In this issue of Blood, Turcotte et al1 demonstrate in the largest cohort to date of childhood acute myelogenous leukemia (AML) survivors that treatment intensification and improved supportive care measures have led to dramatically better long-term survival over time. However, they also show the unwanted effects of treatment intensification, that being a greater burden of late effects and toxicity that have persisted even in patients treated in the most recent time period.
The evolution in treatment of childhood leukemia over the past 60 years has been one of the greatest successes in the field of oncology, with 5-year overall survival rates now surpassing 90%.2 Yet this triumph is due primarily to advances in the treatment of acute lymphoblastic leukemia (ALL), in which dose-intensive combination chemotherapy followed by a prolonged maintenance phase achieves long-term remissions for most patients. For AML, however, improving cure rates have lagged substantially behind those of ALL, requiring more intensive treatments, frequently including hematopoietic cell transplantation (HCT).
Higher rates of cure for childhood cancers have led to an estimated 500 000 cancer survivors by 20203 and a greater appreciation of the burden of late effects suffered by patients related to their prior treatments. Much of this understanding originated from the Childhood Cancer Survivor Study, a robust cohort of patients from 31 institutions with longitudinal data dating to 1970.2 This recognition has resulted in earlier identification of late effects and prompt treatment, as well as the development of preventative measures to improve long-term outcomes. For example, several treatment protocols since the mid-1990s have included dexrazoxane to reduce anthracycline-related cardiotoxicity without impacting relapse mortality.4 More notably, recent treatment protocols for several childhood cancers have shifted their focus to more nuanced systems of risk stratification, with a goal of decreasing treatment intensity to reduce the risk of developing late effects. Such efforts have maintained excellent treatment outcomes while leading to reductions in late mortality.5
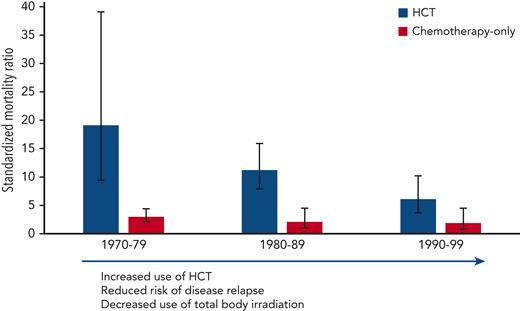
Given that treatment plans for childhood AML have required intensification rather than deescalation to improve survival, many have suggested that these patients may also suffer an undue burden of late effects. However, data for AML survivors are limited.6 In this report from the Childhood Cancer Survivor Study comparing outcomes of 5-year survivors of childhood AML treated between 1970 and 1999, Turcotte et al1 illustrate the double-edged sword of this treatment approach, notably that the risk of relapse has decreased substantially over time while the chance of developing a chronic health condition is more than threefold higher in survivors than healthy siblings. The authors also conducted analyses based on selected treatment groups: (1) HCT recipients, allogeneic or autologous; (2) chemotherapy with cranial radiation; and (3) chemotherapy only. Strikingly, the incidence of late effects has decreased over time in patients who underwent HCT, yet late mortality and chronic health conditions have not changed significantly in the chemotherapy-only group among patients treated in different time periods (see figure). Still, overall most childhood survivors of AML reported good health outcomes regardless of treatment group.
Standardized mortality ratios by decade, comparing standardized mortality ratios by decade of diagnosis and treatment group (hematopoietic cell transplantation vs chemotherapy only).
Standardized mortality ratios by decade, comparing standardized mortality ratios by decade of diagnosis and treatment group (hematopoietic cell transplantation vs chemotherapy only).
These findings demonstrate that although the majority of AML survivors are living without significant perceived impairment, close monitoring and surveillance for late effects are critical during long-term follow-up, and the follow-up should be adapted on the basis of changes in treatment through the years. In addition, more preventative measures and early interventions during survivorship could be effective in reducing the burden in patients, especially those at high risk of late effects. Practical approaches, such as exercise interventions to improve cardiovascular health, could impact many childhood cancer survivors, yet to be successful and widely adopted, they will need to engage this particular patient population, likely by incorporating digital and mobile technology.
The more challenging question is how can we modify upcoming treatment protocols to reduce late toxicity in a disease where 5-year survival outcomes are still suboptimal. Development and incorporation of targeted therapies is likely the best approach to improve efficacy while minimizing toxicity. FLT3 inhibitors have shown efficacy in adult patients with this specific mutation without significant adverse effects,7 and there are ongoing studies in pediatric AML (NCT04293562). Immune-based approaches, such as chimeric antigen receptor T-cell therapy, have demonstrated great success in ALL but have been more difficult to target in AML, though there are some promising studies in development.8 Nevertheless, late toxicities may still be seen with targeted therapies; therefore, further studies are needed to ascertain the long-term effects for immune-based therapies.9
Although targeted therapies are designed primarily to reduce the risk of disease recurrence, other approaches should focus on mitigating treatment-related mortality. First, investigators should strive to identify low-risk AML patients, who do not benefit from more intensive treatment, particularly HCT. Second, advances in molecular profiling including whole exome sequencing and RNA sequencing, as well as more sensitive techniques to assess disease status such as next-generation sequencing, will allow for more individualized and less toxic treatments in the future.10 Third, given that a large population of patients will still require HCT for cure, novel strategies to decrease regimen related toxicity, such as the development of personalized pharmacokinetic-guided dosing algorithms, are needed.
The findings from Turcotte et al’s1 study are limited by the facts that the cohort stretches over nearly 3 decades and treatment of AML has changed substantially through the years. For example, in patients in this study who received HCT, one-third were autologous and nearly half received total body irradiation, neither of which are part of standard treatment today. Yet the long-term outcome data presented here are essential to our understanding of the late toxicity seen in AML patients and will be helpful in designing the next phase of treatment protocols. Ultimately, such protocols should optimize cure rates and long-term quality-of-life outcomes while reducing the risk of late effects.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal