Key Points
Forty percent of patients with iTTP relapse after 5 years of follow-up.
Preemptive anti-CD20 therapy is efficacious in 96% of ADAMTS13 relapses regardless of relapse frequency to achieve ADAMTS13 levels of >20%.
Abstract
Disease relapse is recognized as a risk in immune-mediated thrombotic thrombocytopenic purpura (iTTP) after treatment of the acute presenting episode. Identification of patients at risk of relapse and its patterns are yet to be clearly established. We reviewed patients with iTTP having had >3 years of follow-up over 10 years in the United Kingdom to identify patient characteristics for relapse, assess relapse rates and patterns, and response to anti-CD20 therapy in those with a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS13) relapses (ADAMTS13 activity of <20% without thrombocytopenia). We identified 443 patients demonstrating relapse rates of 40% at 5-year follow-up. At 10-year follow-up, no difference in relapse was observed irrespective of whether rituximab was used at acute presentation (P = .39). Black Caribbean ethnicity increased the risk of disease relapse in the British population. There was a distinct population of patients (6%) that relapsed early with subsequent frequent relapses occurring on average within 2 years (average time to relapse in subgroup, 1.7 years). Overall, nearly 60% of relapses described were ADAMTS13 relapses, with subsequent treatment reducing the risk of progression to clinical relapses. We demonstrate that iTTP diagnosed in the latter part of the study period had lower rates of clinical relapses (22.6% vs 11.1%, P = .0004) with the advent of regular monitoring and preemptive rituximab. In ADAMTS13 relapses, 96% responded to anti-CD20 therapy, achieving ADAMTS13 activity of >20%. Anti-CD20 therapy was demonstrated to be an effective long-term treatment regardless of relapse pattern and there was no loss of this treatment response after subsequent treatment episodes.
Medscape Continuing Medical Education online

In support of improving patient care, this activity has been planned and implemented by Medscape, LLC and the American Society of Hematology. Medscape, LLC is jointly accredited with commendation by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.0 MOC points in the American Board of Internal Medicine's (ABIM) Maintenance of Certification (MOC) program. Participants will earn MOC points equivalent to the amount of CME credits claimed for the activity. It is the CME activity provider's responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at https://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 321.
Disclosures
Laurie Barclay, MD, freelance writer and reviewer, Medscape, LLC, has disclosed the following relevant financial relationships: stock, stock options, or bonds: AbbVie Inc. (former).
Learning objectives
Upon completion of this activity, participants will:
Describe patient characteristics linked to Immune-mediated thrombotic thrombocytopenic purpura (iTTP) relapse and the rates and patterns of relapse, according to a study of 443 patients with iTTP relapses having > 3 years’ follow-up over a 10-year period in the United Kingdom
Determine clinical features during ADAMTS13 relapses and response to anti-cluster of differentiation (CD)20 therapy in persons with ADAMTS13 relapses, according to a study of 443 patients with iTTP relapses having > 3 years’ follow-up over a 10-year period in the United Kingdom
Identify clinical implications of patient characteristics linked to iTTP relapse, relapse rates and patterns, and response to anti-CD20 therapy in persons with ADAMTS13 relapses, according to a study of 443 patients with iTTP having > 3 years’ follow-up over a 10-year period in the United Kingdom
Release date January 19, 2023; Expiration date: January 19, 2024
Introduction
Immune-mediated thrombotic thrombocytopenic purpura (iTTP) is a rare, life-threatening condition associated with significant morbidity and mortality if not treated promptly. Immunoglobulin G (IgG) autoantibodies cause an acquired deficiency of the von Willebrand factor cleaving protein, a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS13) by mediating either an increased clearance of the protein or inhibition of its enzymatic function.1 A key component of treatment in the acute phase of the condition is the early eradication of B lymphocytes producing anti-ADAMTS13 antibodies.2 The use of rituximab, a chimeric anti-CD20 monoclonal antibody, has become established in iTTP over the last decade.2-4
Relapse in iTTP can present as either a “clinical relapse” with an associated thrombocytopenia (platelets <150 × 109/L) or an “ADAMTS13 relapse” with ADAMTS13 activity levels of <20%, without thrombocytopenia or microangiopathic hemolytic anemia.5 ADAMTS13 relapse usually progresses to clinical relapse if treatment is not initiated, with an increased risk of morbidity and mortality associated with the latter.5,6 Relapses of iTTP occur in 15% to 30% of patients by 2 years with rates increasing with the duration of follow-up.5-8 The use of rituximab has been shown to prolong the time to the first episode of disease relapse in the short term, compared with those who did not have rituximab during their acute presentation.2,5,9-11 As such, patients with iTTP require long-term monitoring of ADAMTS13 levels to allow for early recognition of relapsing disease.
At present, the mechanism for disease relapse of iTTP has not yet been clearly elucidated, but the presence of CD19+ B lymphocytes is seen at the time of relapse.5,12 Although B-cell recovery is recognized as a preceding event to relapse, not all patients that have B-cell recovery after immunosuppression develop relapses of iTTP. There are limited clinical parameters that can be used to identify or prognosticate patients for risk of relapse, and therefore, guide intensity of ADAMTS13 surveillance. Potential risk factors for relapse in 1 case series were age of <25 years, non-O blood group, having previous episodes of TTP relapse, and not receiving rituximab at initial presentation.6 Patients having 1 relapse episode are at an increased risk of having subsequent relapses: 25% of patients at 1 year and 49% at 3 years.5,9,10
Preemptive rituximab has been shown by various groups as being a treatment option for ADAMTS13 relapse, with 94% of patients achieving normalization of ADAMTS13 levels.5,10,13 After anti-CD20 treatment, B lymphocytes are initially significantly depleted but become detectable after 6 to 9 months with normalized levels after 18 months.2,10,12 Relapse-free survival is prolonged in the short term by rituximab but after 5 years, these rates show no difference.9
At present, national registry data has been limited by small cohort sizes or short duration of follow-up in order to detect meaningful data on relapse in iTTP. We aimed to review the long-term follow-up of patients with iTTP from a large national, multicenter registry over a 10-year period with a minimum of 3 years of follow-up per patient. We evaluated the features of iTTP relapse in all identified patients and reviewed the responses to anti-CD20 antibody treatment in patients that had ADAMTS13 relapses.
Methods
Material and methods
A retrospective review of patients registered to the United Kingdom (UK) Thrombotic Thrombocytopenic Purpura (TTP) registry, a nationwide registry based at 30 sites across the country, was performed. The UK TTP registry has been approved by appropriate ethics committee review (a database and biobank of UK TTP: Multicentre Research Ethics Committee: 08/H0810/54). The registry database was interrogated using REDCap (Nashville, TN). Cases registered between 1 January 2009 and 31 December 2017 were reviewed, allowing for a minimum of 3 years of follow-up for all patients. As part of registry data collection, information on the acute presentation of TTP, annual follow-up, and additional treatment episodes are recorded.
Definitions of relapse and response
Relapses were assessed by the treating clinician at each center and categorized as either (1) clinical relapse, defined by a platelet count of <150 × 109/L attributable to TTP requiring emergency treatment or (2) ADAMTS13 relapse, defined as an ADAMTS13 activity level of <20% without thrombocytopenia; however, some cases with higher ADAMTS13 activity levels may have been treated if there were clinical concerns of rapid disease relapse, which were also included in this analysis.11 Relapses were reviewed retrospectively by the authors according to platelet count and ADAMTS13 activity. In the sections to follow, “relapse” alone, is defined as both clinical relapse and ADAMTS13 relapse unless otherwise defined.
Regular patient follow-up was undertaken to monitor treatment response, typically weekly during therapy and until remission was achieved. Subsequently, frequency of ADAMTS13 monitoring was guided by the treating clinician. Remission after treatment was defined as an ADAMTS13 activity of ≥20%; complete remission (CR) was defined as ≥60%, a partial remission (PR) was 20% to 59%, and minimal response was <20% after completion of treatment.11 Follow-up was censored on the date of registry review (17 September 2021), date of last registry follow-up, or at death.
Treatment of acute and relapse episodes
Acute presentations and clinical relapses were treated with plasma exchange using solvent detergent fresh frozen plasma until platelet counts were sustained at >150 × 109/L. Steroids were given to patients as per local practice by treating clinicians. As the last inclusion date was December 2017, caplacizumab was not established in the acute treatment of TTP in this patient cohort apart from in clinical studies or compassionate use.
ADAMTS13 relapses were treated with preemptive rituximab when activity levels were <20%, although higher thresholds may have been used if there was clinical concern, for example, previous rapid progression to relapse in the past or severe disease phenotype in acute presentation episode.
Rituximab use was dependent upon the clinicians’ choice with dosing strategies reported. For acute presentation episodes or clinical relapses, a dose strategy of 375 mg/m2 once or twice weekly for a total of 4 to 8 doses was used. For ADAMTS13 relapses, rituximab was typically given as a fixed dose of 200 g or 500 mg, or a dose of 375 mg/m2 once weekly for 4 weeks. All dosing regimens were included in this study.
Assays and laboratory testing
ADAMTS13 activity assays were different according to each site, using either a fluorescence resonance energy transfer, collagen binding assay, or a chemiluminescent immunoassay. Circulating B lymphocytes were assessed by Aquios CL flow cytometry (Beckman Coulter) using anti-CD19 antibodies (a B-lymphocyte marker) to assess suppression by anti-CD20 treatment.
Follow-up and outcome measures
The primary outcome was the frequency and type of relapse during long-term follow-up of iTTP according to the definitions described previously. Patients were defined as having “frequent disease relapses” if the average time between treatments was <2 years (ie, relapse episode ≥0.5 per year), or as having “infrequent disease relapses” if the average time between treatments was >2 years (ie, relapse episode <0.5 per year). Secondary outcomes were (1) the time from acute presentation to first relapse, (2) new symptom burden in those with an ADAMTS13 relapse preceding treatment, and (3) hemolytic markers, ADAMTS13 activity, and CD19+ B-lymphocyte levels before and after receiving anti-CD20 treatment for ADAMTS13 relapse. Safety data of anti-CD20 treatments were collected with rates of infection, hypogammaglobulinemia and hypersensitivity-associated reactions.
Patients were followed up at least once a year as part of the UK TTP registry, although more frequent follow-up occurred to assess treatment response after disease relapse. ADAMTS13 relapse was confirmed by 2 consecutive ADAMTS13 activity tests of <20% within 1 to 2 weeks. During treatment for ADAMTS13 relapse, patients were typically reviewed weekly until ADAMTS13 activity was maintained at >30%, at which point the time between follow-up was decided by the treating clinician.
Statistical analysis
For normally distributed data, 2-sample t tests and analysis of variance tests were used to assess differences between groups. For nonparametric data, Mann-Whitney U test was used for unpaired data and Wilcoxon signed-rank test for paired data. χ2 test was used to compare frequencies between groups. Log-rank test was used to assess differences in relapse-free survival between patients according to whether rituximab was given during their acute presentation episode. An α value of <0.05 was considered statistically significant. Statistical analysis was performed on Prism GraphPad (San Diego, CA).
Results
Study population
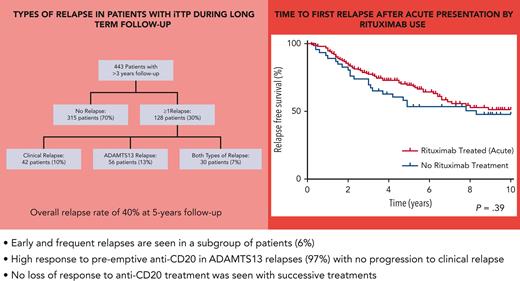
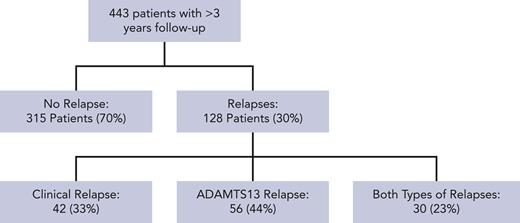
Seven hundred ninety-five patients were registered on the UK TTP registry at the time of review, with 443 patients having a minimum of 3 years follow-up and adequate clinical information to assess for disease relapse. Twelve patients died during the reviewed follow-up period. Three hundred twelve patients (70%) had no relapse episodes reported and 128 patients (30%) had a minimum of 1 relapse episode. Figure 1 shows the nature and type of disease relapses in this patient cohort. There was a median overall follow-up of 8.6 years in this cohort (interquartile range [IQR], 5.7-10.7 years; range, 3.0-37.7 years).
Consort flow diagram of relapses in patients with iTTP during follow-up.
There was a total of 307 reported relapses in 443 patients, with 107 (35%) being clinical relapses and 200 (65%) being ADAMTS13 relapses. There was a median of 2 relapses (IQR, 1-3 episodes; range, 1-12 episodes) during the follow-up period for each patient that relapsed.
Outcomes from the time from acute presentation to first relapse in iTTP
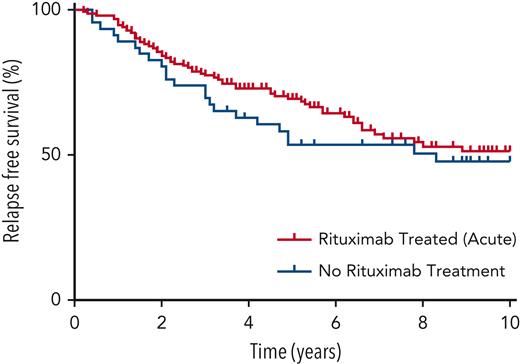
The relapse rate, inclusive of both ADAMTS13 and clinical relapses, after the acute presentation episode was 4% at 1 year, 15% at 2 years, 24% at 3 years, 33% at 4 years, and 40% at 5 years. Figure 2 shows the time from acute presentation to first relapse according to whether rituximab was given or not during the acute presentation episode with no significant difference between the 2 groups at 10-year follow-up (log-rank test, P = .39).
Kaplan-Meier survival plot of patients’ relapse-free survival after acute presentation episode.
Kaplan-Meier survival plot of patients’ relapse-free survival after acute presentation episode.
The clinical features and treatments of patients that relapsed after acute presentation are compared with those that did not relapse are shown in Table 1. Rituximab was given to 249 out of 443 (55%) of the total patient cohort. The dosing of rituximab used was 375 mg/m2 for 210 patients (84%), flat dose of 500 mg for 6 patients (2%), and other/unknown dosing for 33 patients (13%). Risk of relapse was not influenced by the type of immunosuppressive treatment given during the acute presentation episode. There was a greater proportion of patients of Black Caribbean ethnicity that relapsed than those that did not (17% vs 7%; P = .017; odds ratio, 2.66; 95% confidence interval, 1.42-5.00). However, there was no difference in the proportion of patients of Black African ethnicity that relapsed compared with those that did not (9.9% vs 10.2%, P = .92). Other than ethnicity, there were no other phenotypic risk factors identified in this patient cohort. There was a significantly higher proportion of reversible/treatable causes of iTTP (drug-induced, pancreatitis, HIV, and other infections) in patients that did not relapse (16%) than those that relapsed (8%; P = .022; odds ratio, 0.46; 95% confidence interval, 0.23-0.95).
Clinical features and treatment of patients according to relapse status after acute presentation
| Clinical feature . | No relapse (n = 312, 70.4%) . | Relapse (n = 131, 29.6%) . | P . |
|---|---|---|---|
| Duration of follow-up (IQR), y | 8.4 (5.7-10.4) | 8.7 (5.6-10.9) | .39 |
| Age at presentation, y | 45.0 | 45.1 | .49 |
| Age <25 y at presentation, n (%) | 27 (8.7) | 11 (8.4) | .93 |
| Gender (male/female), n (%) | 79 (25.3)/233 (74.7) | 43 (32.8)/88 (67.2) | .11 |
| Blood counts at presentation | |||
| Hemoglobin, g/L | 82 | 95 | <.001 |
| Platelets, ×109/L | 20 | 34 | <.001 |
| Absolute reticulocytes, ×109/L | 158 | 159 | .95 |
| Ethnicity, n (%) | .019 | ||
| Caucasian | 197 (63.1) | 69 (52.7) | |
| Black African | 32 (10.2) | 13 (9.9) | |
| Black Caribbean | 22 (7.1) | 22 (16.8) | |
| Asian | 26 (8.3) | 14 (10.7) | |
| Mixed | 5 (1.6) | 4 (3.1) | |
| Other | 6 (1.9) | 3 (2.3) | |
| Unknown | 24 (7.7) | 6 (4.6) | |
| Associated conditions, n (%) | <.001 | ||
| Idiopathic | 202 (64.7) | 87 (66.4) | |
| HIV | 17 (5.4) | 3 (2.3) | |
| Drug-induced | 5 (1.6) | 0 (0) | |
| Other autoimmune disease | 51 (16.3) | 27 (20.6) | |
| Pancreatitis | 11 (3.5) | 0 (0) | |
| Pregnancy | 10 (3.2) | 8 (6.1) | |
| Other | 16 (5.1) | 7 (5.3) | |
| Treatment at acute presentation, n (%) | |||
| Corticosteroid | 267 (85.6) | 116 (88.5) | .97 |
| Rituximab | 169 (54.2) | ||
| Mycophenolate mofetil | 2 (0.6) | 0 | |
| Caplacizumab | 7 (2.2) | 3 (2.3) |
| Clinical feature . | No relapse (n = 312, 70.4%) . | Relapse (n = 131, 29.6%) . | P . |
|---|---|---|---|
| Duration of follow-up (IQR), y | 8.4 (5.7-10.4) | 8.7 (5.6-10.9) | .39 |
| Age at presentation, y | 45.0 | 45.1 | .49 |
| Age <25 y at presentation, n (%) | 27 (8.7) | 11 (8.4) | .93 |
| Gender (male/female), n (%) | 79 (25.3)/233 (74.7) | 43 (32.8)/88 (67.2) | .11 |
| Blood counts at presentation | |||
| Hemoglobin, g/L | 82 | 95 | <.001 |
| Platelets, ×109/L | 20 | 34 | <.001 |
| Absolute reticulocytes, ×109/L | 158 | 159 | .95 |
| Ethnicity, n (%) | .019 | ||
| Caucasian | 197 (63.1) | 69 (52.7) | |
| Black African | 32 (10.2) | 13 (9.9) | |
| Black Caribbean | 22 (7.1) | 22 (16.8) | |
| Asian | 26 (8.3) | 14 (10.7) | |
| Mixed | 5 (1.6) | 4 (3.1) | |
| Other | 6 (1.9) | 3 (2.3) | |
| Unknown | 24 (7.7) | 6 (4.6) | |
| Associated conditions, n (%) | <.001 | ||
| Idiopathic | 202 (64.7) | 87 (66.4) | |
| HIV | 17 (5.4) | 3 (2.3) | |
| Drug-induced | 5 (1.6) | 0 (0) | |
| Other autoimmune disease | 51 (16.3) | 27 (20.6) | |
| Pancreatitis | 11 (3.5) | 0 (0) | |
| Pregnancy | 10 (3.2) | 8 (6.1) | |
| Other | 16 (5.1) | 7 (5.3) | |
| Treatment at acute presentation, n (%) | |||
| Corticosteroid | 267 (85.6) | 116 (88.5) | .97 |
| Rituximab | 169 (54.2) | ||
| Mycophenolate mofetil | 2 (0.6) | 0 | |
| Caplacizumab | 7 (2.2) | 3 (2.3) |
Patterns of relapse
In this cohort, relapse was not seen within 6 months of diagnosis and only occurred in a small number of patients by 1 year (4% of patient cohort, n = 17/443). The proportion of patients that had frequent disease relapses (≥0.5 per year) was 28 out of 443 (6% of total cohort) compared with 103 out of 443 (23%) who had infrequent disease relapses (<0.5 per year). The clinical features of these patients are described in Table 2. The median time to first relapse was shorter in those with frequent relapse episodes compared with those with infrequent relapse (median, 1.7 vs 4.9 years). In this cohort, relapse frequency was different according to ethnicity, with a higher proportion of patients of Black Caribbean ethnicity having frequent relapses in comparison with infrequent relapses (21% vs 11%, P < .001).
Clinical features of patients according to relapse frequency
| Demographics . | Frequent relapses (n = 28) . | Infrequent relapses (n = 103) . | P . |
|---|---|---|---|
| Duration of follow-up (IQR), y | 7.8 (4.4-9.5) | 9.1 (6.5-11.1) | .02 |
| Age at initial presentation, y (range) | 48.9 (38.4-53.2) | 44.0 (32.8-54.3) | .36 |
| Age <25 y at presentation, n (%) | 2 (7.1) | 9 (8.7) | .27 |
| Gender (male/female), n (%) | 9 (32.1)/19 (67.9) | 34 (33.0)/69 (67.0) | .93 |
| Ethnicity, n (%) | <.001 | ||
| Caucasian | 13 (46) | 56 (54) | |
| Black African | 5 (17) | 7 (7) | |
| Black Caribbean | 6 (21) | 11 (11) | |
| Asian | 2 (7) | 12 (12) | |
| Mixed | 1 (4) | 3 (3) | |
| Other | 1 (4) | 2 (2) | |
| Unknown | 0 (0) | 6 (6) | |
| Treatment at acute presentation, n (%) | |||
| Corticosteroid | 24 (86) | 86 (83) | |
| Rituximab | 16 (57) | 60 (58) | |
| Mycophenolate mofetil | 0 (0) | 1 (1) | |
| Caplacizumab | 0 (0) | 3 (3) |
| Demographics . | Frequent relapses (n = 28) . | Infrequent relapses (n = 103) . | P . |
|---|---|---|---|
| Duration of follow-up (IQR), y | 7.8 (4.4-9.5) | 9.1 (6.5-11.1) | .02 |
| Age at initial presentation, y (range) | 48.9 (38.4-53.2) | 44.0 (32.8-54.3) | .36 |
| Age <25 y at presentation, n (%) | 2 (7.1) | 9 (8.7) | .27 |
| Gender (male/female), n (%) | 9 (32.1)/19 (67.9) | 34 (33.0)/69 (67.0) | .93 |
| Ethnicity, n (%) | <.001 | ||
| Caucasian | 13 (46) | 56 (54) | |
| Black African | 5 (17) | 7 (7) | |
| Black Caribbean | 6 (21) | 11 (11) | |
| Asian | 2 (7) | 12 (12) | |
| Mixed | 1 (4) | 3 (3) | |
| Other | 1 (4) | 2 (2) | |
| Unknown | 0 (0) | 6 (6) | |
| Treatment at acute presentation, n (%) | |||
| Corticosteroid | 24 (86) | 86 (83) | |
| Rituximab | 16 (57) | 60 (58) | |
| Mycophenolate mofetil | 0 (0) | 1 (1) | |
| Caplacizumab | 0 (0) | 3 (3) |
To investigate the effect of modern TTP management (plasma exchange, steroids, and rituximab), the type of relapse for each patient was compared in those presenting up to 2012 (n = 217) with those presenting after 2012 (n = 226). In total, 151 out of 217 (70%) diagnosed in and before 2012 vs 164 out of 226 (73%) diagnosed after 2012 had no episodes of relapse (P = .48). There was a statistically significant reduction in patients having at least 1 episode of clinical relapse from 49 out of 217 (23%) when diagnosed before 2012 compared with 25 out of 226 (11%) diagnosed after 2012. This contrasted with an increase in ADAMTS13 relapses from 17 out of 217 (8%) in the earlier time period in comparison with 37 out of 226 (16%) in the later period (P = .0004). The number of relapse episodes per patient year was similar in both time periods at 8 and 9 relapse episodes per 100 patient years, respectively, in all patients.
Clinical features during ADAMTS13 relapse
Patients were assessed for symptoms and clinical features at the time of ADAMTS13 relapse. A total of 200 ADAMTS13 relapse episodes in 89 patients were reviewed. Table 3 describes the laboratory parameters of these patients at the time of ADAMTS13 relapse (all relapse episodes included), showing an absence of thrombocytopenia and frank hemolysis but normalized levels of CD19+ lymphocytes before treatment.
Laboratory parameters in patients receiving anti-CD20 treatment for ADAMTS13 relapse of TTP
| Blood parameter . | Before treatment (range) . | Remission (range) . | Δ Change (range) . | P . |
|---|---|---|---|---|
| Hemoglobin (g/L) | 133 (122-145) | 136 (125-150) | 2 (−2 to 8) | .19 |
| Reticulocytes (×109/L) | 60.2 (46.1-82.3) | 57.1 (45.0-79.8) | −0.7 (−12.3 to 9.3) | .41 |
| Platelets (×109/L) | 254 (203-297) | 272 (230-308) | 18 (0-43) | <.001 |
| Lactate dehydrogenase (IU/L) | 209 (179-243) | 191 (171-215) | −19 (−43 to 7) | .0008 |
| ADAMTS13 activity (%) | 9.2 (5.0-14.0) | 86.6 (66.2-100.5) | 76.0 (60.6-89.0) | <.001 |
| Absolute no. CD19+ lymphocytes (×109/L) | 0.2 (0.1-0.3) | 0 (0-0) | −0.1 (−0.3 to −0.1) | <.001 |
| CD19+ lymphocyte proportion (%) | 10.8 (6.5-16.4) | 0.1 (0-0.2) | −11 (−15.1 to 6.6) | <.001 |
| Blood parameter . | Before treatment (range) . | Remission (range) . | Δ Change (range) . | P . |
|---|---|---|---|---|
| Hemoglobin (g/L) | 133 (122-145) | 136 (125-150) | 2 (−2 to 8) | .19 |
| Reticulocytes (×109/L) | 60.2 (46.1-82.3) | 57.1 (45.0-79.8) | −0.7 (−12.3 to 9.3) | .41 |
| Platelets (×109/L) | 254 (203-297) | 272 (230-308) | 18 (0-43) | <.001 |
| Lactate dehydrogenase (IU/L) | 209 (179-243) | 191 (171-215) | −19 (−43 to 7) | .0008 |
| ADAMTS13 activity (%) | 9.2 (5.0-14.0) | 86.6 (66.2-100.5) | 76.0 (60.6-89.0) | <.001 |
| Absolute no. CD19+ lymphocytes (×109/L) | 0.2 (0.1-0.3) | 0 (0-0) | −0.1 (−0.3 to −0.1) | <.001 |
| CD19+ lymphocyte proportion (%) | 10.8 (6.5-16.4) | 0.1 (0-0.2) | −11 (−15.1 to 6.6) | <.001 |
In total, 120 (60%) ADAMTS13 relapse episodes had no preceding symptoms. The most commonly described new-onset symptoms were headaches (n = 40, 20%) and lethargy (n = 25, 13%), with various neurological features seen in 58 patients (29%). Other less frequently recorded features were visual disturbances (n = 15, 8%), gastrointestinal disturbance (n = 8, 4%), acute confusional state (n = 7, 4%), mucocutaneous bleeding (n = 6, 3%), and collapse (n = 3, 2%). Thrombotic events were recorded at the time of ADAMTS13 relapse in 3 patients (1 ischemic stroke, 1 transient ischemic attack, and 1 myocardial infarction; 2%). Intracranial hemorrhage was seen in 1 patient without thrombocytopenia.
Responses to anti-CD20 treatment for ADAMTS13 relapse
The most common treatment received by patients for ADAMTS13 relapse was rituximab in 181 (91%) patients, although other anti-CD20 monoclonal antibodies were used: obinutuzumab in 1 (1%), ofatumumab in 15 (8%), and unknown in 3 (2%). Most of the patients received 4 doses of rituximab (180/200, 80%). The dosing of rituximab during treatment episodes were: 375 mg/m2, 35 patients (19%); fixed dose of 500 mg, 72 patients (40%); fixed dose of 200 mg, 42 patients (23%); combination of doses, 21 patients (12%); and other/unknown dosing, 10 patients (6%).
In 177 treatment episodes for ADAMTS13 relapse with available results, disease remission (ADAMTS13 activity ≥ 20%) was achieved in 171 out of 177 (97%) treatment episodes in 85 out of 89 (96%) of patients. CR (ADAMTS13 activity ≥60%) was achieved in 149 out of 177 (84%) and PR (ADAMTS13 activity 20%-59%) in 22 out of 177 (12%). However, minimal ADAMTS 13 activity response (ADAMTS13 activity <20%, after treatment) was seen in 6 out of 177 (3%) treatment episodes. Table 3 shows the change in laboratory parameters after anti-CD20 antibody treatment. The median time to achieve remission (ADAMTS13 activity ≥20%, after treatment) was 21 days (IQR, 14-21 days). After achievement of remission, ADAMTS13 activity after treatment continued to increase, with a median time to peak ADAMTS13 activity of 91 days (IQR, 61-125 days). All the patients that achieved remission with anti-CD20 therapy initially achieved further remission with subsequent anti-CD20 therapy treatments. Table 4 shows the demographics, anti-CD20 treatment dosing, and posttreatment immunosuppression for each treatment episode according to treatment response. The treatment response to ADAMTS13 relapses was compared between those with frequent relapses and those with infrequent relapses of TTP. Of 77 episodes in 23 patients with frequent relapses and 123 episodes in 69 patients with infrequent relapses, there were similar levels of ADAMTS13 remission, at 78% vs 77%, respectively (CR, 66% vs 66%; PR, 12% vs 11%; and minimal response, 6% vs 7%; P = .94). The time to remission (ADAMTS13 activity ≥20%) in those with frequent relapses was 15 days (IQR, 11-23 days), whereas in infrequent relapses it was 21 days (IQR, 14-34 days; P = .11). At the end of treatment, there was no significant difference between those with frequent relapses compared with those with infrequent relapses in the reduction in absolute CD19+ lymphocyte counts (median, −0.2 × 106/L vs −0.2 × 106/L; P = .093) and the reduction in CD19+ lymphocytes proportion of total lymphocytes (median, −9.9% vs −12.4%; P = .23). In total, 108 treatment episodes for ADAMTS13 relapses had subsequent treatment, with a median time between treatments of 1.5 years (IQR, 1.1-2.3 years) of which 54 (50%) were recognized in patients with frequent relapses. At time of review, 78 treatment episodes had yet to relapse, with a median follow-up of 2.1 years (IQR, 1.1-3.4 years), of which 22 (28%) were patients with frequent relapses.
Clinical demographics, anti-CD20 therapy dosing, and CD19+ lymphocytes according to treatment response by ADAMTS13 activity
| Clinical feature . | Complete response (n = 149) . | Partial response (n = 22) . | Minimal response (n = 6) . | P . |
|---|---|---|---|---|
| Age at treatment, y | 46.6 | 55.8 | 59.0 | <.001 |
| Gender (male/female), n | 45/104 | 8/14 | 4/2 | .16 |
| Ethnicity, n | .09 | |||
| Caucasian | 50 | 8 | 2 | |
| Black African | 16 | 4 | 4 | |
| Black Caribbean | 36 | 3 | 0 | |
| Asian | 21 | 3 | 0 | |
| Mixed | 2 | 0 | 0 | |
| Other | 6 | 0 | 0 | |
| Unknown | 18 | 4 | 0 | |
| Median no. of rituximab doses | 4 | 4 | 4 | — |
| Median dose of rituximab per cycle (mg) | 500 | 500 | 500 | — |
| Median total dose of rituximab (mg) | 2000 | 1500 | 2000 | — |
| Absolute no. CD19+ lymphocytes (×109/L) | 0.001 | 0.002 | 0 | .05 |
| CD19+ lymphocyte proportion (%) | 0.05 | 0.07 | 0.02 | .33 |
| Clinical feature . | Complete response (n = 149) . | Partial response (n = 22) . | Minimal response (n = 6) . | P . |
|---|---|---|---|---|
| Age at treatment, y | 46.6 | 55.8 | 59.0 | <.001 |
| Gender (male/female), n | 45/104 | 8/14 | 4/2 | .16 |
| Ethnicity, n | .09 | |||
| Caucasian | 50 | 8 | 2 | |
| Black African | 16 | 4 | 4 | |
| Black Caribbean | 36 | 3 | 0 | |
| Asian | 21 | 3 | 0 | |
| Mixed | 2 | 0 | 0 | |
| Other | 6 | 0 | 0 | |
| Unknown | 18 | 4 | 0 | |
| Median no. of rituximab doses | 4 | 4 | 4 | — |
| Median dose of rituximab per cycle (mg) | 500 | 500 | 500 | — |
| Median total dose of rituximab (mg) | 2000 | 1500 | 2000 | — |
| Absolute no. CD19+ lymphocytes (×109/L) | 0.001 | 0.002 | 0 | .05 |
| CD19+ lymphocyte proportion (%) | 0.05 | 0.07 | 0.02 | .33 |
In those having a minimal response to anti-CD20 treatment, there were 4 patients that had 6 treatment episodes for ADAMTS13 relapses. In this cohort, the median age at presentation was 58.3 years, with the median age at treatment being higher in those with a minimal response compared with in those achieving CR. Of these 4 patients, 50% were male, and 1 out of 4 (25%) was of Caucasian ethnicity and 3 out of 4 (75%) of Black ethnicity. Three out of 4 patients had previously received mycophenolate mofetil and 2 out of 4 patients had received bortezomib as part of treatment for iTTP. The median pretreatment and posttreatment ADAMTS13 activity level was 5% and 15%, respectively. The median pretreatment and posttreatment absolute CD19 count was 0.16 × 106/L and 0.001 × 106/L, respectively. A progression to thrombocytopenia was not seen during treatment in this group.
Adverse events
Adverse events related to rituximab were described in 82 (41%) episodes of treatment in 48 patients (46%). Eight patients had >1 adverse events. There were 21 infective episodes after treatment (7 viral, 9 bacterial, and 5 unreported), which were predominantly respiratory tract (n = 6) and urinary (n = 3). Seven patients required hospitalization owing to adverse events during 1 treatment episode each (n = 7, 4%): 3 owing to infection, 2 owing to severe allergy symptoms, 1 owing to acute serum sickness, and 1 owing to delayed serum sickness. Hypogammaglobulinemia (defined by IgG immunoglobulin levels of <7 g/L) was seen after 10 treatment episodes in 6 patients. No patient required subsequent immunoglobulin replacement, although 1 patient had persistent hypogammaglobulinemia. Acute infusion reactions were seen in 33 treatment episodes in 23 patients and anaphylactoid-type reactions were seen in 14 episodes in 13 patients. There were 14 episodes of delayed rituximab-related reactions in 13 patients. No deaths were recorded during treatment for ADAMTS13 relapse. No cases of progressive multifocal leukoencephalopathy was reported.
Discussion
Clinical relapse after diagnosis of iTTP is a recognized life-threatening complication of the condition. Our large national patient cohort with a prolonged follow-up period demonstrates that relapse is a long-term risk. The data also reinforce that anti-CD20 treatments, particularly with extensive experience using rituximab, are efficacious in preventing clinical relapse in the majority of patients.
With regard to the nature of disease relapse in iTTP, these data provide several novel findings. Firstly, we reviewed the characteristics of patients with disease relapse in comparison with those without. As expected, there was a lower incidence of relapse in patients with reversible/treatable causes of the acute episodes of iTTP. Furthermore, there was a higher proportion of patients of Black Caribbean ethnicity having subsequent disease relapse, with ethnicity previously recognized as affecting relapse-free survival in the United States.14 In the UK population, the proportion of Black Caribbean is 1.1% and Black African is 1.8% of the total population compared with 9.9% and 10.2%, respectively, in this cohort of patients with iTTP.15 Black ethnicity has also previously been recognized by Kremer Hovinga et al as being more prevalent in patients with TTP than with other causes of thrombotic microangiopathy.8 In other autoimmune diseases, there is heterogeneous prevalence and severity according to ethnicity. Systemic lupus erythematosus (SLE) similarly has a two- to fourfold higher incidence in the Black population than Caucasian population, with an increased risk in disease severity in the former. SLE is seen in patients with iTTP, suggesting a similar potentially immune pathophysiology, such as specific HLA genotypes.13,16-18 Given the differences between ethnic groups in HLA and other immune components, we purport that this may be a mechanism of interest in understanding relapse in iTTP. Although not extensively described in iTTP, it has been recognized in other autoimmune conditions such as SLE.19 However, we failed to identify younger age as being a risk factor for relapse, unlike that described by Sun et al, although younger patients may have a longer follow-up period, increasing the period in which relapse could occur.6
Secondly, we highlight that there was a small group of patients with iTTP that relapse frequently, comprising 6% of this study cohort. Similar to results of previous studies,5,12 we demonstrated that relapse within the first 12 months of acute presentation is uncommon, although relapses occurring every 1 to 2 years are reported.5,12 As well as relapsing regularly, these patients also relapsed earlier, suggesting a pattern of early and frequent disease relapse despite appropriate eradication of B lymphocytes with preceding treatment. This may correlate with the relatively rapid recurrence of anti-ADAMTS13 B lymphocytes after anti-CD20 treatment, as suggested by others showing normalization of B-lymphocytes counts typically after 6 to 12 months and a subsequent decrease in ADAMTS13 activity in some patients. However, unlike other autoimmune conditions, patients with iTTP do not relapse at the time of B-cell reconstitution.5,18 In this cohort, a small number of patients (4%) failed to achieve remission with anti-CD20 treatment, similar to previously described rates of 4% to 14% of patients.5,10,13 A focus on other immunosuppression therapies may be required in this cohort. However, this group showed no evidence of progression to clinical relapse after preemptive treatment, with modest increments in ADAMTS13 activity levels, potentially explaining these results.
We demonstrated that after treatment for acute presentation, there was no difference in long-term relapse risk whether rituximab was given or not. Rituximab has been demonstrated to prevent early relapses and increase the time to first relapse with short-term follow-up.2 We suggest that once there is B-cell recovery, there is a subsequent potential for relapse to occur. A “second hit” may then trigger further anti-ADAMTS13 autoantibody stimulation, such as a conformational change of ADAMTS13 from a “closed” to an “open” formation, which could explain this finding, although further research is needed.5,20
We also saw a reduced proportion of clinical relapses in the later period of this study after 2012, which is likely attributable to better recognition of the natural history of the disease and subsequent targeted monitoring and preemptive treatment during ADAMTS13 relapse. We feel that the increased proportion of ADAMTS13 relapses is likely due to increased surveillance after 2012 in the United Kingdom. Although most patients with ADAMTS13 relapse are asymptomatic, we identified that 40% of patients had new-onset symptoms with lethargy and headache being the most common. These are also the predominant symptoms seen in patients with congenital TTP from the UK TTP registry and other cohorts; lethargy being a feature in 19% and headaches in 23% of patients.21,22 In congenital TTP, symptoms improved with ADAMTS13 replacement therapy in 88% of patients and may raise the question of whether earlier treatment could be based on symptoms in addition to ADAMTS13 thresholds in iTTP.21 We recognize that nonspecific symptoms such as headache and fatigue are common features chronically in iTTP. Therefore, although awareness of these symptoms is important for detecting potential ADAMTS13 relapse, caution should be used to ensure these are not acute exacerbations of chronic symptoms or unrelated to iTTP.
Our results demonstrate the efficacy of anti-CD20 treatment in iTTP in treating ADAMTS13 relapse in the long term, confirming data from other cohorts.5,10 Repeated relapses are seen in iTTP, but responses to anti-CD20 treatment are not lost and patients continue to respond well to further anti-CD20 treatment courses. Severe complications with rituximab use in iTTP are uncommon and infection is generally not recognized to occur to the same extent as in other lymphoproliferative and autoimmune conditions.5,18,23 However, there is still the unmet need of a small number of patients with iTTP with inadequate responses to treatment, which include those that do not eradicate the anti-ADAMTS13 antibody and relapse early or frequently. Alternative anti-CD20 monoclonal antibodies, such as obinutuzumab and ofatumumab, or plasma cell–targeted treatments may be an alternative strategy for those that have a short relapse-free survival with rituximab.24
A strategy of repeated anti-CD20 therapy to treat recurrent ADAMTS13 relapses appears to be a long-term therapeutic option in patients with iTTP with a decreased risk of progression to clinical relapse and hospitalization. This has important implications both for patients in terms of reduced morbidity and mortality, and for health economics avoiding expensive hospital and intensive care stays with plasma exchange (PEX) A and other treatments. Unlike earlier reports describing iTTP clinical relapses as being less severe than initial presentations, Masias et al have described that clinical outcomes after acute presentation and clinical relapses are similar, supporting the need for early recognition and preemptive treatment.25-28
These results reinforce that regular monitoring of patients with iTTP is required, with increased frequency during the first 2 years from the acute presentation to ensure monitoring for early relapse, which, from our data, may also predict a more frequent relapse pattern in the long term. Given that later relapses are also seen, longer term/lifelong monitoring is also recommended. Given the higher frequency of disease relapse in the Black population, we feel that this group would benefit from increased frequency of monitoring, particularly during the first 2 years after diagnosis, and appropriate counseling regarding the natural history of the condition.
B-lymphocyte suppression is seen after successful treatment, which typically persists for 6 to 12 months during which time relapses are not typically seen in iTTP.5 B-cell reconstitution and normalization appears to be a preceding event to subsequent relapse, particularly in those with frequent relapses. However, in the majority of patients there is a period of months to years from B-lymphocyte reconstitution to relapse, therefore, additional processes are therefore likely implicated in this progression. There is conflicting data as to whether the presence of detectable anti-ADAMTS13 antibodies are associated with disease relapse.29,30 Therefore, other immune mechanisms that mediate relapse may provide further potential monitoring and therapeutic targets.31-33
This study is limited by its retrospective nature causing reporting bias. Data collection during early recruitment in the UK TTP registry was difficult to ascertain and therefore patients were excluded from further analysis, when the use of rituximab was more limited in iTTP. In addition, we were unable to ascertain a progression-free survival from the end of treatment and, therefore, the time from acute presentation to first relapse was used as a surrogate definition. As data were collected over a long period and before the current definitions of TTP-associated relapses, in some cases these could not fully be defined and may therefore limit interpretation of understanding relapse patterns, particularly when directly comparing clinical relapse to ADAMTS13 relapse. Underreporting of adverse reactions to anti-CD20 treatments owing to the retrospective nature of the study may also have occurred. Similarly, mortality rates may not have been reported appropriately owing to nature of data collection at time of clinical review.
In conclusion, we recognize that there are distinct patterns in patients relapsing with iTTP. Black Caribbean ethnicity appears to be a risk factor for iTTP relapse in the UK population. In this cohort, 6% of patients had frequent relapses, although, reassuringly, responded similarly to anti-CD20 treatment to those who relapse less frequently. Four percent of patients did not achieve a remission state according to ADAMTS13 activity after elective anti-CD20 treatment but did not progress to clinical relapses when treated preemptively. This is reinforced by lower proportions of patients with clinical relapses in the later part of the study period with regular relapse surveillance. At present, there are no clear parameters to identify which patients are at risk of early relapse or failure to respond, although ethnicity should be a consideration in the former. Therefore, we advise that regular disease monitoring by ADAMTS13 activity is required to prevent progression to clinical relapse. Further studies are needed to identify whether there are disease markers to recognize these at-risk cohorts more effectively and identify other modalities of treatment.
Acknowledgment
The authors thank the contribution and support of all participants in the United Kingdom Thrombotic Thrombocytopenic Purpura registry.
Authorship
Contribution: A.J.D. and M.J.S. designed the study, collected and analyzed the data, and wrote the manuscript; T.D., W.L., W.T., J.v.V., J.H., T.C., Q.A.H., A.C., C.B., and S.A. contributed the data and critically reviewed the manuscript; M.T. and J.-P.W. designed the study, contributed the data, and critically reviewed the manuscript; and M.S. led the study, analyzed the data, and reviewed the manuscript.
Conflict-of-interest disclosure: T.D. received speaker fees from Sanofi and Alexion. W.L. received speaker fees from Alexion and served on advisory boards for Sanofi and Alexion. W.T. received speaker fees from Alexion, Bayer, CSL, Daichii Sankyo, Grifols, LFB, Novo Nordisk, Portola, Pfizer, Sanofi, and Takeda and served on advisory boards for Sanofi and Ablynx. J.v.V. received speaker fees from Sanofi, Alexion, and Ablynx and served on advisory board for Ablynx. T.C. received an educational grant from AbbVie. Q.A.H. received speaker or advisory fees from Alexion, Amgen, Apellis, Argenx, Grifols, Novartis, ReAlta, and Sanofi. A.C. received speaker fees from Alexion and served on advisory boards for Sanofi and Ablynx. S.A. received speaker fees from Alexion. M.T. received speaker fees from Bayer AG and Sanofi Genzyme; served on advisory boards for Ablynx, Sanofi Genzyme, and Bayer; and provided consultancy to Bayer. M.S. received speaker fees and served on advisory boards for Novartis, Takeda, Sanofi, and Octapharma and received grants from Shire and Alexion. The remaining authors declare no competing financial interests.
Correspondence: Marie Scully, Department of Haematology, University College Hospitals London, 250 Euston Rd, London NW1 2PG, United Kingdom; e-mail: m.scully@nhs.net.
References
Author notes
Data are available on request from corresponding author, Marie Scully (m.scully@nhs.net).
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal