In this issue of Blood, Cheminant et al report on the results of allogeneic hematopoietic stem cell transplantation (allo-HSCT) and conservative management for adults with inborn errors of immunity (IEI).1
Their study compared 79 adults (patients [cases]: age 15 years or older at transplantation) who received HSCT with 202 adults (controls) who were managed with other treatments for various combined immune disorders and who were selected from an IEI registry. Cases and controls were matched on birth decade; at last review, age older than that at transplantation; diagnosis of chronic granulomatous disease or combined immunodeficiency; and autoimmune or lymphoproliferative complications. They concluded that HSCT offers an advantage for disease-free survival despite the risk for death from the transplantation procedure.
The IEI include a heterogenous group of diseases caused by monogenic germ line mutations in more than 400 genes that regulate the immune system.2 Patients’ age at presentation varied. The more severe clinical phenotypes are present in childhood and are treated with HSCT or gene therapy. In contrast, IEIs that present later in life have a milder clinical phenotype and may not have a genetic diagnosis. Conservative management has been the accepted standard for treatment, especially for those without disease-related complications. The preference for disease-modifying treatments is influenced by a desire to avoid morbidity and mortality that is attributed directly to the transplantation procedure and that may outweigh the benefit of cure. Thus, when considering transplantation, it is prudent to consider an individual patient’s risk from the transplantation per se and balance that with the risk of disease-related morbidities that can eventually lead to death. However, the clinical application of the above-mentioned approach presents unique challenges, the most important being patient selection and the timing of the intervention (transplantation). The availability of a donor who is suitably HLA-matched to a patient with minimal comorbidities at transplantation will ensure the best survival in children and adults.3-5 In the context of inherited diseases when considering a relative as a potential donor, the workup must include screening for germ line mutations because only those without mutations are eligible to donate. Although HLA-matched unrelated donors are readily available for Whites, availability remains a challenge for non-Whites,6 and published reports have shown HLA-mismatched donor transplantation increases the risk for graft failure and mortality.3,4 With higher mortality risks for patients with 2 or more comorbidities5 (a surrogate for disease severity), timing of referral to be considered for transplantation is critical to ensure a successful outcome.
Treatment choices are best studied prospectively to ensure comparable selection of patients and random assignment to treatments.7 Such an approach is impractical in the context of studying rare diseases for the following reasons: lengthy accrual time, financial burden, patients’ reluctance to be assigned very different treatments, and an inability to sustain the interest of patients and the scientific community for several years while awaiting the results of a lengthy trial. Thus, leveraging data that are available through a disease registry to identify suitable controls is an appealing strategy that was used by Cheminant et al. The authors duly acknowledge the observed differences between their cases and controls. Notably, those who underwent transplantation had a more severe clinical phenotype and more active complications at transplantation. The authors recognized the limitations of their approach and performed carefully controlled analyses, including validation of their observations through sensitivity analyses. Their findings will influence clinical decision-making for adults with IEIs.
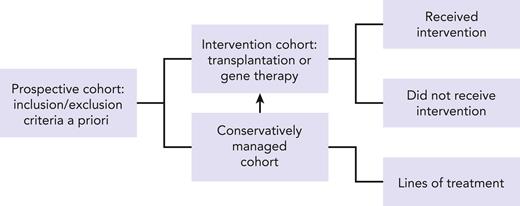
Our challenge is to improve upon the rigor for conducting studies that use existing registries. One option is to design a prospective observational cohort (see figure) based on eligibility criteria established a priori upon entry to a disease registry coupled with reporting of annual longitudinal follow-up data on standardized report forms to capture disease-specific information, choice of treatments, response to treatment, changes in treatment (and reasons for change) or lack thereof when clinically indicated, assessment of disease status, and survival. This would in essence capture not only those who were fit enough to receive HSCT or another intervention such as gene therapy but also those who met disease severity for an intervention but were not offered the treatment or were unable to tolerate the intervention because of multiple comorbidities or a because a suitable donor was not available. Adult patients with IEI are understudied and underrepresented in registries but are likely to offer relevant information on when the timing of a treatment strategy should be modified for the best possible outcome. Another factor that will ensure a robust nested cohort within a registry would be limiting participation to those clinical sites willing to report consecutive patients with IEI and are committed to continue longitudinal follow-up throughout a patient’s life span. Supportive care measures will no doubt evolve and so will strategies for treatment intervention. A prime example is the adoption of less intense conditioning regimens for HSCT. This approach extends access to less fit patients with progressive disease and is intended to lower the burden of morbidity associated with HSCT. Striking an appropriate balance between intensity of conditioning regimen for HSCT and disease control remains a challenge.8,9 Consequently, only through careful study of strategies among similar disease groups and/or donor types (in the case for transplantation) can we begin to make appropriate recommendations for treatments.
The proposed approach would require substantial monetary investment and participation of existing stakeholders. It is particularly important that consecutive patients with IEI be registered from each of the participating sites to minimize biases. Finally, participation in the registry and in associated research requires patients to understand the important role they play in advancing the treatments for their disease, which underscores the need for sustained longitudinal follow-up, and a recognition that registry-led studies impact future generations of patients in addition to themselves.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal