18-49 | 22 |
| 50-59 | 21 |
| 60-64 | 14 |
| 65-69 | 19 |
| 70-74 | 14 |
| 75-79 | 9 |
| ≥80 | 1 |
| Augmented HCT-CI | |
| 0-1 | 15 |
| 2-3 | 29 |
| 4-5 | 23 |
| ≥6 | 33 |
| 2017 ELN cytogenetic risk | |
| Favorable | 21 |
| Intermediate | 43 |
| Adverse | 36 |
| KPS | |
| >70 | 83 |
| ≤70 | 17 |
| AML composite model | |
| 0-3 | 13 |
| 4-6 | 32 |
| 7-9 | 32 |
| ≥10 | 23 |
| Diagnosis | |
| Newly diagnosed AML | 77 |
| Relapsed/refractory AML | 14 |
| High-risk MDS | 9 |
| Status posttreatment | |
| Never achieved CR | 44 |
| Achieved CR | 56 |
KPS, Karnofsky performance status.
Study size
Power calculations were made based on a total enrollment of 640 patients. This study accrued 705 patients. The addition of patients was meant to take into account the possibility of missing data or incomplete surveys. Power calculations were guided by results that show that the 1-year survival rate of patients that are older with AML given allogeneic HCT is ∼65%.27 In contrast, patients receiving non-HCT therapies were shown to have a 1-year survival rate of ∼40%.28 From these 2 studies, the 1-year survival rate for HCT and non-HCT recipients had a difference of 25%; 2-year survival rates were 48% and 26%, respectively, with a difference of 22%. Hence, assuming a comparison group of at least equal size for patients who were treated with nontransplant therapies, we then had 80% power to detect an improvement of 19% (absolute difference) in survival in the transplanted group after the enrollment of at least 640 patients.
Statistical methods
Frequency counts and percentages for categorical variables (diagnosis and ELN cytogenetic risk category) and means and standard deviations for baseline continuous outcome variables were calculated.
Primary outcome
The start of induction therapy was taken as “time zero.” Patients who received HCT had to survive long enough to undergo transplantation, and this potential bias (immortal time bias or lead-time bias) needs to be considered in the assessment of the effect of HCT on outcome. This bias was dealt with by modeling HCT as a time-dependent covariate.29 In such a model, patients were considered in the non-HCT group until receipt of HCT, at which time they entered the HCT group. To account for variables other than HCT that could potentially affect OS, we first utilized Cox regression models to identify baseline and time-dependent risk factors associated with mortality in the overall population. The same factors are thought to influence physicians’ choices of therapies for their patients. Factors identified as significantly associated with mortality (P < .05) were considered in the development of a set of multivariable models examining the association between HCT and mortality within the aforementioned study cohorts. In addition, achievement of CR1 was modeled as a time-dependent covariate in 281 patients treated at the Fred Hutchinson Cancer Center, who achieved CR1 because MRD assessments were complete and had been performed uniformly in this population, generally within 1 to 2 weeks of blood count documentation of CR, using 10-color flow cytometry. In this subset, the potential interaction between MRD status and HCT was assessed by including an interaction term in the regression model. The potential statistical interaction of other factors with HCT was also examined in the same manner.
Secondary outcomes
To estimate the effect of HCT on QOL, functional status, frailty, and other important outcomes, we used logistic regression with generalized estimating equations and robust variances to account for multiple observations per person. Given the time lag between diagnosis of AML and receipt of transplant, patients were categorized into 1 of 3 groups: (1) patients who had never received HCT (never-HCT), (2) those who were scheduled to receive an HCT (pre-HCT), and (3) those already having had received a transplant (post-HCT). This categorization allowed an examination of how the receipt of HCT may change longitudinal outcomes, in addition to whether patients who receive HCT differ from those who never receive HCT. Secondary outcomes were modeled as a function of group, along with time and group-by-time interactions. Curves were also generated to illustrate the pattern of probability of a favorable outcome over time for each group.
Handling of missing data
The handling of missing data and loss of follow-up is described in the supplemental Materials and methods.
Results
Descriptive data
Participant characteristics and exposure
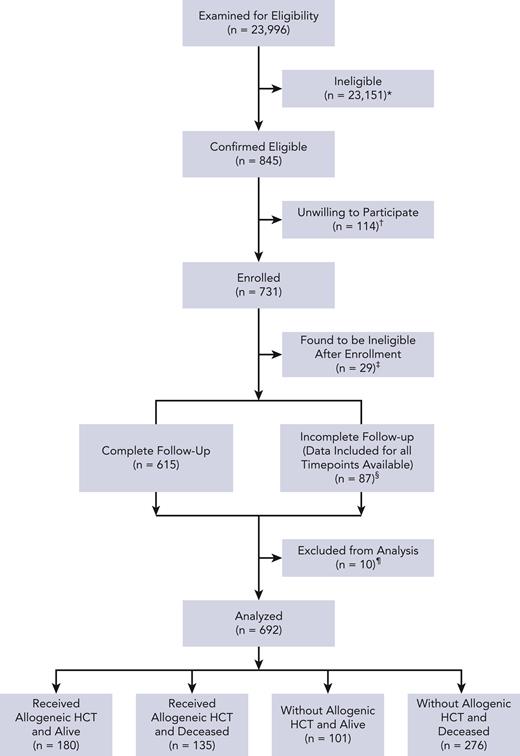
Patient characteristics are reported in Table 1. Details of study flow are in Figure 1 as well as in supplemental Materials and methods. Data from 692 patients were analyzed. Patients aged ≥65 years constituted 43% of the cohort, whereas those with augmented HCT-CI scores of ≥4 constituted 56%; 68.2% of patients (n = 472) were aged ≥65 and/or had augmented HCT-CI scores of ≥4.
Study flow diagram. ∗ indicates patients were deemed ineligible if their diagnosis was not listed on inclusion criteria, they were not within the allowed age range, the patient was not offered an AML-like therapy with curative intent, and/or the patient was not able to speak/read English; †, patient was too tired (n = 18), overwhelmed with diagnosis (n = 33), too occupied with treatment (n = 30), not interested in the study (n = 24), did not like the way the study was presented (n = 1), did not understand why the study was important (n = 1), and/or had a different reason (n = 24); ‡, patient transferred to receive initial treatment at another institution (n = 1), patient not within allowed age range (n = 1), did not have eligible diagnosis or disease status at enrollment (n = 10), received palliative care as main treatment or did not receive treatment (n = 6), and/or consented >7 days after day 1 of treatment (n = 11); §, received HCT as initial treatment (n = 10); and ¶, unable to reach patient (n = 59), withdrawn per patient request (n = 28), and/or data included for time points available.
Study flow diagram. ∗ indicates patients were deemed ineligible if their diagnosis was not listed on inclusion criteria, they were not within the allowed age range, the patient was not offered an AML-like therapy with curative intent, and/or the patient was not able to speak/read English; †, patient was too tired (n = 18), overwhelmed with diagnosis (n = 33), too occupied with treatment (n = 30), not interested in the study (n = 24), did not like the way the study was presented (n = 1), did not understand why the study was important (n = 1), and/or had a different reason (n = 24); ‡, patient transferred to receive initial treatment at another institution (n = 1), patient not within allowed age range (n = 1), did not have eligible diagnosis or disease status at enrollment (n = 10), received palliative care as main treatment or did not receive treatment (n = 6), and/or consented >7 days after day 1 of treatment (n = 11); §, received HCT as initial treatment (n = 10); and ¶, unable to reach patient (n = 59), withdrawn per patient request (n = 28), and/or data included for time points available.
Diagnoses were mostly newly diagosed AML (77%), whereas relapsed/refractory AML (14%) and high-risk MDS (9%) were less frequent. Most patients had intermediate (43%) or adverse (36%) cytogenetic risk per the ELN classification.22 The majority (83%) had KPS scores of >70%. After initial treatment, 56% achieved CR. Induction regimens are shown in supplemental Table 2. The most common intensive regimens used were cytarabine (≥1 g/m2 per dose) ± others (in 46.6% of patients) and “7 + 3” ± others (in 46.1%); the most common less-intensive regimens were azacitidine ± others (in 33.0%) and decitabine ± others (in 28.7%).
Of the 315 (45.5%) patients who received an HCT, 241 (76.5%) were given HCT in CR1. Among the 472 patients that were older and/or had comorbidities, 171 (36.2%) received an HCT and 137 (80.1%) were in CR1; 31.2% of the 295 patients aged ≥65 years received an HCT, with 83.7% of these in CR1. Of the 296 patients at ELN intermediate risk, 156 (52.7%) received an HCT, with 124 (79.5%) in CR1; and 42.7% of the 248 patients at ELN adverse risk received an HCT, with 82 (77.4%) in CR1.
Length of follow-up and missing data
Median follow-up among survivors was 53.6 months (range, 1.1-88.5 months) after start of treatment. Supplemental Table 1 shows low rates of missing data in general. As would be expected, data collected via medical charts were less frequently missing relative to patient-reported tools and other assessments.
Reasons for declining enrollment
Among the 114 patients who were unable or unwilling to participate in the study, 99 patients completed a declination survey. Patients were allowed to endorse more than 1 reason for declining the study. Reasons for declination included being too tired (15.7%), too overwhelmed with diagnosis (28.4%), too occupied with treatment (26.3%), not interested in the study (21.1%), and other reasons (21.1%). Other reasons included in order of frequency, “did not want to complete assessments,” “overwhelmed by diagnosis,” “too occupied with treatment,” “not interested in study,” “unwell or too tired,” “participating in too many other research studies,” “prior negative experience in another research study,” “too busy,” and “concerned about commitment for study follow-up.”
Patient preferences and patients’ and physicians’ estimates of chances of cure
At study enrollment, 80% of patients ranked cure as their main treatment goal, followed by QOL (36%) and length of life (33%) (Table 2). When we assessed these parameters later, either at 3 or 6 months after enrollment for non-HCT recipients or at the closest time point before receiving HCT among recipients of HCT, we found cure remained the main goal regardless of type of treatment (74% and 87%, respectively), followed in importance by QOL (51% and 44%, respectively).
Treatment preferences and values of physician and general patients with AML population, as stratified by HCT and non-HCT recipients
| . | . | All patients at enrollment . | Non-HCT at 3- or 6-mo follow-up time point∗ . | HCT at closest time point before transplant . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Rank of importance, n (%) . | Rank of importance, n (%) . | Rank of importance, n (%) . | ||||||
| Patient survey | Objective | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 |
| Cure | 430 (80) | 57 (11) | 52 (10) | 134 (74) | 29 (16) | 17 (9) | 201 (87) | 19 (8) | 10 (4) | |
| QOL | 188 (36) | 217 (41) | 123 (23) | 90 (51) | 55 (31) | 32 (18) | 99 (44) | 85 (38) | 40 (18) | |
| LOL | 173 (33) | 143 (27) | 207 (40) | 56 (32) | 56 (32) | 65 (36) | 84 (38) | 59 (26) | 81 (36) | |
| . | . | All patients at enrollment . | Non-HCT at 3- or 6-mo follow-up time point∗ . | HCT at closest time point before transplant . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Rank of importance, n (%) . | Rank of importance, n (%) . | Rank of importance, n (%) . | ||||||
| Patient survey | Objective | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 |
| Cure | 430 (80) | 57 (11) | 52 (10) | 134 (74) | 29 (16) | 17 (9) | 201 (87) | 19 (8) | 10 (4) | |
| QOL | 188 (36) | 217 (41) | 123 (23) | 90 (51) | 55 (31) | 32 (18) | 99 (44) | 85 (38) | 40 (18) | |
| LOL | 173 (33) | 143 (27) | 207 (40) | 56 (32) | 56 (32) | 65 (36) | 84 (38) | 59 (26) | 81 (36) | |
| . | . | More important objective, n (%) . | More important objective, n (%) . | More important objective, n (%) . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient survey . | Cure vs QOL . | Cure . | Equal . | QOL . | Cure . | Equal . | QOL . | Cure . | Equal . | QOL . |
| 313 (60) | 130 (25) | 80 (15) | 81 (47) | 52 (30) | 40 (23) | 121 (54) | 82 (37) | 21 (9) | ||
| . | . | More important objective, n (%) . | More important objective, n (%) . | More important objective, n (%) . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient survey . | Cure vs QOL . | Cure . | Equal . | QOL . | Cure . | Equal . | QOL . | Cure . | Equal . | QOL . |
| 313 (60) | 130 (25) | 80 (15) | 81 (47) | 52 (30) | 40 (23) | 121 (54) | 82 (37) | 21 (9) | ||
| . | . | More important objective, n (%) . | More important objective, n (%) . | More important objective, n (%) . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient survey . | Cure vs LOL . | Cure . | Equal . | LOL . | Cure . | Equal . | LOL . | Cure . | Equal . | LOL . |
| 318 (61) | 129 (25) | 73 (14) | 101 (58) | 50 (29) | 22 (13) | 130 (58) | 79 (35) | 15 (7) | ||
| . | . | More important objective, n (%) . | More important objective, n (%) . | More important objective, n (%) . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient survey . | Cure vs LOL . | Cure . | Equal . | LOL . | Cure . | Equal . | LOL . | Cure . | Equal . | LOL . |
| 318 (61) | 129 (25) | 73 (14) | 101 (58) | 50 (29) | 22 (13) | 130 (58) | 79 (35) | 15 (7) | ||
| . | . | More important objective, n (%) . | More important objective, n (%) . | More important objective, n (%) . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient survey . | QOL vs LOL . | QOL . | Equal . | LOL . | QOL . | Equal . | LOL . | QOL . | Equal . | LOL . |
| 240 (46) | 132 (25) | 149 (29) | 90 (51) | 49 (28) | 36 (21) | 94 (42) | 82 (37) | 46 (21) | ||
| . | . | More important objective, n (%) . | More important objective, n (%) . | More important objective, n (%) . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient survey . | QOL vs LOL . | QOL . | Equal . | LOL . | QOL . | Equal . | LOL . | QOL . | Equal . | LOL . |
| 240 (46) | 132 (25) | 149 (29) | 90 (51) | 49 (28) | 36 (21) | 94 (42) | 82 (37) | 46 (21) | ||
| . | . | Chance of cure, n (%) . | Chance of cure, n (%) . | Chance of cure, n (%) . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Good (>75%) . | Maybe (25%-74%) . | Poor (<25%) . | Good (>75%) . | Maybe (25%-74%) . | Poor (<25%) . | Good (>75%) . | Maybe (25%-74%) . | Poor (<25%) . |
| Patient perception | 338 (65) | 162 (31) | 22 (4) | 110 (60) | 63 (35) | 10 (6) | 140 (61) | 87 (38) | 1 (<1) | |
| Physician perception | 36 (7) | 309 (64) | 137 (28) | 14 (7) | 90 (47) | 89 (46) | 13 (6) | 179 (78) | 34 (15) | |
| . | . | Chance of cure, n (%) . | Chance of cure, n (%) . | Chance of cure, n (%) . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Good (>75%) . | Maybe (25%-74%) . | Poor (<25%) . | Good (>75%) . | Maybe (25%-74%) . | Poor (<25%) . | Good (>75%) . | Maybe (25%-74%) . | Poor (<25%) . |
| Patient perception | 338 (65) | 162 (31) | 22 (4) | 110 (60) | 63 (35) | 10 (6) | 140 (61) | 87 (38) | 1 (<1) | |
| Physician perception | 36 (7) | 309 (64) | 137 (28) | 14 (7) | 90 (47) | 89 (46) | 13 (6) | 179 (78) | 34 (15) | |
LOL, length of life.
Data selected from either 3- or 6-month follow-up assessment per patient, whichever had earliest responses.
Patients’ and physicians’ perceptions of patients’ chances of cure were clearly discordant. For example, although 65% of patients estimated at enrollment that they might have a “good” (>75%) chance of cure, only 7% of physicians agreed.
At later time points, 78% of physicians estimated a “maybe” (25% to 74%) chance of cure for recipients of HCT, whereas only 47% of physicians estimated the same for those not given HCT. A “poor” (<25%) chance of cure was estimated by only 15% of physicians for recipients of HCT, whereas 46% of physicians estimated such a low chance for non-HCT recipients. Patients’ perceptions of chance of cure differed less according to whether they had received HCT or not (38% vs 35%, respectively).
Primary outcome (OS)
Potential confounders
Table 3 describes results of Cox regression models for associations between mortality and baseline and time-dependent potential confounding variables in all 692 patients. Variables that had statistically significant associations with mortality included increasing augmented HCT-CI scores (2-3, 4-5, and ≥6 vs 0-1); increasing age (60-64, 65-69, and ≥70 vs <60 years); intermediate and adverse ELN risk (vs favorable risk); relapsed/refractory AML (vs de novo AML) at enrollment; relapse or refractory; CR2 or CR3 (vs CR1) responses to treatment given after enrollment; frailty as measured by 4-MWT; impaired QOL as measured by FACT-G scores; increased depressive symptoms as measured by PHQ-9 scores; and dependent status as measured by ADL scores <14.
Multivariable analysis of risk factors associated with mortality in the general AML population
| Risk factor . | HR (95% CI)∗ . | P . |
|---|---|---|
| Augmented HCT-CI | ||
| 0-1 | 1.0 | |
| 2-3 | 1.79 (1.05-3.06) | .03 |
| 4-5 | 2.42 (1.44-4.07) | .0009 |
| ≥6 | 3.69 (2.24-6.06) | <.0001 |
| Age, y | ||
| 0-59 | 1.0 | |
| 60-64 | 1.50 (1.05-2.13) | .02 |
| 65-69 | 1.36 (0.99-1.86) | .06 |
| ≥70 | 2.19 (1.65-2.91) | <.0001 |
| ELN cytogenetic risk† | ||
| Low | 1.0 | |
| Intermediate | 1.53 (1.03-2.28) | .03 |
| Adverse | 2.35 (1.56-3.54) | <.0001 |
| Status at enrollment | ||
| Newly diagnosed AML | 1.0 | |
| Relapsed/refractory AML | 1.65 (1.24-2.20) | .0005 |
| Status after treatment | ||
| Never reached CR | 1.0 | |
| CR1 | 0.29 (0.23-0.37) | <.0001 |
| Relapsed after CR1 | 1.66 (1.24-2.23) | .0007 |
| CR2 | 0.78 (0.44-1.37) | .38 |
| Relapsed after CR2 | 3.41 (1.81-6.44) | .0002 |
| CR3 | 3.80 (1.19-12.07) | .02 |
| FACT-G (per 10 points)‡ | 0.89 (0.81-0.98) | .02 |
| PHQ-9 depressive symptoms (per point) | 1.03 (1.00-1.06) | .03 |
| ADL (per point) | 0.95 (0.90-1.00) | .05 |
| 4-MWT mean time (per doubling) | 1.31 (1.09-1.57) | .004 |
| Risk factor . | HR (95% CI)∗ . | P . |
|---|---|---|
| Augmented HCT-CI | ||
| 0-1 | 1.0 | |
| 2-3 | 1.79 (1.05-3.06) | .03 |
| 4-5 | 2.42 (1.44-4.07) | .0009 |
| ≥6 | 3.69 (2.24-6.06) | <.0001 |
| Age, y | ||
| 0-59 | 1.0 | |
| 60-64 | 1.50 (1.05-2.13) | .02 |
| 65-69 | 1.36 (0.99-1.86) | .06 |
| ≥70 | 2.19 (1.65-2.91) | <.0001 |
| ELN cytogenetic risk† | ||
| Low | 1.0 | |
| Intermediate | 1.53 (1.03-2.28) | .03 |
| Adverse | 2.35 (1.56-3.54) | <.0001 |
| Status at enrollment | ||
| Newly diagnosed AML | 1.0 | |
| Relapsed/refractory AML | 1.65 (1.24-2.20) | .0005 |
| Status after treatment | ||
| Never reached CR | 1.0 | |
| CR1 | 0.29 (0.23-0.37) | <.0001 |
| Relapsed after CR1 | 1.66 (1.24-2.23) | .0007 |
| CR2 | 0.78 (0.44-1.37) | .38 |
| Relapsed after CR2 | 3.41 (1.81-6.44) | .0002 |
| CR3 | 3.80 (1.19-12.07) | .02 |
| FACT-G (per 10 points)‡ | 0.89 (0.81-0.98) | .02 |
| PHQ-9 depressive symptoms (per point) | 1.03 (1.00-1.06) | .03 |
| ADL (per point) | 0.95 (0.90-1.00) | .05 |
| 4-MWT mean time (per doubling) | 1.31 (1.09-1.57) | .004 |
4-MWT, National Institutes of Health Toolbox 4-Meter Walk Gait Speed Test; ADL, activities of daily living; FACT-G, functional assessment of cancer therapy–general; PHQ-9, patient health questionnaire 9; HR, hazard ratio; CI, confidence interval.
Cox regression analysis based on time since start of treatment on enrolled study.
Missing data indicator included.
For time-dependent QOL measures, missing data indicator until first known data and analysis uses last available observation.
Univariate associations between HCT and mortality
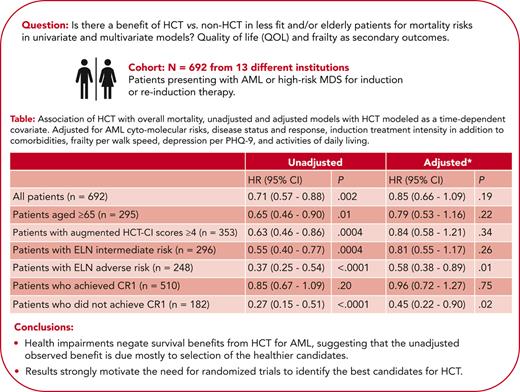
For patients given HCT, the 4-year (following HCT) OS estimate was 54% (95% CI, 48-59). Unadjusted analysis among all patients showed a 29% reduced risk of mortality for HCT relative to no HCT (HR, 0.71; 95% CI, 0.57-0.88; P = .002). Patients that were older (aged ≥65 years) had a 35% reduction in risk of mortality when given HCT (HR, 0.65; 95% CI, 0.46-0.90; P = .01). Patients with augmented HCT-CI scores of ≥4 had a 37% reduction in risk of mortality when given HCT (HR, 0.63; 95% CI, 0.46-0.86; P = .0004). Corresponding figures were 45% in patients at intermediate (HR, 0.55; 95% CI, 0.40-0.77; P = .0004) and 63% in patients at adverse (HR, 0.37; 95% CI, 0.25-0.54; P < .0001) ELN risk, respectively (Table 4).
Association of HCT with overall mortality, unadjusted, and adjusted models with HCT modeled as a time-dependent covariate
| . | Unadjusted . | Adjusted∗ . | ||
|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | |
| All patients (N = 692) | 0.71 (0.57-0.88) | .002 | 0.85 (0.66-1.09) | .19 |
| Patients aged ≥65 y (n = 295) | 0.65 (0.46-0.90) | .01 | 0.79 (0.53-1.16) | .22 |
| Patients with augmented HCT-CI scores ≥4 (n = 353) | 0.63 (0.46-0.86) | .0004 | 0.84 (0.58-1.21) | .34 |
| Patients with ELN intermediate risk (n = 296) | 0.55 (0.40-0.77) | .0004 | 0.81 (0.55-1.17) | .26 |
| Patients with ELN adverse risk (n = 248) | 0.37 (0.25-0.54) | <.0001 | 0.58 (0.38-0.89) | .01 |
| Patients who achieved CR1 (n = 510) | 0.85 (0.67-1.09) | .20 | 0.96 (0.72-1.27) | .75 |
| Patients who did not achieve CR1 (n = 182) | 0.27 (0.15-0.51) | <.0001 | 0.45 (0.22-0.90) | .02 |
| . | Unadjusted . | Adjusted∗ . | ||
|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | |
| All patients (N = 692) | 0.71 (0.57-0.88) | .002 | 0.85 (0.66-1.09) | .19 |
| Patients aged ≥65 y (n = 295) | 0.65 (0.46-0.90) | .01 | 0.79 (0.53-1.16) | .22 |
| Patients with augmented HCT-CI scores ≥4 (n = 353) | 0.63 (0.46-0.86) | .0004 | 0.84 (0.58-1.21) | .34 |
| Patients with ELN intermediate risk (n = 296) | 0.55 (0.40-0.77) | .0004 | 0.81 (0.55-1.17) | .26 |
| Patients with ELN adverse risk (n = 248) | 0.37 (0.25-0.54) | <.0001 | 0.58 (0.38-0.89) | .01 |
| Patients who achieved CR1 (n = 510) | 0.85 (0.67-1.09) | .20 | 0.96 (0.72-1.27) | .75 |
| Patients who did not achieve CR1 (n = 182) | 0.27 (0.15-0.51) | <.0001 | 0.45 (0.22-0.90) | .02 |
Adjusted for the augmented HCT-CI, age, ELN cytogenetic risk, relapsed/refractory AML at enrollment, posttreatment CR1 status, treatment intensity, sum PHQ-9, KPS, ADL, FACT-G, and 4-MWT (posttreatment CR1 status, sum PHQ-9, KPS, ADL, FACT-G, and 4-MWT modeled as time-dependent variables, with missing indicator to account for those without data).
The unadjusted risk of mortality was lower among patients who failed to achieve CR1 and who were given HCT compared with patients who failed to achieve CR1 and were not given HCT (HR, 0.27; 95% CI, 0.15-0.51; P < .0001). Among patients who achieved CR1, the unadjusted HR of mortality for patients who received HCT compared with those who did not receive HCT was 0.85 (95% CI, 0.61-1.09; P = .20).
Multivariable associations between HCT and mortality
After adjusting for variables shown to be associated with mortality (Table 3), there was no definitive evidence that receiving HCT improved OS in the entire cohort or any of the previously described subgroups (Table 4), with the exception of patients at adverse ELN risk (HR, 0.58; 95% CI, 0.38-0.89; P = .01) and those who never achieved CR1 (HR, 0.45; 95% CI, 0.22-0.90; P = .02), although the number of transplants in this later group was relatively small (30 such patients received HCT).
Effect of HCT based on MRD status and induction intensity
Unadjusted models indicated that receipt of HCT was associated with at least a numerically improved OS among the Fred Hutchinson Cancer Center CR1 subset (HR, 0.79; 95% CI, 0.57-1.08; P = .14). However, this numerical advantage was diminished after adjustment for risk factors as detailed in previous sections in addition to the presence of MRD (HR, 0.96; 95% CI, 0.66-1.40; P = .82). A test of interaction between the impact of HCT among patients who achieved CR1 and MRD status yielded P = .78 (adjusted HR = 1.01 for patients with MRD and adjusted HR = 0.94 for patients without MRD). Regardless of HCT status, however, the HR of mortality for patients with MRD compared with those without MRD was 1.47 (95% CI, 1.00-2.17; P = .05).
A statistical test of this interaction between impact of HCT and induction regimen intensity (intensive vs less intensive) yielded P = .41. Furthermore, the adjusted HR of mortality for HCT vs non-HCT among patients who received intensive induction therapy was 0.87. That adjusted HR was 0.68 for patients who received less-intensive induction.
Secondary outcomes
Multivariable associations between HCT and secondary outcomes
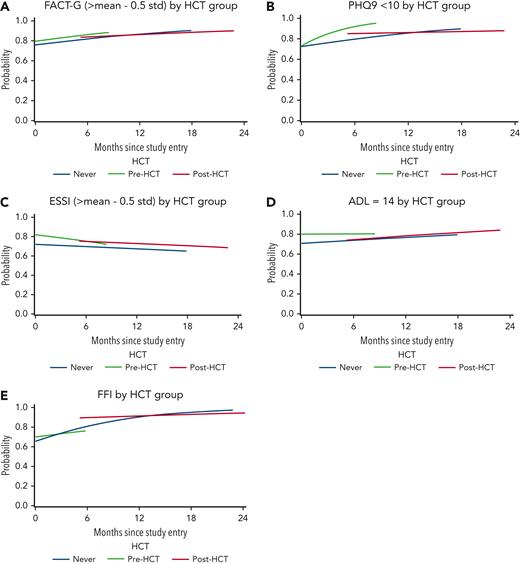
In adjusted models, there were no statistically significant differences between pre-HCT and never-HCT groups for scores on FACT-G, EuroQOL 5-dimensions, PHQ-9, Fried frailty index, 4-MWT, or instrumental activities of daily living scores at enrollment. However, pre-HCT group social activity log (P < .001), ENRICHD social support instrument (P = .003) and ADL (P = .004) scores, and performance status (<0.001) were significantly better when compared with never-HCT group scores at enrollment.
When analyzing group changes over time, the pre-HCT and never-HCT groups showed no significant differences for any of the outcomes with the exception of faster improvement in PHQ-9 (depressive symptoms) and worsening of SAL among the pre-HCT group relative to the never-HCT group (P = .005 and P = .034, respectively). In contrast and when considering changes over time, the post-HCT group showed no differences on any of the tools compared with the never-HCT group (Table 5; Figure 2).
Generalized estimating equation models examining secondary outcomes across never-HCT, pre-HCT, and post-HCT groups after adjusting for covariates plus/minus change over time
| Measure . | Parameter . | Comparison . | Estimate (95% CI) . | P . |
|---|---|---|---|---|
| QOL (FACT-G) (greater than mean - 0.5 standard) | Group | Never-HCT | Reference | |
| Group | Pre- vs never-HCT | 1.25 (0.86-1.82) | .2512 | |
| Group | Post- vs never-HCT | 1.37 (0.77-2.45) | .2859 | |
| Group change over time∗ | Never-HCT | Reference | ||
| Group change over time∗ | Pre- vs never-HCT | 1.02 (0.94-1.10) | .6542 | |
| Group change over time∗ | Post- vs never-HCT | 0.97 (0.92-1.03) | .3057 | |
| QOL (EuroQOL 5-dimensions index) (>33rd percentile) | Group | Never-HCT | Reference | |
| Group | Pre- vs never-HCT | 1.26 (0.89-1.79) | .1859 | |
| Group | Post- vs never-HCT | 1.41 (0.82-2.43) | .2098 | |
| Group change over time∗ | Never-HCT | Reference | ||
| Group change over time∗ | Pre- vs never-HCT | 1.00 (0.92-1.10) | .9112 | |
| Group change over time∗ | Post- vs never-HCT | 1.00 (0.95-1.04) | .8568 | |
| Depressive symptoms (PHQ-9) (<10) | Group | Never-HCT | Reference | |
| Group | Pre- vs never-HCT | 1.04 (0.72-1.49) | .8368 | |
| Group | Post- vs never-HCT | 2.01 (1.06-3.81) | .0323 | |
| Group change over time∗ | Never-HCT | Reference | ||
| Group change over time∗ | Pre- vs never-HCT | 1.19 (1.05-1.35) | .0055 | |
| Group change over time∗ | Post- vs never-HCT | 0.95 (0.89-1.01) | .0913 | |
| Social activities (social activity log) (≥1.13) | Group | Never-HCT | Reference | |
| Group | Pre- vs never-HCT | 2.06 (1.40-3.04) | <.001 | |
| Group | Post- vs never-HCT | 1.27 (0.70-2.32) | .4344 | |
| Group change over time∗ | Never-HCT | Reference | ||
| Group change over time∗ | Pre- vs never-HCT | 0.93 (0.87-0.99) | .0346 | |
| Group change over time∗ | Post- vs never-HCT | 1.00 (0.95-1.05) | .9789 | |
| Perceived social support (ENRICHD social support inventory) (greater than mean - 0.5 standard) | Group | Never-HCT | Reference | |
| Group | Pre- vs never-HCT | 1.76 (1.21-2.57) | .0033 | |
| Group | Post- vs never-HCT | 1.31 (0.76-2.25) | .3341 | |
| Group change over time∗ | Never-HCT | Reference | ||
| Group change over time∗ | Pre- vs never-HCT | 0.95 (0.89-1.02) | .1838 | |
| Group change over time∗ | Post- vs never-HCT | 1.00 (0.96-1.04) | .97 | |
| Instrumental activities of daily living (=14) | Group | Never-HCT | Reference | |
| Group | Pre- vs never-HCT | 0.98 (0.71-1.36) | .9177 | |
| Group | Post- vs never-HCT | 0.39 (0.24-0.63) | <.001 | |
| Group change over time∗ | Never-HCT | Reference | ||
| Group change over time∗ | Pre- vs never-HCT | 0.99 (0.93-1.06) | .8508 | |
| Group change over time∗ | Post- vs never-HCT | 1.02 (0.98-1.07) | .3102 | |
| ADL (=14) | Group | Never-HCT | Reference | |
| Group | Pre- vs never-HCT | 1.67 (1.17-2.37) | .0043 | |
| Group | Post- vs never-HCT | 0.98 (0.58-1.67) | .9542 | |
| Group change over time∗ | Never-HCT | Reference | ||
| Group change over time∗ | Pre- vs never-HCT | 0.98 (0.91-1.05) | .4973 | |
| Group change over time∗ | Post- vs never-HCT | 1.01 (0.96-1.06) | .7107 | |
| Frailty (4-MWT) (≥0.8 m/s) | Group | Never-HCT | Reference | |
| Group | Pre- vs never-HCT | 1.33 (0.94-1.89) | .1119 | |
| Group | Post- vs never-HCT | 2.14 (1.14-3.99) | .0171 | |
| Group change over time∗ | Never-HCT | Reference | ||
| Group change over time∗ | Pre- vs never-HCT | 1.11 (0.97-1.27) | .1222 | |
| Group change over time∗ | Post- vs never-HCT | 0.95 (0.89-1.01) | .1092 | |
| Frailty (Fried frailty index) (=0) | Group | Never-HCT | Reference | |
| Group | Pre- vs never-HCT | 1.22 (0.77-1.93) | .4019 | |
| Group | Post- vs never-HCT | 3.69 (1.48-9.23) | .0051 | |
| Group change over time∗ | Never-HCT | Reference | ||
| Group change over time∗ | Pre- vs never-HCT | 0.93 (0.81-1.06) | .2599 | |
| Group change over time∗ | Post- vs never-HCT | 0.91 (0.81-1.02) | .1022 | |
| Performance status (KPS) (>70) | Group | Never-HCT | Reference | |
| Group | Pre- vs never-HCT | 3.00 (2.01-4.46) | <.001 | |
| Group | Post- vs never-HCT | 0.66 (0.41-1.05) | .0804 | |
| Group change over time∗ | Never-HCT | Reference | ||
| Group change over time∗ | Pre- vs never-HCT | 0.94 (0.88-1.00) | .0464 | |
| Group change over time∗ | Post- vs never-HCT | 1.00 (0.96-1.04) | .9939 |
| Measure . | Parameter . | Comparison . | Estimate (95% CI) . | P . |
|---|---|---|---|---|
| QOL (FACT-G) (greater than mean - 0.5 standard) | Group | Never-HCT | Reference | |
| Group | Pre- vs never-HCT | 1.25 (0.86-1.82) | .2512 | |
| Group | Post- vs never-HCT | 1.37 (0.77-2.45) | .2859 | |
| Group change over time∗ | Never-HCT | Reference | ||
| Group change over time∗ | Pre- vs never-HCT | 1.02 (0.94-1.10) | .6542 | |
| Group change over time∗ | Post- vs never-HCT | 0.97 (0.92-1.03) | .3057 | |
| QOL (EuroQOL 5-dimensions index) (>33rd percentile) | Group | Never-HCT | Reference | |
| Group | Pre- vs never-HCT | 1.26 (0.89-1.79) | .1859 | |
| Group | Post- vs never-HCT | 1.41 (0.82-2.43) | .2098 | |
| Group change over time∗ | Never-HCT | Reference | ||
| Group change over time∗ | Pre- vs never-HCT | 1.00 (0.92-1.10) | .9112 | |
| Group change over time∗ | Post- vs never-HCT | 1.00 (0.95-1.04) | .8568 | |
| Depressive symptoms (PHQ-9) (<10) | Group | Never-HCT | Reference | |
| Group | Pre- vs never-HCT | 1.04 (0.72-1.49) | .8368 | |
| Group | Post- vs never-HCT | 2.01 (1.06-3.81) | .0323 | |
| Group change over time∗ | Never-HCT | Reference | ||
| Group change over time∗ | Pre- vs never-HCT | 1.19 (1.05-1.35) | .0055 | |
| Group change over time∗ | Post- vs never-HCT | 0.95 (0.89-1.01) | .0913 | |
| Social activities (social activity log) (≥1.13) | Group | Never-HCT | Reference | |
| Group | Pre- vs never-HCT | 2.06 (1.40-3.04) | <.001 | |
| Group | Post- vs never-HCT | 1.27 (0.70-2.32) | .4344 | |
| Group change over time∗ | Never-HCT | Reference | ||
| Group change over time∗ | Pre- vs never-HCT | 0.93 (0.87-0.99) | .0346 | |
| Group change over time∗ | Post- vs never-HCT | 1.00 (0.95-1.05) | .9789 | |
| Perceived social support (ENRICHD social support inventory) (greater than mean - 0.5 standard) | Group | Never-HCT | Reference | |
| Group | Pre- vs never-HCT | 1.76 (1.21-2.57) | .0033 | |
| Group | Post- vs never-HCT | 1.31 (0.76-2.25) | .3341 | |
| Group change over time∗ | Never-HCT | Reference | ||
| Group change over time∗ | Pre- vs never-HCT | 0.95 (0.89-1.02) | .1838 | |
| Group change over time∗ | Post- vs never-HCT | 1.00 (0.96-1.04) | .97 | |
| Instrumental activities of daily living (=14) | Group | Never-HCT | Reference | |
| Group | Pre- vs never-HCT | 0.98 (0.71-1.36) | .9177 | |
| Group | Post- vs never-HCT | 0.39 (0.24-0.63) | <.001 | |
| Group change over time∗ | Never-HCT | Reference | ||
| Group change over time∗ | Pre- vs never-HCT | 0.99 (0.93-1.06) | .8508 | |
| Group change over time∗ | Post- vs never-HCT | 1.02 (0.98-1.07) | .3102 | |
| ADL (=14) | Group | Never-HCT | Reference | |
| Group | Pre- vs never-HCT | 1.67 (1.17-2.37) | .0043 | |
| Group | Post- vs never-HCT | 0.98 (0.58-1.67) | .9542 | |
| Group change over time∗ | Never-HCT | Reference | ||
| Group change over time∗ | Pre- vs never-HCT | 0.98 (0.91-1.05) | .4973 | |
| Group change over time∗ | Post- vs never-HCT | 1.01 (0.96-1.06) | .7107 | |
| Frailty (4-MWT) (≥0.8 m/s) | Group | Never-HCT | Reference | |
| Group | Pre- vs never-HCT | 1.33 (0.94-1.89) | .1119 | |
| Group | Post- vs never-HCT | 2.14 (1.14-3.99) | .0171 | |
| Group change over time∗ | Never-HCT | Reference | ||
| Group change over time∗ | Pre- vs never-HCT | 1.11 (0.97-1.27) | .1222 | |
| Group change over time∗ | Post- vs never-HCT | 0.95 (0.89-1.01) | .1092 | |
| Frailty (Fried frailty index) (=0) | Group | Never-HCT | Reference | |
| Group | Pre- vs never-HCT | 1.22 (0.77-1.93) | .4019 | |
| Group | Post- vs never-HCT | 3.69 (1.48-9.23) | .0051 | |
| Group change over time∗ | Never-HCT | Reference | ||
| Group change over time∗ | Pre- vs never-HCT | 0.93 (0.81-1.06) | .2599 | |
| Group change over time∗ | Post- vs never-HCT | 0.91 (0.81-1.02) | .1022 | |
| Performance status (KPS) (>70) | Group | Never-HCT | Reference | |
| Group | Pre- vs never-HCT | 3.00 (2.01-4.46) | <.001 | |
| Group | Post- vs never-HCT | 0.66 (0.41-1.05) | .0804 | |
| Group change over time∗ | Never-HCT | Reference | ||
| Group change over time∗ | Pre- vs never-HCT | 0.94 (0.88-1.00) | .0464 | |
| Group change over time∗ | Post- vs never-HCT | 1.00 (0.96-1.04) | .9939 |
Adjusted for age group, sex, comorbidity score group per AML composite model, disease type (MDS/myeloproliferative disorder or AML), and induction treatment regimen intensity as appropriate.
Comparisons of quality of life, function, depression, frailty and social activity among recipients and nonrecipients of hematopoietic cell transplantation. (A-E) Generalized estimating equation comparisons of secondary outcomes among never-HCT, pre-HCT, and post-HCT groups final models. ESSI, ENRICHD social support inventory; FFI, Fried frailty index.
Comparisons of quality of life, function, depression, frailty and social activity among recipients and nonrecipients of hematopoietic cell transplantation. (A-E) Generalized estimating equation comparisons of secondary outcomes among never-HCT, pre-HCT, and post-HCT groups final models. ESSI, ENRICHD social support inventory; FFI, Fried frailty index.
Discussion
This US-based multisite prospective observational longitudinal trial showed significant survival benefit associated with HCT. However, this benefit was diminished among all patients and in most of the various prespecified subgroups once we accounted for confounding AML- and patient-specific variables that impacted mortality and, likely, treatment assignment, because these variables disproportionately favored recipients of HCT (Table 4). Specifically, we found no definitive survival advantage for HCT considering either all 692 patients, those aged ≥65 years, those with augmented HCT-CI scores of ≥4, the group with intermediate ELN risk, or among patients who achieved a CR1. Moreover, the impact of HCT on outcome was not demonstrably different among patients who had MRD at CR1 vs those who did not have MRD at CR1, nor was there sufficient evidence to conclude that there was a differential impact of HCT based on induction intensity. Two patient groups, specifically those with the highest AML risks for relapse, sustained benefit from HCT after multivariate model adjustments: patients with ELN-based high risk as well as those who had received transplants in a status beyond CR1 benefited from HCT regardless of their baseline differential risks, when compared with patients who did not receive transplants. The study has >4 years of follow-up, therefore, it is unlikely that any late benefits of allogeneic HCT were missed. To the best of our knowledge, our comparisons are the first to entail the use of validated models to include a comprehensive assessment of patients’ overall health.
Our results might reflect improvement in supportive care and non-HCT therapies and/or the highly selective nature of the current HCT eligibility process. The latter is probably the most important because we accounted for variables that are usually ignored in clinical trials but yet strongly influenced physicians’ decisions about referral to HCT. We acknowledge, however, that it is very difficult to account for all confounding variables in a nonrandomized setting, and the selection bias associated with HCT is very difficult to accurately model.
Our study has the advantage of describing real-world data from a large number of institutions without restrictions to patient accrual. It includes pre- and posttreatment data on QOL, function, and geriatric health; information that is routinely lacking from comparative effectiveness studies in HCT because these data are not generally collected in routine care and are unavailable in most registries and retrospective studies. Recent reports suggest a lack of significant difference in effect estimates when comparing observational and randomized studies, regardless of the specific observational study design.30 Perhaps accordingly, the US Food and Drug Administration currently accepts real-world evidence31,32 in accordance with the 21st Century Cures Act, approved by the US Congress in 2016.33
Nonetheless, we are very aware of the well-recognized limitations of observational studies. As a matter of principle, we do not consider these as substitutes but rather motivators for randomized trials. To date, randomized studies comparing outcomes between HCT and alternative treatments in older patients have been very limited,17 with, to the best of our knowledge, only two published randomized studies. The first study, focusing on patients aged 50 to 70 years, showed lack of benefit of allogeneic HCT from sibling donors after reduced intensity conditioning.34 The second study was from the French Innovative Leukemia Organization and that study suggested improved survival after allogeneic HCT;35 however, it did not account for patient-related factors such as comorbidities, frailty, cognitive health, and other parameters that were included in our study, which, in part, might explain the different results. In particular, in our study after adjusting only for clinical factors (ie, HCT-CI, age, ELN, achievement of CR1, relapsed/refractory disease at time of treatment, and use of intensive induction therapy) the HR for mortality for HCT vs non-HCT in all patients was 0.71 (95% CI, 0.55-0.90; P = .005). However, after additionally adjusting for the factors that measure frailty, cognitive health, comorbidities, and other patient-specific parameters, the HR was increased to 0.85 (95% CI, 0.66-1.09; P = .19). This further shows the importance of taking into account patient risk factors when comparing treatment approaches. We believe the findings of our study support our contention that randomized studies are sorely needed to clarify the role of allogeneic HCT in patients with AML that are older and/or medically infirm, similar to what has recently been done for patients with advanced MDSs.36,37
Indeed, we believe that observational and randomized trials are complementary,38 with the former serving to help motivate and design the latter. It seems that decisions regarding HCT might not be currently evidence based in 2 large groups of older patients with AML. First, patients who are at intermediate risk of relapse and who are apparently in good health, who had been systematically chosen for HCT without knowledge of how they will fare without it, thus, exposing them to potentially unnecessary long-term complications of HCT such as chronic graft-versus-host disease and second malignancies. Second, patients who at high risk of relapse but who are not considered for HCT because they are subjectively considered unfit. This occurs despite reports of continued improvements in allogeneic HCT outcomes7,39 and despite current trials focused on improving evaluation and management of vulnerabilities such as older age, comorbidity burden, and/or frailty (#NCT03992352 and #NCT03870750, respectively). We conducted this 8-year-long study hoping to change these practices.
Patients enrolled in this prospective trial have received 38 different combinations for induction therapy (supplemental Table 2). No patient was excluded from the study based on the type of initial therapy. This was done to ensure that we account for current practice and to minimize the impact of initial therapy on study outcomes. Clearly, our study predated the approval of venetoclax. Randomization to venetoclax plus azacitidine rather than azacitidine alone improved median survival from 9 to 15 months;40 likewise, randomization to venetoclax plus low-dose Ara-c rather than low-dose Ara-c alone increased median survival from 4.1 to 8.4 months.41 Although, when compared with standard less-intensive therapy, these improvements are statistically significant, they have been less obviously clinically significant. Hence, it does not seem that introduction of venetoclax changed practice in a way to obviate the need for a randomized trial to better understand the role of allogeneic HCT in management of patients with AML that are older and/or medically infirm.
Taking into consideration our real-world results as well as the current availability of different choices for donor source,42 we suggest 3 possible randomized, rather than biologic assignment, studies (Figure 3). The first would randomize older patients without MRD at intermediate risk between immediate vs delayed HCT. In addition to our results, a report from the United Kingdom suggested that for patients at intermediate risk, similar survival can be achieved by delaying HCT until relapse.43 Other studies found that patients who achieve a MRD-negative CR have a relapse probability of only ∼30% even without HCT in CR1.44 This relatively low rate suggests that it is possible to spare patients with MRD-negative CR and ELN intermediate-risk group HCT complications. A second potential trial might address whether older patients with MRD before a proposed HCT should proceed directly to HCT followed by MRD-directed treatment, or first receive treatment intended to reduce MRD. The third possible trial would focus on patients that are older and apparently unfit, who are currently considered to be at a relatively higher risk of morbidity and mortality post-HCT, but who are also at very high risk of relapse without HCT because they are in the ELN adverse risk group or are in CR2. The use of an outcome-adaptive randomization approach to ensure randomization of more patients to the demonstrated more successful treatment option would be of interest for these patient groups.45,46 Finally, consideration for maintenance treatment after the nontransplant arm might be beneficial to further improve outcomes.
Before HCT, patients who were selected by their treating physicians to receive HCT had better function, social support, social activity, less depression, and better performance status compared with those who were not selected to receive HCT. These findings suggest that seemingly fitter patients with a higher perceived chance of cure may have been preferentially identified by physicians to receive HCT. Yet, our results show such QOL and geriatric outcomes were similar among patients post-HCT vs never-HCT, suggesting that the benefits of improved general health before transplant are lost once HCT is given. These results imply that HCT could come at a relatively high price by early deterioration in general health. This is in alignment with the knowledge that health impairments after HCT are linked to chronic graft-versus-host disease that tend to last for a median of 30 months (range, 5.5-119 months).8 Lastly, this study showed that patients who did or did not receive HCT had similar goals of therapy and similar expectations from therapy, that is, cure. On the contrary, physicians believed that HCT offered better chances of cure, yet at the same time, they appear to have selected the “better” patients for this treatment. This further highlights the need for thoughtful studies to better understand the role of HCT in patients with AML that are vulnerable. Furthermore, the discrepancies between physicians’ perspectives and patients’ expectations of outcomes raises the need for future studies to better understand these discrepancies. This could include prospective monitoring of communications between patients and physicians in the clinic setting, voice or video recording of conversations, and receiving and providing feedback from patients to further understand how the gap can be narrowed.
In conclusion, this large observational study provides evidence that the current belief that HCT is preferable to no HCT is subject to a large degree of contradiction, making the assessment of HCT difficult outside a randomized clinical trial. This is owing to, in large part, the selection bias associated with the decision to receive or not receive HCT and/or the lack of consideration of patients/overall health using modern means to examine vulnerabilities. We believe the role of HCT needs to be formally studied in randomized trials for the groups, described herein, to definitively address the efficacy of HCT in AML. This is particularly of importance given the continued improvements in HCT outcomes, the better current understanding of patient health risks, the novel trials directed toward improving resilience to HCT, and the results of this study that showed equal desires for cure by those who were and were not selected by their physicians to receive an HCT.
Acknowledgments
The authors are grateful to all research nurses and data coordinators for implementation of protocols. The authors also thank the study staff and administrative staff for their assistance with manuscript preparation. The authors are grateful to the many physicians, nurses, physician assistants, nurse practitioners, pharmacists, and support staff who cared for our patients, and to the patients who allowed us to care for them and who participated in our ongoing clinical research. The authors are also grateful to Helen Crawford, Sophie Fluent, and Indira Dastan for their help in assembling data and preparing the manuscript.
This study was, in part, funded by a Patient-Centered Outcome Research Institute award (CE-1304-7451); in part, by a Research Scholar grant from the American Cancer Society (grant RSG-13-084-01-CPHPS); and, in part, supported by an American Society of Hematology Bridge Award.
Funding organizations had absolutely no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or in the preparation, review, or approval of the manuscript. The statements and findings in this article are solely the responsibility of the authors and do not necessarily represent the views of any funding organization, neither the Patient-Centered Outcomes Research Institute, its Board of Governors, or Methodology Committee; nor the American Cancer Society; nor the America Society of Hematology.
Elihu H. Estey died on 8 October 2021.
Authorship
Contribution: M.L.S., T.A.G., and B.E.S. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis; M.L.S. and E.H.E. conceptualized and designed the study; M.L.S., T.A.G., A.T.G., M.A.S., B.C.M., E.S.W., P.J.S., K.A., S.L., M.R.B., D.A.R., T.M.W., J.K., J.S., M.G., K.K., W.M.L., L.E.O., P.S.B., J.S.M., S.J.L., B.M.S., F.R.A., and E.H.E. performed data acquisition, analysis, and/or interpretation and critically reviewed the manuscript for important intellectual content; B.E.S., T.A.G., W.M.L., and L.E.O. performed statistical analysis; M.L.S. obtained funding and drafted the manuscript; and M.L.S. and E.H.E. supervised the study.
Conflict-of-interest disclosure: M.L.S. reports consultancy and receiving honorarium from Jazz Pharmaceuticals. A.T.G. reports grants from Patient-Centered Outcome Research Institute, during the conduct of the study and personal fees from AstraZeneca, Incyte, and CTI BioPharma, outside the submitted work. P.J.S. serves as a member of the clinical research support/data safety monitoring board of Amgen Inc, Aptevo, Cantex, and Chimerix; serves on the scientific advisory boards of, consultants for, or serves as expert witness for AbbVie Inc, Agios Inc, and Jazz Pharmaceuticals Inc; and reports employment/governing board member, patent, equity, or royalty for panel member or dependent of JSK Therapeutics and Lone Star Thiotherapies Takeda. S.L. received honoraria from Daiichi-Sankyo, Pfizer, Bristol-Myers Squibb, Acceleron, and Agios and receives research funding from Onconova, Kura, Hoffman La Roche, Ariad, and Biosight. T.M.W. received research funding from Janssen and consults for Carevive Systems and Seattle Genetics. P.S.B. reports research support (to institution) from AbbVie, Bristol-Myers Squibb, Cardiff Oncology, Glycomimetics, JW Pharmaceutical, Novartis, Pfizer, SecuraBio, and Tolero; serves in an adviser role for Accordant Health Services/Caremark; served as speaker for France Foundation (CME); and consults for CVS Caremark and McKesson. S.J.L. reports serving on the National Marrow Donor Program Board of Directors. The remaining authors declare no competing financial interests.
Correspondence: Mohamed L. Sorror, Clinical Research Division (D5-285), Fred Hutchinson Cancer Center, 1100 Fairview Ave North, Seattle, WA 98109-1024; e-mail: msorror@fredhutch.org.
References
Author notes
This is not an intervention clinical trial. There is no data sharing plan for this study because the requirements by the International Committee of Medical Journal Editors are not applicable to this study.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal