In this issue of Blood, Icheva et al describe a new and highly predictive approach for the laboratory diagnosis of acquired von Willebrand syndrome (aVWS) in neonates and infants undergoing surgery for congenital heart disease (CHD).1 Although pediatric aVWS is a rare disease, it appears most commonly in the clinical setting of CHD, in which the shear stress–induced increase in von Willebrand factor (VWF) proteolysis causes the loss of high molecular weight multimers (HMWM).2 Understanding possible risk factors of bleeding in infants with CHD is crucial for this patient population because neonates and small infants, in particular, are susceptible to the coagulopathic effects of cardiopulmonary bypass, almost invariably requiring the use of blood components and other procoagulant interventions.3,4
Historically, a significant barrier to the timely diagnosis of aVWS has been the lack of readily available and accurate laboratory testing. Individual and preanalytical variables affect the sensitivity of traditional laboratory testing for aVWS, particularly with ristocetin-based activity testing.5 The gold standard for diagnosing aVWS, the VWF multimer analysis, is time consuming and unavailable on-site at many institutions. A key advancement in recent years has been in the measurement of functional assessment of VWF, with ristocetin-based activity tests gradually being supplemented or replaced by assays based on the binding of VWF to a recombinant platelet glycoprotein (GP1bM), which show greater precision and higher sensitivity.6 In this prospective cohort study, Icheva et al take the next step of investigating how this new testing can be used in the identification of aVWS.
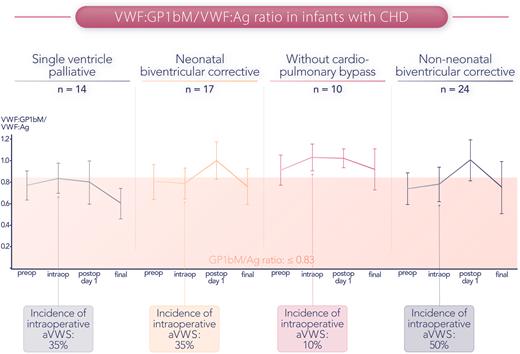
The investigators screened all patients with CHD aged 0 to 12 months requiring corrective or palliative cardiac surgery over a 17-month time frame and achieved a high enrollment percentage (95% of eligible infants enrolled in the study). Participants underwent detailed coagulation testing at 4 standardized time points (preoperative, intraoperative, postoperative day 1, and final testing, typically within the first 2 weeks after surgery) (figure). VWF:GP1bM testing was performed using a commercially available test, and at the authors’ institution, only 1 hour elapses from blood collection to the result. In their analysis, the authors compared the predictive value of the GP1bM/VWF:antigen (Ag) ratio, the VWF:collagen binding/VWF:Ag ratio, and peak systolic echocardiographic gradients with the gold standard HMWM ratios. Among the algorithms studied, the GP1bM/VWF:Ag ratio provided the best predictive value for identifying aVWS and correlated strongly with the HMWM ratio. Another key finding from this work was that a GP1bM/VWF:Ag cutoff value of <0.83, as opposed to the generally recommended cutoff of <0.7, was found to be the optimal cutoff because it provided better sensitivity. To transfer these research findings to the bedside, more institutions will need to adopt a VWF:GP1bM activity assay as an on-site test. The authors also highlight that institutions should invest time and resources to develop in-house cut-off values for identifying low GP1bM/VWF:Ag ratios.
GP1bM/VWF:Ag ratio over the perioperative course of infants with congenital heart disease. The intraoperative incidence of aVWS (defined as a ratio ≤0.83) ranged from 10% in the subgroup undergoing surgery without cardiopulmonary bypass to 50% in non-neonates undergoing biventricular corrective procedures. Professional illustration by Somersault18:24.
GP1bM/VWF:Ag ratio over the perioperative course of infants with congenital heart disease. The intraoperative incidence of aVWS (defined as a ratio ≤0.83) ranged from 10% in the subgroup undergoing surgery without cardiopulmonary bypass to 50% in non-neonates undergoing biventricular corrective procedures. Professional illustration by Somersault18:24.
The large sample size of this study (n = 65 in the final analysis) compared with historical literature in this field also improves our understanding of the epidemiology of aVWS in infants with CHD. In this cohort, a substantial number (approximately one-third) of neonates and infants undergoing various surgical procedures for palliation or correction of CHD were found to have laboratory evidence of aVWS. However, only 1 patient subgroup (Group II, neonates undergoing biventricular corrective surgery) showed possible significant differences in blood loss (indirectly measured by supplemented blood components and chest closure times) according to aVWS status. However, it is impossible to know how much of this difference (fresh frozen plasma, platelets, packed red blood cells, and fibrinogen were all given more commonly in those with aVWS) was based on correction of laboratory values as opposed to perceived blood loss. In addition, all patients receiving cardiopulmonary bypass (Groups I, II, and IV) received intraoperative tranexamic acid as standard care. Postoperative bleeding occurred in <10% of patients (n = 6 patients total), and the incidence of bleeding events (nor the amount of chest tube drainage over the first 24 postoperative hours) did not differ between aVWS+ and aVWS− patients.
These findings highlight continued knowledge gaps unable to be filled by the current study. What is the clinical significance of a laboratory diagnosis of aVWS? Would routine correction with VWF concentrate in such patients provide clinical benefit or simply increase costs and thrombosis risk for this patient population? Indeed, clinical trials have not evaluated the value of screening for and treating aVWS in previously asymptomatic patients,7 and experts have argued that the diagnosis should only be made in the presence of bleeding symptoms. On the other hand, these are very young patients facing a highly invasive surgery who may not have had time to manifest bleeding symptoms.5,7 In fact, in a cohort of older pediatric patients with aVWS (aged 5 months to 19 years), 15 of 16 (94%) had bleeding symptoms at the time of presentation, with heavy menstrual bleeding and epistaxis being most common.8 And unlike adults, cardiopulmonary-related aVWS remains uncorrected in most pediatric patients after surgery (as supported by the current study and initially demonstrated in the authors’ initial case series).3,9
This work by Icheva et al represents a significant step towards a more accurate and timely diagnosis of aVWS. This advance should lead to more routine preoperative screening for aVWS and an increasing understanding of clinical bleeding risk. It is intriguing to wonder whether standardized use of antifibrinolytics in combination with targeted replacement of VWF concentrate could help to change the current paradigm, in which large volumes of donated blood components (an increasingly scarce global resource) are routinely given to neonates and infants undergoing repair of CHD.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal