Key Points
Morbidity and mortality have decreased over time following HCT for childhood AML.
Self-reported health status was good to excellent for most childhood AML survivors.
Abstract
Five-year survival following childhood acute myeloid leukemia (AML) has increased following improvements in treatment and supportive care. Long-term health outcomes are unknown. To address this, cumulative incidence of late mortality and grades 3 to 5 chronic health condition (CHC) were estimated among 5-year AML survivors diagnosed between 1970 and 1999. Survivors were compared by treatment group (hematopoietic cell transplantation [HCT], chemotherapy with cranial radiation [chemo + CRT], chemotherapy only [chemo-only]), and diagnosis decade. Self-reported health status was compared across treatments, diagnosis decade, and with siblings. Among 856 survivors (median diagnosis age, 7.1 years; median age at last follow-up, 29.4 years), 20-year late mortality cumulative incidence was highest after HCT (13.9%; 95% confidence interval [CI], 10.0%-17.8%; chemo + CRT, 7.6%; 95% CI, 2.2%-13.1%; chemo-only, 5.1%; 95% CI, 2.8%-7.4%). Cumulative incidence of mortality for HCT survivors diagnosed in the 1990s (8.5%; 95% CI, 4.1%-12.8%) was lower vs those diagnosed in the 1970s (38.9%; 95% CI, 16.4%-61.4%). Most survivors did not experience any grade 3 to 5 CHC after 20 years (HCT, 45.8%; chemo + CRT, 23.7%; chemo-only, 27.0%). Furthermore, a temporal reduction in CHC cumulative incidence was seen after HCT (1970s, 76.1%; 1990s, 38.3%; P = .02), mirroring reduced use of total body irradiation. Self-reported health status was good to excellent for 88.2% of survivors; however, this was lower than that for siblings (94.8%; P < .0001). Although HCT is associated with greater long-term morbidity and mortality than chemotherapy-based treatment, gaps have narrowed, and all treatment groups report favorable health status.
Introduction
Acute myeloid leukemia (AML) is the second most common pediatric leukemia. The 5-year overall survival has improved from <30% in the 1970s1 to nearly 70% in the current treatment era.2 Treatment has evolved during this time, progressing from chemotherapy-based remission induction followed by a prolonged maintenance course in the 1970s and early 1980s, to dose-intensive, shorter-duration chemotherapy regimens in the late 1980s. Hematopoietic cell transplantation (HCT) has been widely used since the 1980s, whereas routine use of cranial radiation (CRT) in newly diagnosed patients is now reserved for rare cases of refractory central nervous system disease.3,4 In addition, postinduction intensification with high-dose cytarabine and daunorubicin emerged as a standard component of therapy in the mid-1990s. These therapeutic modifications have led to improvements in the 5-year survival because of decreased relapse,5 but survival improvements are also attributable to better supportive care and decreased treatment-related mortality.6 Nevertheless, there is concern that recent survivors may experience a greater burden of late effects because of the treatment intensification, despite improved 5-year survival.
Although reports have been published of compelling decreases in late mortality,7 serious chronic diseases,8 and subsequent malignant neoplasms (SMNs)9 among survivors of childhood cancer treated in more recent years, results specific to survivors of AML are limited.10-19 Small sample sizes, variable inclusion of patients undergoing HCT, and the inability to describe the impact of therapeutic changes over time are all limitations of previously published reports. To address these gaps, this study aims to examine long-term morbidity, mortality, and health status among >800 5-year survivors of childhood AML included in the Childhood Cancer Survivor Study (CCSS) cohort, based on treatment and treatment era.
Materials and methods
Population
The CCSS cohort includes 5-year survivors of childhood cancer diagnosed between 1 January 1970 and 31 December 1999 at one of the 31 participating centers in North America. Participants were diagnosed with AML at <21 years of age. Participating centers received human subjects committee approval before subject recruitment, and participants or parents of children <18 years of age provided informed consent. Participants completed baseline and up to 5 follow-up questionnaires. Closest-age siblings of a random sample of survivors were invited to participate in the study as a control comparison group. CCSS study design and methods have been described previously.20,21
Treatment exposures and health outcomes
Cumulative chemotherapy doses received during the 5 years following diagnosis were abstracted from medical records. Cumulative alkylating agent doses are reported as cyclophosphamide equivalent dose,22 and cumulative anthracycline doses are based on doxorubicin equivalence doses.23 For each participant, radiotherapy treatment fields were classified according to direct delivery to 8 body regions; for this analysis, those treated with CRT were described. Participants were classified into 1 of 3 mutually exclusive treatment groups: (1) HCT recipients, allogeneic or autologous; (2) chemotherapy and CRT without HCT (ie, chemo + CRT); and (3) chemotherapy only, without CRT or HCT (ie, chemo-only).
Late mortality, defined as death ≥5 years after diagnosis, was obtained through linkage with the National Death Index (NDI), current through 2017. The NDI provided causes of death according to the International Classification of Diseases, 9th and 10th Revisions. The underlying cause of death was then grouped as mutually exclusive categories: recurrence, health-related, and external/other causes of death.
Chronic health conditions (CHCs) and age at first occurrence were identified by self-report from baseline and follow-up questionnaires, which included questions on multiple organ system–based conditions. The US National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE v4.03) grading criteria were adapted to categorize reported conditions as mild (grade 1), moderate (grade 2), severe or disabling (grade 3), life threatening (grade 4), or fatal (grade 5).24,25 All conditions and grades were reviewed by a physician panel, and in cases where information was not sufficient to distinguish between 2 grades, the lower grade was selected. Fatal conditions were ascertained based on cause of death from an NDI search.
A data linkage was performed between the CCSS and the Center for International Blood and Marrow Transplant (CIBMTR) for participants treated with HCT to supplement CCSS data and to evaluate the presence and severity of chronic graft-versus-host disease (cGVHD) among CCSS participants treated with allogeneic HCT. The CIBMTR collects longitudinal outcome data on US and international patients who receive cellular therapies. Beginning in the early 1970s, these data were submitted voluntarily by centers. The CIBMTR was subsequently charged with prospectively collecting data for all allogeneic HCTs performed in the United States. Since 2005, data submission for allogeneic transplants has been mandatory. The CIBMTR also collects data for most (>85%) autologous transplants performed in the United States, although data submission is optional. To perform the linkage, computerized matching was performed by an honest broker between CCSS and CIBMTR data to determine individuals included in both data sources. Variables used for data linkage included treating institution, date of birth, name, social security number (when available), and sex. Once initial matches were identified, a manual review was carried out.
SMNs were initially identified through self or proxy report or death certificate and were validated through pathology report review, or if a pathology report was unavailable, by review of death certificate, medical records, or both. Potential SMNs were independently reviewed by a pathologist and an oncologist. SMNs were defined as neoplasms histologically unique from the primary childhood cancer, classified as International Classification of Diseases for Oncology (third version) behavior code of 3,26 excluding nonmelanoma skin cancers. Recurrences of primary cancers or SMNs were excluded. Only SMNs occurring ≥5 years after childhood cancer diagnosis were included.
Health status variables were based on self-reported responses to baseline and follow-up questionnaires and were based on 6 domains: general health, mental health, functional impairment, activity limitations, pain as a result of cancer treatment, and anxiety/fears related to cancer and/or treatment. Definitions for adverse health status were based on previously established definitions.27-29 Participants were classified as having poor general health if they responded Poor or Fair to the question “Would you say that your health is excellent, very good, good, fair, or poor?” Poor mental health was defined as a sex-specific T score ≥ 63 on the Brief Symptom Inventory-18 Global Severity Index or on the depression, anxiety or somatization subscales. Activity limitations were defined as present when participants answered that their health limited moderate activities for ≥3 months of the past 2 years. Participants who endorsed needing help with personal care, routine needs, or difficulty attending school or work were considered to have a functional impairment. Survivors who rated pain as medium, a lot, or very bad or excruciating were classified as having cancer-related pain, and those who answered medium, a lot, or very many or extreme anxiety/fears were classified as having cancer-related anxiety.
Statistical methods
Cumulative incidence of all-cause and cause-specific mortality, SMNs, and CTCAE grades 3 to 5 CHCs were estimated for the overall AML survivor population and stratified by treatment group and treatment era. P values comparing cumulative incidence curves were determined by applying Gray’s test.30 Standardized mortality ratios (SMRs) were calculated for all-cause and cause-specific mortality with the expected number of deaths calculated using calendar year-, age-, and sex-specific mortality rates for the US population from the National Center for Health Statistics.31 Multivariable Poisson regression models, including decade of diagnosis, HCT status (yes/no), anthracycline exposure (yes/no), and sex, were used to estimate mortality rates. Cox regression models were used to estimate hazard ratios (HRs) for grade 3 to 5 CHCs based on treatment group and decade of diagnosis. These models included sex, HCT (yes/no), CRT (yes/no), and anthracyclines (yes/no). Additional models separately included total body irradiation (TBI; yes/no) and cGVHD (yes/no). Dose of anthracyclines, alkylating agents, and epipodophyllotoxins were examined within the models, but no significant effects were found. HRs for CHCs were also estimated for AML survivors compared with a non–cancer sibling comparison group.
The prevalence of adverse health status outcomes in each domain were calculated, and rates of adverse health status outcomes were compared between AML survivors and siblings. Among survivors, rates were compared across treatment groups and decade of diagnosis. Multivariable regression models, including treatment group and decade of diagnosis, adjusted for sex and age at baseline, and using the chemo-only group and diagnosis between 1990 and 1999 as the reference group, were used to understand how treatment group and decade of diagnosis are associated. Odds ratios (ORs) for health status outcomes were estimated for AML treatment groups compared with those of siblings, as well. Participants were censored at the date of death or most recent contact before 30 November 2016, whichever occurred first.
Results
There were 856 5-year AML survivors (1970s, 110; 1980s, 317; 1990s, 429) with 16 668 person-years of follow-up, median age of 7.1 years (range, 1-20 years) at diagnosis, and median age of 29.4 years (range, 8-60 years) at last follow-up. There were 5059 siblings used as the comparison group, with median age of 37 years (range, 3-72 years) at last follow-up. Among survivors, 339 (40%) were treated with HCT (238 allogeneic, 81 autologous, 20 unknown transplant type), 93 (11%) with chemo + CRT, and 424 (50%) with chemo-only. Treatment with CRT decreased over time, whereas the number of survivors treated with HCT increased from the 1970s (n = 18) to 1990s (n = 201), particularly with allogeneic HCT. Use of TBI with HCT decreased from the 1970s (76.5%) to the 1990s (46.4%) (supplemental Table 1, available on the Blood website). Additional survivor and treatment characteristics are shown in Table 1, and cumulative treatment exposures by decade of diagnosis and treatment group are shown in supplemental Table 1.
Demographic and treatment characteristics of survivors of AML, overall and by treatment group
| . | Treatment group . | Overall . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HCT . | Chemo + CRT . | Chemo-only . | |||||||
| n . | % . | n . | % . | n . | % . | N . | % . | ||
| Sex | Female | 164 | 48.4 | 50 | 53.8 | 222 | 52.4 | 436 | 50.9 |
| Age at diagnosis, y | 0-4 | 121 | 35.7 | 43 | 46.2 | 216 | 50.9 | 380 | 44.4 |
| 5-9 | 74 | 21.8 | 15 | 16.1 | 71 | 16.7 | 160 | 18.7 | |
| 10-14 | 84 | 24.8 | 23 | 24.7 | 72 | 17.0 | 179 | 20.9 | |
| 15-20 | 60 | 17.7 | 12 | 12.9 | 65 | 15.3 | 137 | 16.0 | |
| Down syndrome | Yes | 1 | 0.3 | 3 | 3.2 | 81 | 19.1 | 85 | 9.9 |
| Race/ethnicity | White non-Hispanic | 256 | 75.5 | 75 | 80.6 | 313 | 73.8 | 644 | 75.2 |
| White Hispanic | 19 | 5.6 | 7 | 7.5 | 25 | 5.9 | 51 | 6.0 | |
| Black | 22 | 6.5 | 6 | 6.5 | 37 | 8.7 | 65 | 7.6 | |
| Other | 39 | 11.5 | 3 | 3.2 | 43 | 10.1 | 85 | 9.9 | |
| Unknown | 3 | 0.9 | 2 | 2.2 | 6 | 1.4 | 11 | 1.3 | |
| Decade of diagnosis | 1970-79 | 18 | 5.3 | 31 | 33.3 | 61 | 14.4 | 110 | 12.9 |
| 1980-89 | 120 | 35.4 | 55 | 59.1 | 142 | 33.5 | 317 | 37.0 | |
| 1990-99 | 201 | 59.3 | 7 | 7.5 | 221 | 52.1 | 429 | 50.1 | |
| Anthracyclines (doxorubicin-equivalent dose), mg/m2 | None | 15 | 4.8 | 7 | 8.4 | 29 | 7.2 | 51 | 6.0 |
| >0-100 | 89 | 28.3 | 18 | 21.7 | 46 | 11.5 | 153 | 17.9 | |
| >100-249 | 162 | 51.4 | 31 | 37.3 | 235 | 58.6 | 428 | 50.0 | |
| ≥250 | 49 | 15.6 | 27 | 32.5 | 91 | 22.7 | 167 | 19.5 | |
| Epipodophyllotoxins, mg/m2 | None | 80 | 25.3 | 59 | 64.1 | 133 | 32.4 | 272 | 31.8 |
| >0-1000 | 106 | 33.5 | 3 | 3.3 | 109 | 26.6 | 218 | 25.5 | |
| >1000-3999 | 116 | 36.7 | 14 | 15.2 | 136 | 33.2 | 266 | 31.1 | |
| ≥4000 | 14 | 4.4 | 16 | 17.4 | 32 | 7.8 | 62 | 7.2 | |
| Alkylating agents (cyclophosphamide equivalent dose), mg/m2 | None | 73 | 24.9 | 29 | 34.1 | 255 | 61.9 | 357 | 41.7 |
| >0-3999 | 81 | 27.6 | 12 | 14.1 | 101 | 24.5 | 194 | 22.7 | |
| ≥4000-7999 | 57 | 19.5 | 26 | 30.6 | 31 | 7.5 | 114 | 13.3 | |
| ≥8000 | 82 | 28.0 | 18 | 21.2 | 25 | 6.1 | 125 | 14.6 | |
| Any radiation | Yes | 192 | 56.6 | 93 | 100.0 | 6 | 1.4 | 291 | 34.0 |
| TBI | Yes | 157 | 49.5 | 0 | 0.0 | 0 | 0.0 | 157 | 18.3 |
| CRT (not TBI) | Yes | 15 | 9.4 | 93 | 100.0 | 0 | 0.0 | 108 | 12.6 |
| Other site radiation (not TBI) | Yes | 12 | 7.5 | 18 | 19.4 | 6 | 1.4 | 36 | 4.2 |
| HCT | No HCT | 0 | 0.0 | 93 | 100.0 | 424 | 100.0 | 517 | 60.4 |
| Allogeneic | 238 | 70.2 | 0 | 0.0 | 0 | 0.0 | 238 | 27.8 | |
| Autologous | 81 | 23.9 | 0 | 0.0 | 0 | 0.0 | 81 | 9.5 | |
| HCT type unknown | 20 | 5.9 | 0 | 0.0 | 0 | 0.0 | 20 | 2.3 | |
| Chronic GVHD, by grade∗ (among survivors treated with allogeneic HCT) | None | 77 | 32.4 | 93 | 100.0 | 424 | 100.0 | 594 | 69.4 |
| Limited | 23 | 9.7 | 0 | 0.0 | 0 | 0.0 | 23 | 2.7 | |
| Extensive | 12 | 5.0 | 0 | 0.0 | 0 | 0.0 | 12 | 1.4 | |
| Unknown grade | 2 | 0.8 | 0 | 0.0 | 0 | 0.0 | 2 | 0.2 | |
| Unavailable data | 124 | 52.1 | 0 | 0.0 | 0 | 0.0 | 124 | 14.5 | |
| Vital status | Alive | 274 | 80.8 | 79 | 84.9 | 393 | 92.7 | 746 | 87.1 |
| Follow-up, y | 5-9 | 24 | 7.1 | 1 | 1.1 | 8 | 1.9 | 33 | 3.9 |
| 10-19 | 91 | 26.9 | 11 | 11.8 | 110 | 26.0 | 212 | 24.7 | |
| 20-29 | 181 | 53.4 | 30 | 32.3 | 200 | 47.1 | 411 | 48.0 | |
| ≥30 | 43 | 12.6 | 51 | 54.8 | 106 | 25.0 | 200 | 23.4 | |
| . | Treatment group . | Overall . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HCT . | Chemo + CRT . | Chemo-only . | |||||||
| n . | % . | n . | % . | n . | % . | N . | % . | ||
| Sex | Female | 164 | 48.4 | 50 | 53.8 | 222 | 52.4 | 436 | 50.9 |
| Age at diagnosis, y | 0-4 | 121 | 35.7 | 43 | 46.2 | 216 | 50.9 | 380 | 44.4 |
| 5-9 | 74 | 21.8 | 15 | 16.1 | 71 | 16.7 | 160 | 18.7 | |
| 10-14 | 84 | 24.8 | 23 | 24.7 | 72 | 17.0 | 179 | 20.9 | |
| 15-20 | 60 | 17.7 | 12 | 12.9 | 65 | 15.3 | 137 | 16.0 | |
| Down syndrome | Yes | 1 | 0.3 | 3 | 3.2 | 81 | 19.1 | 85 | 9.9 |
| Race/ethnicity | White non-Hispanic | 256 | 75.5 | 75 | 80.6 | 313 | 73.8 | 644 | 75.2 |
| White Hispanic | 19 | 5.6 | 7 | 7.5 | 25 | 5.9 | 51 | 6.0 | |
| Black | 22 | 6.5 | 6 | 6.5 | 37 | 8.7 | 65 | 7.6 | |
| Other | 39 | 11.5 | 3 | 3.2 | 43 | 10.1 | 85 | 9.9 | |
| Unknown | 3 | 0.9 | 2 | 2.2 | 6 | 1.4 | 11 | 1.3 | |
| Decade of diagnosis | 1970-79 | 18 | 5.3 | 31 | 33.3 | 61 | 14.4 | 110 | 12.9 |
| 1980-89 | 120 | 35.4 | 55 | 59.1 | 142 | 33.5 | 317 | 37.0 | |
| 1990-99 | 201 | 59.3 | 7 | 7.5 | 221 | 52.1 | 429 | 50.1 | |
| Anthracyclines (doxorubicin-equivalent dose), mg/m2 | None | 15 | 4.8 | 7 | 8.4 | 29 | 7.2 | 51 | 6.0 |
| >0-100 | 89 | 28.3 | 18 | 21.7 | 46 | 11.5 | 153 | 17.9 | |
| >100-249 | 162 | 51.4 | 31 | 37.3 | 235 | 58.6 | 428 | 50.0 | |
| ≥250 | 49 | 15.6 | 27 | 32.5 | 91 | 22.7 | 167 | 19.5 | |
| Epipodophyllotoxins, mg/m2 | None | 80 | 25.3 | 59 | 64.1 | 133 | 32.4 | 272 | 31.8 |
| >0-1000 | 106 | 33.5 | 3 | 3.3 | 109 | 26.6 | 218 | 25.5 | |
| >1000-3999 | 116 | 36.7 | 14 | 15.2 | 136 | 33.2 | 266 | 31.1 | |
| ≥4000 | 14 | 4.4 | 16 | 17.4 | 32 | 7.8 | 62 | 7.2 | |
| Alkylating agents (cyclophosphamide equivalent dose), mg/m2 | None | 73 | 24.9 | 29 | 34.1 | 255 | 61.9 | 357 | 41.7 |
| >0-3999 | 81 | 27.6 | 12 | 14.1 | 101 | 24.5 | 194 | 22.7 | |
| ≥4000-7999 | 57 | 19.5 | 26 | 30.6 | 31 | 7.5 | 114 | 13.3 | |
| ≥8000 | 82 | 28.0 | 18 | 21.2 | 25 | 6.1 | 125 | 14.6 | |
| Any radiation | Yes | 192 | 56.6 | 93 | 100.0 | 6 | 1.4 | 291 | 34.0 |
| TBI | Yes | 157 | 49.5 | 0 | 0.0 | 0 | 0.0 | 157 | 18.3 |
| CRT (not TBI) | Yes | 15 | 9.4 | 93 | 100.0 | 0 | 0.0 | 108 | 12.6 |
| Other site radiation (not TBI) | Yes | 12 | 7.5 | 18 | 19.4 | 6 | 1.4 | 36 | 4.2 |
| HCT | No HCT | 0 | 0.0 | 93 | 100.0 | 424 | 100.0 | 517 | 60.4 |
| Allogeneic | 238 | 70.2 | 0 | 0.0 | 0 | 0.0 | 238 | 27.8 | |
| Autologous | 81 | 23.9 | 0 | 0.0 | 0 | 0.0 | 81 | 9.5 | |
| HCT type unknown | 20 | 5.9 | 0 | 0.0 | 0 | 0.0 | 20 | 2.3 | |
| Chronic GVHD, by grade∗ (among survivors treated with allogeneic HCT) | None | 77 | 32.4 | 93 | 100.0 | 424 | 100.0 | 594 | 69.4 |
| Limited | 23 | 9.7 | 0 | 0.0 | 0 | 0.0 | 23 | 2.7 | |
| Extensive | 12 | 5.0 | 0 | 0.0 | 0 | 0.0 | 12 | 1.4 | |
| Unknown grade | 2 | 0.8 | 0 | 0.0 | 0 | 0.0 | 2 | 0.2 | |
| Unavailable data | 124 | 52.1 | 0 | 0.0 | 0 | 0.0 | 124 | 14.5 | |
| Vital status | Alive | 274 | 80.8 | 79 | 84.9 | 393 | 92.7 | 746 | 87.1 |
| Follow-up, y | 5-9 | 24 | 7.1 | 1 | 1.1 | 8 | 1.9 | 33 | 3.9 |
| 10-19 | 91 | 26.9 | 11 | 11.8 | 110 | 26.0 | 212 | 24.7 | |
| 20-29 | 181 | 53.4 | 30 | 32.3 | 200 | 47.1 | 411 | 48.0 | |
| ≥30 | 43 | 12.6 | 51 | 54.8 | 106 | 25.0 | 200 | 23.4 | |
Chronic GVHD data provided through linkage to the CIBMTR database; no data available for survivors diagnosed before 1980 and limited to those included in CIBMTR thereafter; percentages presented relative to 238 allogeneic transplant recipients.
Late mortality
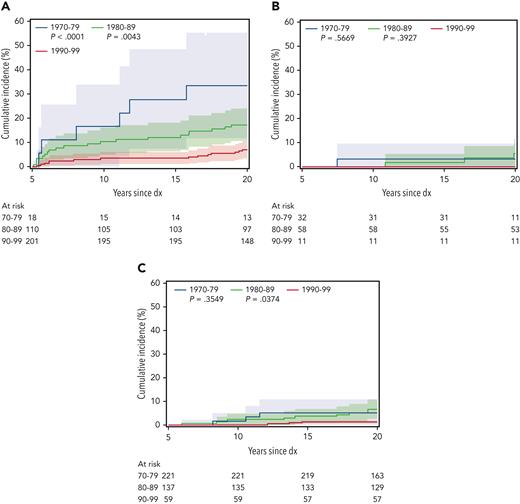
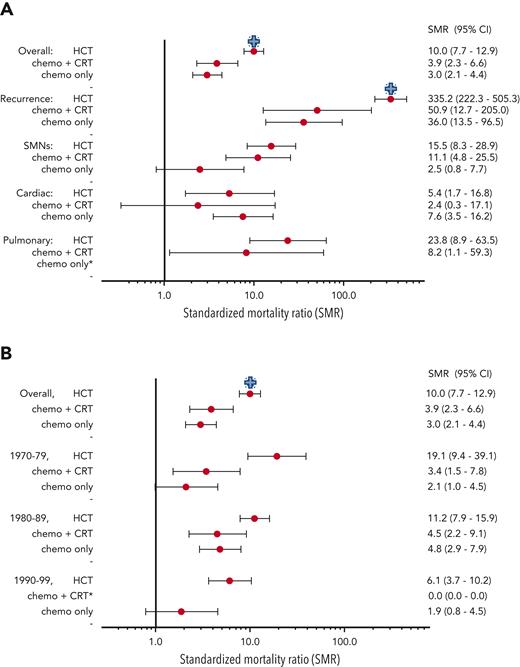
There were 110 deaths among survivors occurring >5 years after diagnosis, including 27 from recurrence or progression of AML, 22 from SMN, 11 each from cardiac and external causes, 5 from pulmonary causes, and the remainder from other or unknown causes. The 20-year cumulative incidence of all-cause late mortality among all 5-year survivors was 8.8% (95% confidence interval [CI], 6.9-10.7), with the highest incidence in those treated with HCT (13.9%; 95% CI, 10.0-17.8; P < .0001 vs chemo-only), compared with incidence in those treated with chemo + CRT (7.6%; 95% CI, 2.2-13.1; P = .45 vs chemo-only), and with incidence in those treated with chemo-only (5.1%; 95% CI, 2.8-7.4). Late mortality incidence decreased over time for those treated with HCT (1970s, 38.9%; 1980s, 19.0%; 1990s, 8.5%) but was fairly similar among individuals with chemo + CRT and chemo-only (Figure 1). Cumulative incidence of 20-year cause-specific mortality by treatment group and decade of diagnosis is shown in Table 2; late recurrence or progression was the most common cause of death among survivors treated with HCT, whereas SMN was the most common cause for survivors treated with chemo + CRT, and cardiovascular deaths were the most common cause for survivors treated with chemo-only. The significant decrease in all-cause mortality incidence over time after HCT was primarily owing to reduced incidence of AML recurrence- or progression-related deaths (1990s, 33.3%; 1980s, 7.8%; 1990s, 3.5%). Compared with the general population, risk for all-cause mortality was the highest among survivors treated with HCT (SMR, 10.0; 95% CI, 7.7-12.9) and was similar among the chemo + CRT (SMR, 3.9; 95% CI, 2.3-6.6) and chemo-only (SMR, 3.0; 95% CI, 2.1-4.4) groups. The highest risk for late mortality was from AML recurrence, SMNs, and pulmonary causes for both the HCT and chemo + CRT groups (Figure 2A). However, AML recurrence and cardiac causes contributed to the highest mortality risk in the chemo-only group. SMRs decreased over time for the HCT group but not for the chemo + CRT or chemo-only groups (Figure 2B). When allogeneic HCT was specifically considered, SMRs decreased over time for recurrence (1970s, SMR 1220.7 vs 1990s, SMR 272.8) and SMNs (1970s, SMR 43.4 vs 1990s, SMR 16.1). The limited number of subjects did not permit examination of changes in mortality among survivors treated with autologous HCT. Multivariable models adjusted for sex and treatment era showed that history of HCT (relative rate [RR], 3.4; 95% CI, 2.1-5.5; vs no HCT) and anthracycline exposure (RR, 2.6; 95% CI, 1.0-6.7) were significantly associated with late mortality (supplemental Table 2).
Cumulative incidence of late mortality by decade of diagnosis (dx) for each treatment group. (A) HCT, (B) chemo + CRT, and (C) chemo-only (reference decade 1990-1999).
Cumulative incidence of late mortality by decade of diagnosis (dx) for each treatment group. (A) HCT, (B) chemo + CRT, and (C) chemo-only (reference decade 1990-1999).
Twenty-year cumulative incidence of mortality by treatment and decade of diagnosis
| . | HCT . | Chemo + CRT . | Chemo-only . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1970-1979 . | 1980-1989 . | 1990-1999 . | 1970-1979 . | 1980-1989 . | 1990-1999 . | 1970-1979 . | 1980-1989 . | 1990-1999 . | ||
| % (95% CI) . | % (95% CI) . | % (95% CI) . | % (95% CI) . | % (95% CI) . | % (95% CI) . | % (95% CI) . | % (95% CI) . | % (95% CI) . | ||
| Cause of death | All cause | 38.9 (16.4-61.4) | 19.0 (11.8-26.1) | 8.5 (4.1-12.8) | 3.2 (0-9.5) | 10.9 (2.7-19.2) | 0 | 6.8 (0.4-13.2) | 8.0 (3.5-12.6) | 2.6 (0-5.6) |
| Relapse | 33.3 (11.6-55.1) | 7.8 (2.9-12.6) | 3.5 (1.0-6.0) | 3.2 (0-9.5) | 0 | 0 | 5.2 (0-10.7) | 0.7 (0-2.2) | 0 | |
| External∗ | 0 | 2.6 (0-5.5) | 1.1 (0-2.5) | 0 | 1.8 (0-5.4) | 0 | 1.7 (0-5.0) | 0.7 (0-2.2) | 0 | |
| SMN | 5.6 (0-16.1) | 1.7 (0-4.1) | 1.9 (0-4.6) | 0 | 5.5 (0-11.5) | 0 | 0 | 0 | 0 | |
| CV | 0 | 0.9 (0-2.5) | 0 | 0 | 0 | 0 | 0 | 2.9 (0.1-5.7) | 1.4 (0-2.9) | |
| Pulmonary | 0 | 0.9 (0-2.5) | 0.5 (0-1.5) | 0 | 1.8 (0-5.4) | 0 | 0 | 0 | 0 | |
| Other | 0 | 5.2 (1.1-9.2) | 1.6 (0-3.4) | 0 | 1.8 (0-5.4) | 0 | 0 | 3.7 (0.5-6.8) | 1.3 (0-3.8) | |
| . | HCT . | Chemo + CRT . | Chemo-only . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1970-1979 . | 1980-1989 . | 1990-1999 . | 1970-1979 . | 1980-1989 . | 1990-1999 . | 1970-1979 . | 1980-1989 . | 1990-1999 . | ||
| % (95% CI) . | % (95% CI) . | % (95% CI) . | % (95% CI) . | % (95% CI) . | % (95% CI) . | % (95% CI) . | % (95% CI) . | % (95% CI) . | ||
| Cause of death | All cause | 38.9 (16.4-61.4) | 19.0 (11.8-26.1) | 8.5 (4.1-12.8) | 3.2 (0-9.5) | 10.9 (2.7-19.2) | 0 | 6.8 (0.4-13.2) | 8.0 (3.5-12.6) | 2.6 (0-5.6) |
| Relapse | 33.3 (11.6-55.1) | 7.8 (2.9-12.6) | 3.5 (1.0-6.0) | 3.2 (0-9.5) | 0 | 0 | 5.2 (0-10.7) | 0.7 (0-2.2) | 0 | |
| External∗ | 0 | 2.6 (0-5.5) | 1.1 (0-2.5) | 0 | 1.8 (0-5.4) | 0 | 1.7 (0-5.0) | 0.7 (0-2.2) | 0 | |
| SMN | 5.6 (0-16.1) | 1.7 (0-4.1) | 1.9 (0-4.6) | 0 | 5.5 (0-11.5) | 0 | 0 | 0 | 0 | |
| CV | 0 | 0.9 (0-2.5) | 0 | 0 | 0 | 0 | 0 | 2.9 (0.1-5.7) | 1.4 (0-2.9) | |
| Pulmonary | 0 | 0.9 (0-2.5) | 0.5 (0-1.5) | 0 | 1.8 (0-5.4) | 0 | 0 | 0 | 0 | |
| Other | 0 | 5.2 (1.1-9.2) | 1.6 (0-3.4) | 0 | 1.8 (0-5.4) | 0 | 0 | 3.7 (0.5-6.8) | 1.3 (0-3.8) | |
CV, cardiovascular.
External causes include accidents, suicides, and poisonings.
SMR. (A) SMR by treatment group and cause of death. (B) SMR by treatment group and decade of diagnosis. Blue “+” indicates HCT is different from other treatment groups, P < .05. ∗Estimates not provided if no mortality events for a given category.
SMR. (A) SMR by treatment group and cause of death. (B) SMR by treatment group and decade of diagnosis. Blue “+” indicates HCT is different from other treatment groups, P < .05. ∗Estimates not provided if no mortality events for a given category.
Chronic health conditions
The 20-year cumulative incidence of grade 3 to 5 CHCs was highest among the HCT group (45.8%; 95% CI, 40.3-51.2; P < .001 vs chemo-only) and was significantly higher among survivors treated with allogeneic (49.2%; 95% CI, 42.5-55.9) than those treated with autologous (30.6%; 95% CI, 20.4-40.8) HCT. Rates were similar between the chemo + CRT (23.7%; 95% CI, 15.1-32.4) and chemo-only (27.0%; 95% CI, 22.7-31.3) groups (P = .69). Numbers of CHCs by treatment group are shown in the supplemental Figure. Notably, nearly half of the allogeneic HCT group (48.2%) and approximately two-thirds of the autologous HCT (66.3%), chemo + CRT (64.5%), and chemo-only (68.1%) groups reported no grade 3 to 5 CHCs, and approximately one-quarter of each group had only 1 condition (allogeneic HCT, 26.6%; autologous HCT, 23.8%; chemo + CRT, 23.7%; chemo-only, 23.7%) at last follow-up.
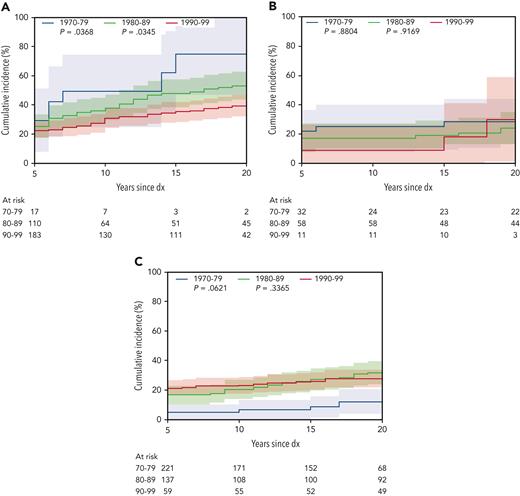
Of the reported conditions, the most common included those affecting the cardiac and respiratory systems and SMNs. Twenty-year cumulative incidence of grade 3 to 5 cardiac conditions was similar across treatment groups (HCT, 10.5%; 95% CI, 7.2-13.8; chemo + CRT, 11.8%; 95% CI, 5.3-18.4; chemo-only, 8.9%; 95% CI, 6.2-11.6). HCT survivors experienced the highest incidence of respiratory conditions (3.0%; 95% CI, 1.2-4.8), whereas the incidence for chemo-only was 1.2% (95% CI, 0.2-2.2), and no grade 3 to 5 respiratory conditions were reported among the chemo + CRT group. Cumulative incidence of SMNs at 20 years was much lower in the chemo-only group (0.2%; 95% CI, 0-0.7) than in the HCT (4.8%; 95% CI, 2.5-7.1) and chemo + CRT (3.2%; 95% CI, 0-6.8) groups. A significant reduction over time in the cumulative incidence of any grade 3 to 5 CHC was seen for the HCT group (1970s, 76.1%; 95% CI, 49.9-102.4 vs 1990s, 38.3%; 95% CI, 31.5-45.0; P = .04), particularly among those treated with allogeneic HCT (1970s, 82.3%; 95% CI, 52.3-111.4 vs 1990s, 43.5%; 95% CI, 35.3-51.7), whereas the chemo + CRT (1970s, 26.0%; 95% CI, 10.5-41.4 vs 1990s, 28.6%; 95% CI, 0-62.0; P = .88) and chemo-only (1970s, 12.2%; 95% CI, 3.7-20.7 vs 1990s, 27.6%; 95% CI, 21.7-33.5; P = .06) groups were not significantly changed over time (Figure 3).
Cumulative incidence of any grade 3 to 5 CHC, by treatment group and decade of diagnosis (dx). (A) HCT, (B) chemo + CRT, and (C) chemo-only (reference decade 1990-1999).
Cumulative incidence of any grade 3 to 5 CHC, by treatment group and decade of diagnosis (dx). (A) HCT, (B) chemo + CRT, and (C) chemo-only (reference decade 1990-1999).
In multivariable Cox models comparing incidence of CHCs in AML survivors with that in siblings, survivors not treated with HCT were more than threefold more likely (HR, 3.6; 95% CI, 3.0-4.2) and those treated with HCT were sevenfold more likely (HR, 7.1; 95% CI, 5.9-8.4) than siblings to have a grade 3 to 5 CHC (supplemental Table 3). In survivor-only models, the risk for developing a grade 3 to 5 CHC was independently associated with HCT (HR, 1.6; 95% CI, 1.2-2.0), anthracycline exposure (HR, 1.9; 95% CI, 1.1-3.4) and CRT (HR, 1.3; 95% CI, 1.0-1.6) (supplemental Table 4). When the impact of TBI as part of the HCT preparative regimen was examined, HCT with TBI was associated with an increased risk for grade 3 to 5 CHCs (HR, 2.2; 95% CI, 1.5-3.2), whereas HCT without TBI had a more marginal association (HR, 1.3; 95% CI, 1.0-1.7) than that of survivors treated with chemo-only (supplemental Table 5). Among a subset of survivors treated with allogeneic HCT with available cGVHD data (n = 114), risk for grade 3 to 5 CHCs was similarly elevated for those with a history of cGVHD (HR, 2.1; 95% CI, 1.3-3.5) and those without (HR, 2.0; 95% CI, 1.4-3.0) when compared with survivors not treated with HCT (supplemental Table 6).
Models were also constructed for specific types of CHCs (supplemental Table 4). Treatment with HCT was associated with increased risks for chronic respiratory (HR, 3.1; 95% CI, 1.1-8.9), hepatic (HR, 1.8; 95% CI, 1.0-3.1), and endocrine (HR, 6.0; 95% CI, 3.5-10.2) conditions, as well as SMNs (HR, 2.9; 95% CI, 1.4-6.2). CRT was associated with an increased risk for SMNs (HR, 2.6; 95% CI, 1.3-5.5). No other significant risk factors were identified for specific types of CHCs.
Health status
Most survivors of AML reported overall excellent, very good, or good health status, regardless of treatment group (HCT, 85% [allo 86%, auto 84%], chemo + CRT, 92%, chemo-only, 90%). However, compared with siblings, survivors of AML treated with HCT or chemo-only were more likely to report poor health status for all outcomes (Table 3), whereas survivors treated with chemo + CRT were more likely to report only functional impairment (OR, 3.7; 95% CI, 1.8-7.7), which may be an artifact of the small sample size of that treatment group. Treatment with HCT (vs chemo-only, OR, 1.7; 95% CI, 1.1-2.7) and diagnosis in the 1980s (vs 1990s, OR, 1.8; 95% CI, 1.2-2.9) were associated with activity limitations, and treatment in the 1970s was associated with decreased likelihood of functional impairment (vs 1990s, OR, 0.4; 95% CI, 0.1-0.8). Allogeneic and autologous HCT were not significantly different. Neither treatment group nor decade of diagnosis was associated with fair or poor general health, poor mental health, cancer pain, or cancer anxiety (supplemental Table 7).
Health status by treatment group, compared with that of siblings
| Health status indicator . | Treatment group . | Yes, n (%) . | No, n (%) . | OR (95% CI) . |
|---|---|---|---|---|
| Fair or poor general health | Sibling | 246 (5) | 4741 (95) | 1.0 (ref) |
| Chemo + CRT | 7 (7.8) | 83 (92.2) | 1.9 (0.9-4.3) | |
| Chemo-only | 40 (9.8) | 369 (90.2) | 2.5 (1.8-3.6) | |
| HCT | 45 (14.8) | 259 (85.2) | 3.8 (2.7-5.4) | |
| Functional impairment | Sibling | 130 (2.6) | 4853 (97.4) | 1.0 (ref) |
| Chemo + CRT | 8 (8.8) | 83 (91.2) | 3.7 (1.8-7.7) | |
| Chemo-only | 83 (20.5) | 322 (79.5) | 9.8 (7.3-13.2) | |
| HCT | 48 (16.2) | 249 (83.8) | 7.1 (5.0-10.2) | |
| Limitations on moderate activities for ≥3 mo | Sibling | 288 (5.8) | 4681 (94.2) | 1.0 (ref) |
| Chemo + CRT | 8 (8.9) | 82 (91.1) | 1.8 (0.9-3.9) | |
| Chemo-only | 42 (10.3) | 365 (89.7) | 2.2 (1.6-3.1) | |
| HCT | 50 (15.8) | 266 (84.2) | 3.5 (2.5-4.9) | |
| Poor mental health | Sibling | 397 (10.1) | 3524 (89.9) | 1.0 (ref) |
| Chemo + CRT | 9 (14.1) | 55 (85.9) | 1.4 (0.7-2.8) | |
| Chemo-only | 46 (18.3) | 205 (81.7) | 1.9 (1.4-2.7) | |
| HCT | 36 (15.9) | 191 (84.1) | 1.6 (1.1-2.4) |
| Health status indicator . | Treatment group . | Yes, n (%) . | No, n (%) . | OR (95% CI) . |
|---|---|---|---|---|
| Fair or poor general health | Sibling | 246 (5) | 4741 (95) | 1.0 (ref) |
| Chemo + CRT | 7 (7.8) | 83 (92.2) | 1.9 (0.9-4.3) | |
| Chemo-only | 40 (9.8) | 369 (90.2) | 2.5 (1.8-3.6) | |
| HCT | 45 (14.8) | 259 (85.2) | 3.8 (2.7-5.4) | |
| Functional impairment | Sibling | 130 (2.6) | 4853 (97.4) | 1.0 (ref) |
| Chemo + CRT | 8 (8.8) | 83 (91.2) | 3.7 (1.8-7.7) | |
| Chemo-only | 83 (20.5) | 322 (79.5) | 9.8 (7.3-13.2) | |
| HCT | 48 (16.2) | 249 (83.8) | 7.1 (5.0-10.2) | |
| Limitations on moderate activities for ≥3 mo | Sibling | 288 (5.8) | 4681 (94.2) | 1.0 (ref) |
| Chemo + CRT | 8 (8.9) | 82 (91.1) | 1.8 (0.9-3.9) | |
| Chemo-only | 42 (10.3) | 365 (89.7) | 2.2 (1.6-3.1) | |
| HCT | 50 (15.8) | 266 (84.2) | 3.5 (2.5-4.9) | |
| Poor mental health | Sibling | 397 (10.1) | 3524 (89.9) | 1.0 (ref) |
| Chemo + CRT | 9 (14.1) | 55 (85.9) | 1.4 (0.7-2.8) | |
| Chemo-only | 46 (18.3) | 205 (81.7) | 1.9 (1.4-2.7) | |
| HCT | 36 (15.9) | 191 (84.1) | 1.6 (1.1-2.4) |
Discussion
Among a large population of survivors of childhood AML diagnosed from 1970 to 1999 and treated with HCT, chemo + CRT, and chemo-only, late mortality, CHCs, and health status outcomes differed based on treatment and decade of diagnosis. Although risks of late mortality and CHCs among AML survivors treated with HCT were consistently higher than risks for those treated with chemotherapy (with or without CRT), significant reductions were observed for the HCT treatment group over time. In contrast, among survivors treated with chemotherapy with or without CRT, risk for mortality and incidence of CHCs have remained stable over time. Health status was overall very good among AML survivors, regardless of treatment group. To our knowledge, this is the largest cohort of long-term survivors of childhood AML described to date. Given current treatment strategies for AML, the findings remain applicable to current HCT and chemo-only–treated populations.
The decrease in late mortality risk over treatment decades among the AML HCT group is consistent with a previous report including the overall population of the CCSS cohort, in which risk for mortality decreased from the 1970s to the 1990s.7 This reduction in mortality risk among HCT recipients is multifactorial. Late recurrence dramatically decreased over time as the proportion of allogeneic transplants increased. Autologous transplantation has previously been reported to be associated with higher rates of late relapse than allogeneic HCT32 and with inferior long-term survival, and its use in AML therapy has been largely abandoned in more recent treatment eras. Additionally, exclusion of TBI from the majority of contemporary allogeneic transplant treatment regimens, post-HCT immune suppression, use of minimal residual disease detection before transplant, and supportive care have evolved over time, leading to fewer complications from acute and chronic GVHD and decreased incidence of end-organ toxicity, disease recurrence, and transplant-related mortality.33-35 However, the risk for late mortality has not decreased significantly for the non-HCT treatment groups. Within these treatment groups, in an effort to improve 5-year survival, there has been intensification of chemotherapy regimens over time. Although these changes have resulted in improvements in relapse rates and short-term survival, they have also led to increased acute toxicity36 and no clear improvements in long-term health-related outcomes, as shown here.
The reporting of CHCs was consistent with those observed for late mortality among AML treatment groups. Survivors who were treated with HCT experienced a significant reduction in cumulative incidence of grades 3 to 5 CHCs in more recent eras despite an increasing proportion of HCT patients receiving allogeneic transplants, whereas incidence in the chemo-only group was unchanged over time. Notably, survivors treated with HCT preparative regimens including TBI experienced significantly higher risk for CHCs than those treated with chemo-only, whereas risk in those treated with non–TBI-based HCT was not significantly different from chemo-only. The temporal reduction in TBI use has been an important contributor to reduced CHCs among HCT patients. Importantly, major differences in risk for CHCs were not observed between survivors who experienced cGVHD following allogeneic HCT and those who did not, suggesting that high-dose chemotherapy was the primary contributor to the development of CHCs in this analysis, although power to explore these associations in depth was limited. Given the increased use of allogeneic HCT among patients with AML, these findings are important, and along with reductions in relapse, are partially responsible for the reduction in late mortality. Despite this favorable trend for the HCT group, they remain at greater risk for CHCs than AML survivors not treated with HCT, and ongoing vigilant clinical monitoring and screening, particularly for high-risk conditions such as cardiotoxicity, remain important for all AML survivors.37
The larger number of AML survivors from the 1990s than that from the 1970s exemplifies the improvements in 5-year survival after initial diagnosis. This is particularly notable for individuals treated with HCT in the 1970s, for which our cohort numbers are small. However, AML is among the childhood cancers that have yet to reach the 5-year survival outcomes observed in diseases such as acute lymphoblastic leukemia (88%), Hodgkin lymphoma (98%), and Wilms tumor (92%),2 and as such, therapy continues to evolve. Efforts to improve AML outcomes, including the introduction of novel therapies, may alter long-term morbidity and mortality risk profiles in survivors treated after 1999. Although our overall findings appear consistent with those previously reported in smaller cohorts,12,15,16 this analysis provides the advantage of greater detail on temporal trends and differences based on treatment groups, with longitudinal follow-up. Contemporary AML studies through the National Clinical Trials Network Children’s Oncology Group have aimed to mitigate the risk for acute and late cardiotoxicity associated with intensive anthracycline exposure by adding dexrazoxane to upfront treatment and by investigating the efficacy of liposomal formulations of daunorubicin. Additionally, efforts have been made to limit the number of patients treated with HCT, through risk stratification, to reduce acute and late toxicities. Encouragingly, despite our observations for mortality and CHCs among different treatment groups, incidence and risk are low across all treatment groups, particularly within the chemo-only group. The majority of AML survivors are living without significant perceived impairment as evidenced by the health status findings presented here. Armenian et al previously reported similar differences in general health and functional impairment between survivors with a variety of childhood cancer diagnoses treated with HCT and conventional chemotherapy; however, they reported greater risk for activity limitations than we observed among HCT survivors, potentially due to a higher proportion of patients receiving allogeneic rather than autologous transplants in their cohorts.38 Continued follow-up as survivors age and treatments evolve will be critical.
Although the presented results provide valuable guidance on how survivorship follow-up should be approached for survivors of AML based on prior treatments, certain limitations must be acknowledged. Therapy has already evolved from the AML treatment groups presented in this analysis. Autologous transplants made up one-third of the presented HCT population, and even in the 1990s, 46% of those treated with HCT received TBI, whereas now, most transplants in pediatric AML are allogeneic, and non–TBI-based preparative regimens are the standard of care.39,40 This analysis was underpowered to examine how temporal changes in cGVHD have altered long-term outcomes; however, an analysis from the CIBMTR examining associations between cGVHD and late effects among survivors of pediatric hematologic malignancy treated between 2000 and 2010 demonstrates persistent associations between cGVHD and nonmalignant late effects.41 Cranial irradiation is now rarely used in AML and would currently only be indicated for patients with central nervous system involvement refractory to intrathecal and systemic chemotherapy.42 Additionally, contemporary AML chemotherapy is now largely based on high-dose cytarabine and intensive anthracycline dosing,43 which became widely adopted in the mid-to-late 1990s, limiting the proportion of the chemo-only population that is representative of current patients with AML (supplemental Table 1). Notably, 80% of this study population received <250 mg/m2 of doxorubicin-equivalent anthracycline dosing. Despite these limitations, we provide valuable data regarding long-term outcomes for AML survivors, which may help shape future treatment protocols.
In conclusion, in the largest population of survivors of childhood AML described to date, long-term outcomes are favorable overall. The risk for late mortality and serious CHCs has decreased over time among survivors treated with HCT, without similar changes in survivors treated with chemotherapy. Although HCT continues to be associated with inferior health status and greater morbidity and mortality than chemotherapy-based treatment, the gaps in late outcomes of AML have narrowed as HCT becomes less toxic and chemotherapy becomes more intense. Long-term outcomes should be monitored in future decades to fully assess the impact of contemporary therapies.
Acknowledgments
This work was supported by the National Cancer Institute (NCI; CA55727, G.T.A., Principal Investigator; CA234232, L.M.T., Principal Investigator). Support to St. Jude Children’s Research Hospital was also provided by the Cancer Center Support (CORE) grant (CA21765, C. Roberts, Principal Investigator) and the American Lebanese-Syrian Associated Charities (ALSAC). The CIBMTR is supported primarily by Public Health Service U24CA076518 from the NCI, the National Heart, Lung and Blood Institute, and the National Institute of Allergy and Infectious Diseases; HHSH250201700006C from the Health Resources and Services Administration; and N00014 to 20-1-2832 and N00014 to 21-1-2954 from the Office of Naval Research; support is also provided by Be the Match Foundation, the Medical College of Wisconsin, the National Marrow Donor Program, and by the following commercial entities: AbbVie; Actinium Pharmaceuticals, Inc; Adaptive Biotechnologies Corporation; ADC Therapeutics; Adienne SA; Allogene; Allovir, Inc; Amgen, Inc; Anthem; Astellas Pharma US; AstraZeneca; Atara Biotherapeutics; BeiGene; bluebird bio, Inc; Bristol Myers Squibb Co; CareDx Inc; CRISPR; CSL Behring; CytoSen Therapeutics, Inc; Eurofins Viracor, DBA Eurofins Transplant Diagnostics; Fate Therapeutics; Gamida-Cell, Ltd; Gilead; GlaxoSmithKline; HistoGenetics; Incyte Corporation; Iovance; Janssen Research & Development, LLC; Janssen/Johnson & Johnson; Jasper Therapeutics; Jazz Pharmaceuticals, Inc; Kadmon, a Sanofi Company; Karius; Kiadis Pharma; Kite, a Gilead Company; Kyowa Kirin; Legend Biotech; Magenta Therapeutics; Mallinckrodt Pharmaceuticals; Medac GmbH; Medexus Pharma; Merck & Co; Millennium, the Takeda Oncology Co; Miltenyi Biotec, Inc; MorphoSys; Novartis Pharmaceuticals Corporation; Omeros Corporation; OptumHealth; Orca Biosystems, Inc; Ossium Health, Inc; Pfizer, Inc; Pharmacyclics, LLC, An AbbVie Company; Priothera; Sanofi; Sanofi-Aventis US Inc; Sobi, Inc; Stemcyte; Takeda Pharmaceuticals; Talaris Therapeutics; Terumo Blood and Cell Technologies; TG Therapeutics; Vertex Pharmaceuticals; and Xenikos BV.
Authorship
Contribution: L.M.T. designed the research, collected data, interpreted data, and wrote the manuscript; J.A.W. performed the statistical analysis, analyzed and interpreted the data, and reviewed and edited the manuscript; W.M.L. designed the research, performed the statistical analysis, analyzed and interpreted the data, and reviewed and edited the manuscript; R.M.H. designed the research and reviewed and edited the manuscript; J.P.N. designed the research, collected data, and reviewed and edited the manuscript; R.P. provided data and reviewed and edited the manuscript; K.C.O. designed the research and reviewed and edited the manuscript; K.K.N. designed the research and reviewed and edited the manuscript; W.G.W. designed the research and reviewed and edited the manuscript; E.A.K. designed the research and reviewed and edited the manuscript; L.L.R. designed the research, collected data, and reviewed and edited the manuscript; G.T.A. designed the research, collected data, and reviewed and edited the manuscript; and E.J.C. designed the research, interpreted data, and reviewed and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lucie M. Turcotte, Division of Pediatric Hematology/Oncology, University of Minnesota Medical School, 420 Delaware St SE, MMC 484, Minneapolis, MN 55455; e-mail: turc0023@umn.edu.
References
Author notes
Data are available on request from the corresponding author, Lucie M. Turcotte (turc0023@umn.edu).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal