Key Points
Quercetin inhibits XIAP deficiency-associated NLRP3 inflammasome dysfunction.
Quercetin may be an effective natural therapeutic for the prevention of XIAP deficiency-associated hyperinflammation.
Abstract
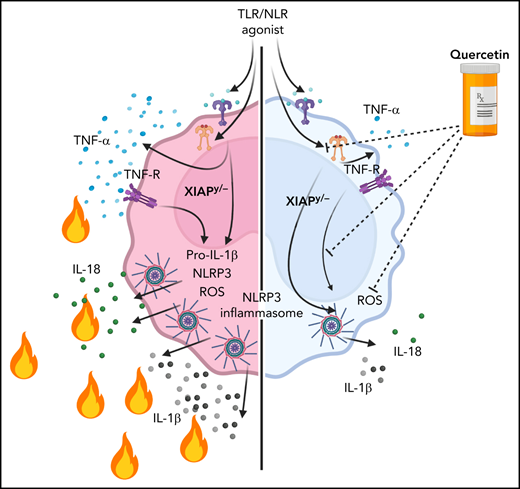
XIAP (X-linked inhibitor of apoptosis) deficiency is a rare inborn error of immunity. XIAP deficiency causes hyperinflammatory disease manifestations due to dysregulated TNF (tumor necrosis factor)-receptor signaling and NLRP3 (NOD- [nucleotide-binding oligomerization domain], LRR- [leucine-rich repeat] and pyrin domain-containing protein 3) inflammasome function. Safe and effective long-term treatments are needed and are especially important to help prevent the need for high-risk allogeneic hematopoietic cell transplantation. Here we evaluated inflammasome inhibitors as potential therapeutics with a focus on the natural flavonoid antioxidant quercetin. Bone marrow (BM)-derived macrophages were derived from XIAP-deficient or wild-type (WT) mice. Human monocytes were obtained from control or XIAP-deficient patients. Cells were stimulated with TLR (Toll-like receptor) agonists or TNF-α ± inhibitors or quercetin. For in vivo lipopolysaccharide (LPS) challenge experiments, XIAP-deficient or WT mice were fed mouse chow ± supplemental quercetin (50 mg/kg per day exposure) for 7 days followed by a challenge with 10 ng/kg LPS. IL-1β (interleukin-1β) and IL-18 were measured by ELISA (enzyme-linked immunosorbent assay). In murine studies, quercetin prevented IL-1β secretion from XIAP knockout cells following TLR agonists or TNF-α stimulation (P < .05) and strongly reduced constitutive production of IL-18 by both WT and XIAP-deficient cells (P < .05). At 4 hours after in vivo LPS challenge, blood levels of IL-1β and IL-18 were significantly decreased in mice that had received quercetin-supplemented chow (P < .05). In experiments using human cells, quercetin greatly reduced IL-1β secretion by monocytes following TNF-α stimulation (P < .05). Our data suggest that quercetin may be an effective natural therapeutic for the prevention of XIAP deficiency-associated hyperinflammation. Clinical trials, including careful pharmacokinetic and pharmacodynamic studies to ensure that effective levels of quercetin can be obtained, are warranted.
Introduction
Pathogenic variants in the XIAP/BIRC4 gene lead to an X-linked inborn error of immunity known as XIAP (X-linked inhibitor of apoptosis) deficiency or historically as XLP2 (X-linked lymphoproliferative syndrome type 2). It was originally described to cause hemophagocytic lymphohistiocytosis (HLH), inflammatory bowel disease, and hypogammaglobulinemia.1 Since then, XIAP deficiency has also been associated with recurrent fever syndromes, cytopenias, infections, granulomatous interstitial lung disease, hepatitis, arthritis, uveitis, and skin abscesses.2-6 Pathogenic variants in XIAP/BIRC4 can cause inflammatory bowel disease in female carriers,7 and HLH may also rarely present in severely skewed females with low percentages of cells expressing normal XIAP.8,9 The pathogenesis of disease is complex and differs from the mechanism that underlies most genetic causes of HLH, which relate fundamentally to crippled cytotoxic lymphocyte granule-mediated cytotoxicity.10 Human studies have revealed that patients with XIAP deficiency have markedly elevated levels of total and free IL-18 (interleukin-18) during both active disease and remission, which suggests that these patients have constitutively active inflammasome function.11,12
Inflammasomes typically consist of oligomeric complexes that include a sensing protein such as NLRP3 (NOD- [nucleotide-binding oligomerization domain], LRR- [leucine-rich repeat] and pyrin domain-containing protein 3), the adaptor protein apoptosis-associated speck-like protein containing a CARD (ASC), and caspase-1.13 The pyrin domain (PYD) domain of ASC binds with the PYD domain of inflammasome sensor proteins, and the CARD (caspase activation and recruitment domain) domain of ASC interacts with the CARD domain of caspase-1.13 Two signals are normally required for the NLRP3 inflammasome to become fully active and produce active IL-1β. In traditionally accepted models, a “priming” signal is induced by agents such as Toll-like-receptor agonists or by endogenous cytokines such as TNF-α (tumor necrosis factor-α) that lead to NF-κB signaling and upregulation of NLRP3 and pro–IL-1β.14 Then, in order for the inflammasome complex to assemble and become activated, a second signal is required: the “activation” signal.14 In the laboratory, this is often achieved with ATP (adenosine triphosphate) or nigericin. Inflammasome activation results in the formation of oligomeric inflammasome complexes with activation of caspase-1, which converts pro–IL-1β to active IL-1β, which is then secreted from cells by a poorly understood mechanism. Pro–IL-18 is also converted to active IL-18 by the NLRP3 inflammasome PYD.
Prior work has demonstrated that mice with XIAP deficiency have dysregulated inflammasome function. Murine XIAP-deficient bone marrow (BM)-derived macrophages and dendritic cells secrete large amounts of IL-1β following inflammasome priming signals without the requirement for a second activation signal.15,16 TNF-receptor signaling is abnormal in the absence of XIAP and is required for observation of abnormal IL-1β processing and secretion.15-18 Given that TNF-α is often secreted from cells following TLR (Toll-like receptor) signaling, IL-1β secretion can occur following a variety of stimulatory signaling pathways involving pattern recognition receptors without a second activation signal. As mentioned, human studies have revealed high levels of IL-18 in patients with XIAP deficiency during both active disease and times of remission.11 Unlike pro–IL-1β, pro–IL-18 is constitutively produced by cells and not dependent on a priming signal. Thus, IL-18 is chronically found at high levels in patients with constitutively active inflammasomes (eg, in patients with activating NLRC4 mutations),19,20 and this observation supports constitutively active inflammasome function in human XIAP deficiency.
It is unknown which inflammasome is specifically dysregulated in XIAP deficiency, though previous reports have suggested that it may be NLRP3.15,16 It is also unknown if inhibition of the requisite inflammasome complex would be an effective therapeutic intervention. Here we show that the NLRP3 inflammasome is specifically dysregulated in XIAP-deficient murine BM-derived macrophages. We further demonstrate that inhibition of the NLRP3 inflammasome with the natural flavonoid quercetin prevents aberrant IL-1β secretion, as do other therapeutics that inhibit the NLRP3 inflammasome. Our work suggests that targeting the NLRP3 inflammasome with quercetin or other NLRP3 inhibitors represents a novel therapeutic strategy for patients with XIAP deficiency. Durable and safe treatment approaches are particularly important in XIAP deficiency because definitive treatment with allogeneic hematopoietic cell transplantation remains challenging. Patients suffer increased mortality with fully myeloablative conditioning approaches, and XIAP deficiency also confers an increased propensity for severe graft-versus-host disease (GVHD).21-24 A benign long-term treatment such as quercetin would offer an attractive alternative if effective. Based on our work, we suggest that clinical trials are warranted.
Methods
Mice
The generation of XIAP-deficient mice has been previously described.25 NLRP3 knockout (KO) mice (B6.129S6-Nlrp3tm1Bhk/J) were obtained from Jackson Labs. These mice were bred to generate XIAP and NLRP3 double KO mice by crossing them with XIAP-deficient mice (XIAP–NLRP3 double KO).
Generation of BM-derived macrophages
BM cells isolated from femur and tibia bones of the above mouse strains between 6 and 12 weeks of age were ficolled using lymphocyte separation media (MP Bio) to eliminate any red blood cells or bone fragments and grown at 37°C in a humidified incubator in DMEM (Dulbecco’s Modified Eagle Medium) containing 1% l-glutamine, 1% Pen-Strep, 10% fetal bovine serum (Gibco), and 30% L929 supernatant (ie, BMDM [BM-derived macrophage] growth media) for 7 to 8 days.
Generation of human adherent monocytes
Deidentified patient samples were obtained following informed consent to a study approved by the Cincinnati Children’s Hospital Institutional Review Board. Blood from healthy control subjects or XIAP-deficient patients was ficolled using lymphocyte separation media (MP Bio), and the resulting peripheral blood mononuclear cells were cultured in 6 well plates for an hour in incomplete RPMI (Roswell Park Memorial Institute) media to allow monocyte attachment to the plates. Wells were then washed twice with phosphate-buffered saline, and the remaining adherent monocyte layer was tested in RPMI medium containing 1% l-glutamine, 1% Pen-Strep, and 10% FCS (all Gibco).
Human adherent monocyte and mouse BMDM priming and activation
Human cells were incubated in media containing human TNF-α (Biolegend, 100 ng/mL) with or without quercetin (Cayman Chemical Company, 100 µM) for 4 hours. Mouse BMDMs were stimulated with various stimulations and/or inhibitors for 18 hours for experiments to study IL-1β (as preliminary experiments revealed that stimulation for 4 hours resulted in undetectable IL-1β). Stimulants used include TNF-α (Biolegend, 100 ng/mL), CpG oligodeoxynucleotides (ODN) (Hycult Biotech, 10 ng/mL), Poly I:C (Invivogen, 10 µg/mL), Pam3CSK (Invivogen, 1 µg/mL), lipopolysaccharide (LPS) (Invivogen, 1 µg/mL), nigericin (Invivogen, 1 µM), and inhibitors included caspase-1 inhibitor (N-Ac-Tyr-Val-Ala-Asp-CMK, Cayman Chemical, 100 µM), MCC950 (NLRP3 inhibitor) (Sigma, 100 µM), quercetin (Cayman Chemical, 50 µM or 100 µM), caspase-8 inhibitor (Z-IETD-FMK, R&D Systems, 10 µM), chloroquine (Sigma, 50 µM), and glyburide (Invivogen, 62.5 µg/mL). Inhibitors were added 30 minutes before TNF-α or TLR agonist stimulation. For mouse ELISAs (enzyme-linked immunosorbent assays), each plotted data point represents the mean of duplicates or triplicates. Experiments were repeated 3 to 9 times independently per condition. Depending on the cell counts per harvest, a different number of conditions could be performed each time, leading to the different numbers of independent runs for each condition.
Cytokine production
Cytokine release in culture supernatants of adherent monocytes and BMDMs stimulated as described above were measured by ELISA using capture and detection antibodies directed against mouse IL-1β (Invitrogen), mouse TNF-α (eBioscience), mouse IL-18 (Invitrogen), human IL-1β (Abcam), and human IL-18 (Abcam) according to manufacturer’s protocols.
Immunoblots
Mouse BMDMs were incubated in RIPA (radio immunoprecipitation assay) buffer with protease inhibitors before being homogenized and spun down to remove debris. Supernatants were then resuspended in LDS buffer (Novex) and heated to 90°C for 5 minutes. Lysates were run on an 8% to 10% sodium dodecyl-sulfate–polyacrylamide gel, transferred onto Immobilon-p polyvinylidene difluoride membrane, and blocked in 5% nonfat powdered milk in tris-buffered saline with Tween 20(TBST) (50 mM Tris, pH 7.5, 150 mM NaCl, 0.01% Tween 20). The membrane was incubated with various primary antibodies in 2.5% milk in TBST and washed extensively with TBST before incubating in 1:1500 diluted anti-rabbit or mouse conjugated to horseradish peroxidase (Santa Cruz). Proteins were visualized with ECL Dura reagents (Fisher Scientific) using a Bio-Rad imager. Antibodies directed against NLRP3 (Adipogen, clone Cryo-2), IL-1β (Santa Cruz, clone H-153), TNF-α (R&D, clone 28401), and β-Actin (Sigma, clone AC-15) were used.
Evaluation of reactive oxygen species (ROS)
BMDM ROS production was measured via H2DCFDA staining (Abcam) after stimulation with similar conditions as described above. Data were acquired using a FACSCanto II flow cytometer (BD Biosciences). The fluorescence intensity of treated cells was normalized in each experiment against the mock-treated cells of each cell type to allow pooling of results for analysis.
Viability
The viability of BMDMs was evaluated via flow cytometric analysis of propidium iodide (BD Bioscience) exclusion. After supernatants were collected, cells were scraped from wells and stained with propidium iodide (PI) before being run on a FACSCanto II flow cytometer (BD Biosciences). Viability for each condition was measured, and the percentage of cell loss of treated cells vs no treatment was calculated.
In vivo quercetin treatment and LPS challenge
XIAP-deficient or wild-type (WT) mice were fed mouse chow with or without quercetin 50 mg/kg per day exposure for 7 days. On day 7, mice were challenged with 10 ng/kg LPS, and terminal bleeds were performed 4 hours following the LPS challenge.
Data analysis
Data were plotted and statistically analyzed with Graphpad Prism v8.3. Unless otherwise stated, the Mann-Whitney U test was used and figures marked with n.s. for not significant, * for P ≤ .05, ** for P ≤ .01, and *** for P ≤ .001. Bars represent means, whereas whiskers represent standard deviation.
Results
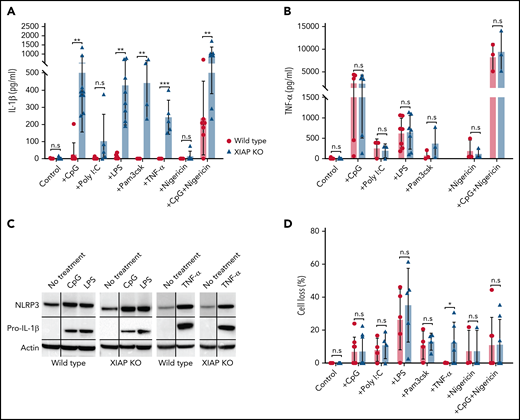
We first performed experiments to confirm that BMDMs from XIAP-deficient mice secrete abnormally large amounts of IL-1β in response to single TLR agonist or TNF-α priming signals without the requirement for a second activation signal. Following CpG ODN (TLR9 agonist), Poly(I:C) (TLR3 agonist), Pam3CSK4 (TLR2 agonist), LPS (TLR4 agonist), or TNF-α, IL-1β was readily detected in the culture supernatants of BMDMs from XIAP-deficient mice (Figure 1A). This was in stark contrast to WT mouse BMDMs which, as expected, did not secrete IL-1β unless stimulated with CpG ODN (or other agonists) together with the potassium ionophore nigericin, which serves as the second activation signal for NLRP3 inflammasome activation (Figure 1A).26 As a control, we measured TNF-α secretion by cells following TLR agonist stimulations (Figure 1B), as well as pro–IL-1β and NLRP3 upregulation in cell lysates following stimulation with CpG ODN, LPS, or TNF-α (Figure 1C) and demonstrated that both WT and XIAP KO cells were similarly stimulated as evidenced by these additional readouts. TNF-α secretion is also of particular importance in the setting of XIAP deficiency, as signaling through TNF-receptors is required for the abnormal IL-1β processing and secretion.15,17 Finally, the cells were evaluated for cell loss, and we observed generally similar viability (Figure 1D) except for the notable observation that TNF-α did not result in any cell death in WT BMDMs but resulted in cell death in a portion of XIAP-deficient BMDMs. Thus the NLRP3 inflammasome pathway of both WT and XIAP KO cells is equally primed but differentially activated.
XIAP-deficient BMDMs hypersecrete IL-1β in response to TLR agonists without the requirement for a second activation signal and in response to TNF-α. (A) Stimulation via various TLR agonists or TNF-α alone leads to IL-1β secretion in XIAP-deficient BMDMs but not WT BMDMs unless WT BMDMs are also stimulated concurrently with nigericin. (B) Comparable levels of TNF-α production indicate that BMDMs from XIAP-deficient or WT mice were stimulated similarly. (C) Pro–IL-1β and NLRP3 were upregulated in both WT and XIAP KO cells upon priming. Horizontal black lines on Western blot images indicate that bands of different molecular weights from the original probe and subsequent reprobes with antibodies against the indicated proteins were cropped together. Vertical black lines indicate cropping to remove an intervening lane between the “No treatment” and either “CpG” or “TNF- α” lanes (the cropped lane contained lysate from quercetin-treated cells). (D) Comparable cell loss in both XIAP and WT BMDMs except following TNF-α. Symbols used: n.s for not significant, * for p≤0.05, ** for p≤0.01, and *** for p≤0.001.
XIAP-deficient BMDMs hypersecrete IL-1β in response to TLR agonists without the requirement for a second activation signal and in response to TNF-α. (A) Stimulation via various TLR agonists or TNF-α alone leads to IL-1β secretion in XIAP-deficient BMDMs but not WT BMDMs unless WT BMDMs are also stimulated concurrently with nigericin. (B) Comparable levels of TNF-α production indicate that BMDMs from XIAP-deficient or WT mice were stimulated similarly. (C) Pro–IL-1β and NLRP3 were upregulated in both WT and XIAP KO cells upon priming. Horizontal black lines on Western blot images indicate that bands of different molecular weights from the original probe and subsequent reprobes with antibodies against the indicated proteins were cropped together. Vertical black lines indicate cropping to remove an intervening lane between the “No treatment” and either “CpG” or “TNF- α” lanes (the cropped lane contained lysate from quercetin-treated cells). (D) Comparable cell loss in both XIAP and WT BMDMs except following TNF-α. Symbols used: n.s for not significant, * for p≤0.05, ** for p≤0.01, and *** for p≤0.001.
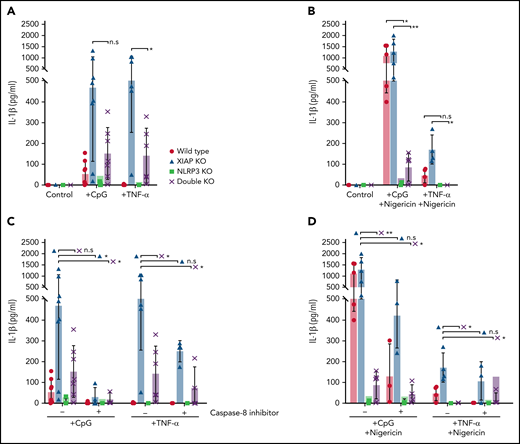
Next, we sought to determine if the NLRP3 inflammasome is specifically dysregulated in XIAP-deficient macrophages. We crossed NLRP3-deficient and XIAP-deficient mice and repeated stimulations. As shown in Figure 2A, the production of IL-1β following CpG ODN or TNF-α priming signals was decreased in XIAP–NLRP3 double KO cells compared with XIAP KO cells, indicating that a dysregulated NLRP3 inflammasome contributes significantly to aberrant IL-1β production in XIAP deficiency. When the stimulation conditions were expanded to include nigericin as the second activation signal together with CpG ODN or TNF-α priming, production of IL-1β by XIAP–NLRP3 double KO cells was still decreased compared with XIAP KO cells and also decreased compared with WT cells (Figure 2B). Consistent with previous literature demonstrating that pro–IL-1β can be converted to active IL-1β by caspase-8 in an inflammasome-independent manner, we found that preincubation with a caspase-8 inhibitor further reduced residual IL-1β production in the XIAP–NLRP3 double KO cells, particularly when the stimulation was with CpG ODN (Figure 2C).15 This was less pronounced when nigericin was present (Figure 2D).
The NLRP3 inflammasome is dysregulated in XIAP deficiency. (A) BMDMs from 4 different mouse strains were preincubated with CpG ODN, TNF-α, or media as control. XIAP KO cells readily produced IL-1β with a single priming stimulation, but this was reduced in XIAP-NLRP3 double KO cells, clearly implying the role of NLRP3 in XIAP deficiency-mediated inflammasome dysregulation. (B) Similarly, the role of the NLRP3 inflammasome in XIAP deficiency-mediated inflammasome dysregulation was also evident in the presence of nigericin. (C) The addition of a caspase-8 inhibitor further reduced residual IL-1beta activation and secretion when cells were primed with CpG ODN or TNF-α alone or (D) additionally activated with nigericin, acting in an inflammasome-independent manner. Symbols used: n.s for not significant, * for p≤0.05, and ** for p≤0.01.
The NLRP3 inflammasome is dysregulated in XIAP deficiency. (A) BMDMs from 4 different mouse strains were preincubated with CpG ODN, TNF-α, or media as control. XIAP KO cells readily produced IL-1β with a single priming stimulation, but this was reduced in XIAP-NLRP3 double KO cells, clearly implying the role of NLRP3 in XIAP deficiency-mediated inflammasome dysregulation. (B) Similarly, the role of the NLRP3 inflammasome in XIAP deficiency-mediated inflammasome dysregulation was also evident in the presence of nigericin. (C) The addition of a caspase-8 inhibitor further reduced residual IL-1beta activation and secretion when cells were primed with CpG ODN or TNF-α alone or (D) additionally activated with nigericin, acting in an inflammasome-independent manner. Symbols used: n.s for not significant, * for p≤0.05, and ** for p≤0.01.
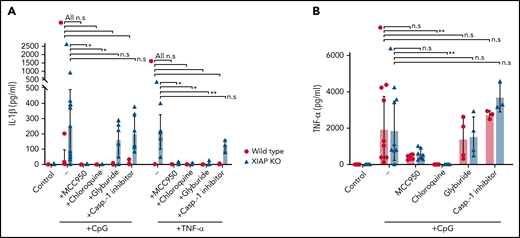
Several mechanisms have been reported to play important roles in activating the NLRP3 inflammasome, including potassium efflux, generation of ROS, and cathepsin B release from lysosomes.27-31 We evaluated known therapeutic agents and inhibitors for their ability to inhibit NLRP3 inflammasome activity and downstream IL-1β secretion in XIAP KO BMDMs. First, the NLRP3 inhibitor MCC950 was found highly effective in preventing IL-1β secretion when cells were primed by either CpG ODN or TNF-α (Figure 3A).32 The same was true for chloroquine, an inhibitor of lysosome acidification (Figure 3A). Glyburide, a potassium efflux inhibitor, and Ac-YVAD-CMK, a caspase-1 inhibitor, were also tested but did not significantly lower IL-1β secretion from XIAP KO macrophages after prestimulation with CpG ODN, though glyburide did have an effect on IL-1β following TNF-α stimulation (Figure 3A).33 Chloroquine treatment also profoundly decreased TNF-α release (Figure 3B).
NLRP3 inflammasome inhibitors have varying effectiveness. WT and XIAP-deficient BMDMs were preincubated with various inhibitors as listed before stimulation with either CpG ODN or TNF-α. Levels of (A) secreted IL-1β and (B) TNF-α were measured. Symbols used: n.s for not significant, * for p≤0.05, and ** for p≤0.01.
NLRP3 inflammasome inhibitors have varying effectiveness. WT and XIAP-deficient BMDMs were preincubated with various inhibitors as listed before stimulation with either CpG ODN or TNF-α. Levels of (A) secreted IL-1β and (B) TNF-α were measured. Symbols used: n.s for not significant, * for p≤0.05, and ** for p≤0.01.
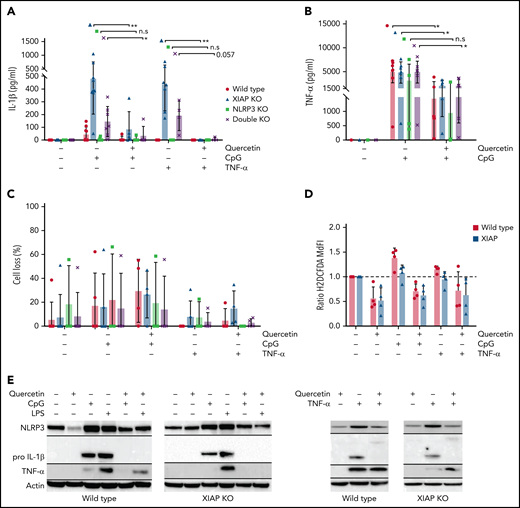
We then examined the natural flavonoid antioxidant quercetin in an effort to discover a benign natural therapeutic with no known adverse consequences following long-term administration. Such an agent would be an ideal therapeutic option for life-long treatment. Quercetin is a potent ROS inhibitor,34,35 which suggests it may attenuate NLRP3 inflammasome-mediated IL-1β activation by inhibiting upstream priming, as other ROS inhibitors are known to inhibit NLRP3 priming,36 and the synthetic antioxidant BHA (butylated hydroxyanisole) inhibits priming in XIAP-deficient cells.17 Additionally, quercetin was shown to interfere with ASC oligomerization independently of any effects on priming and thus prevent IL-1β release via direct inhibition of inflammasome complexes.37 In Figure 4A, we show that quercetin inhibits IL-1β secretion in XIAP KO cells following CpG ODN stimulation as well as residual IL-1β secretion by XIAP–NLRP3 double KO cells. The residual amounts of IL-1β in some experiments could be further blocked by the addition of a caspase-8 inhibitor (Z-IETD-FMK) (supplemental Figure 1, available on the Blood Web site), consistent with previous observations of the ability of caspase-8 to process pro–IL-1β independent of the inflammasome.15 Remarkably, quercetin completely prevented IL-1β secretion in XIAP KO cells following TNF-α stimulation and also completely prevented residual IL-1β secretion in XIAP–NLRP3 double KO cells following TNF-α stimulation (Figure 4A). This is of particular importance in XIAP deficiency as TNF-receptor signaling is required for the observation of abnormal inflammasome activity, and TNF-receptor signaling may be simplistically conceptualized as both a priming and activation signal in the setting of XIAP deficiency.15,17 The ability of quercetin to eliminate residual IL-1β secretion in XIAP–NLRP3 double KO cells supports that quercetin also inhibits IL-1β processing outside of the NLRP3 inflammasome. We noted that quercetin also lowered TNF-α secretion in both WT and XIAP-deficient cells (Figure 4B). This again has particular importance in XIAP deficiency and may offer additional global benefits outside XIAP deficiency as TNF-α is an important transcriptional regulator of the NLRP3 inflammasome.38 Quercetin did not appear to significantly alter cell viability, as shown in Figure 4C, and quercetin decreased ROS as expected, given it is a potent ROS scavenger (Figure 4D). Treatment with quercetin led to decreased NLRP3 and pro–IL-1β upregulation in cell lysates from both WT and XIAP-deficient mice treated with CpG ODN or TNF-α (Figure 4E), indicating that quercetin inhibits NLRP3 inflammasome priming.
Quercetin inhibits NLRP3 inflammasome priming, IL-1beta secretion, and TNF-α production in XIAP-deficient cells. Four strains of mouse BMDMs were preincubated with quercetin before stimulation with either CpG ODN or TNF-α. We then measured the levels of (A) IL-1β, (B) TNF-α, and (C) cell loss. Quercetin reduced the secreted levels of IL-1β and TNF-α. Quercetin reduced levels of (D) ROS (normalized to control), and (E) inhibited upregulation of NLRP3 and pro–IL-1β following CpG ODN or TNF-α priming. Data for lipopolysaccharide (LPS)-treated cells are also shown in block E. Horizontal black lines on Western blot images indicate that bands of different molecular weights from the original probe and subsequent reprobes with antibodies against the indicated proteins were cropped together. Symbols used: n.s for not significant, * for p≤0.05, and ** for p≤0.01.
Quercetin inhibits NLRP3 inflammasome priming, IL-1beta secretion, and TNF-α production in XIAP-deficient cells. Four strains of mouse BMDMs were preincubated with quercetin before stimulation with either CpG ODN or TNF-α. We then measured the levels of (A) IL-1β, (B) TNF-α, and (C) cell loss. Quercetin reduced the secreted levels of IL-1β and TNF-α. Quercetin reduced levels of (D) ROS (normalized to control), and (E) inhibited upregulation of NLRP3 and pro–IL-1β following CpG ODN or TNF-α priming. Data for lipopolysaccharide (LPS)-treated cells are also shown in block E. Horizontal black lines on Western blot images indicate that bands of different molecular weights from the original probe and subsequent reprobes with antibodies against the indicated proteins were cropped together. Symbols used: n.s for not significant, * for p≤0.05, and ** for p≤0.01.
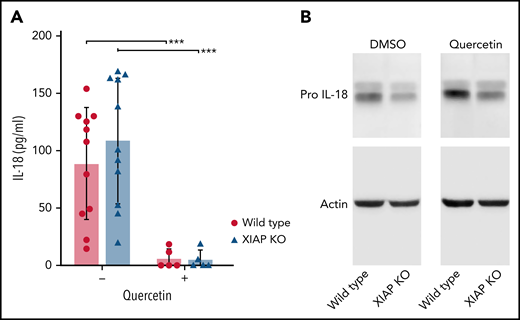
We next evaluated the effect of quercetin on IL-18. Pro–IL-18 production is constitutive and not highly upregulated with inflammasome priming signals. Therefore, we evaluated the effect of quercetin on baseline IL-18 secretion by BMDMs cultured for 7 days. As shown in Figure 5A, quercetin strongly reduced levels of IL-18 in culture supernatants from both WT and XIAP-deficient cells. Cell lysates demonstrated equivalent pro–IL-18 levels with and without quercetin treatment at 1 week (Figure 5B) and later time points (data not shown). These results hint at quercetin reducing IL-18 processing and excretion.
Quercetin inhibits IL-18 secretion but not pro–IL-18 production. BMDM from WT and XIAP KO mice were incubated with or without quercetin for 7 days. (A) IL-18 was measured in culture supernatants and (B) pro–IL-18 was detected in cell lysates. Symbols used: *** indicates p≤0.001.
Quercetin inhibits IL-18 secretion but not pro–IL-18 production. BMDM from WT and XIAP KO mice were incubated with or without quercetin for 7 days. (A) IL-18 was measured in culture supernatants and (B) pro–IL-18 was detected in cell lysates. Symbols used: *** indicates p≤0.001.
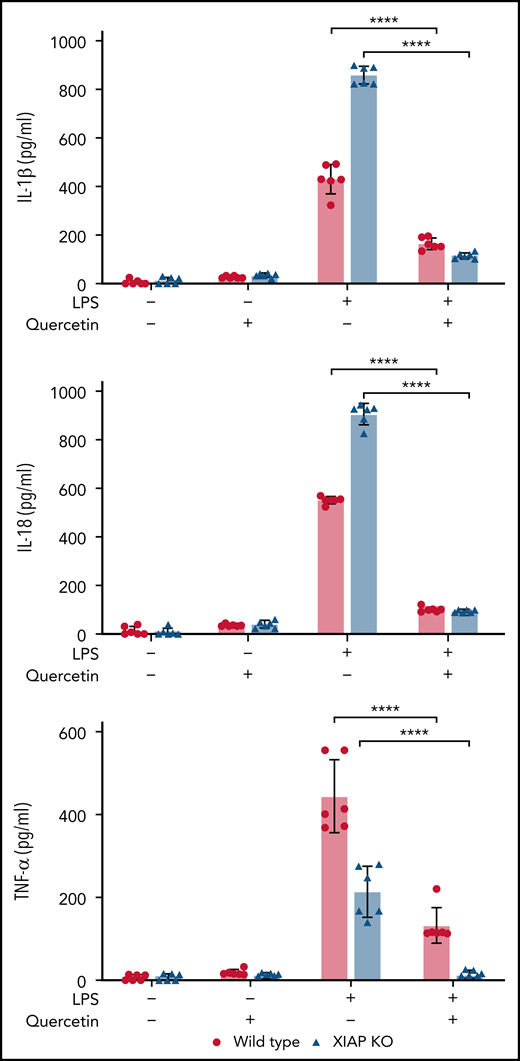
We next wanted to determine if in vivo treatment of mice with quercetin would ameliorate inflammasome-derived cytokines following a challenge with a singular TLR agonist. We made use of the widely used LPS challenge model. XIAP-deficient or WT mice were fed mouse chow with or without quercetin (50 mg/kg per day exposure) for 7 days, and on day 7 were challenged with LPS. At 4 hours after LPS, blood levels of IL-1β, IL-18, and TNF-α were significantly decreased in mice that received quercetin-supplemented chow (Figure 6).
Quercetin inhibits IL-1β, IL-18, and TNF-α following in vivo LPS challenge. XIAP-deficient or WT mice were fed mouse chow with or without quercetin (50 mg/kg per day exposure) for 7 days and, on day 7, were treated with LPS. Blood cytokines were measured at 4 hours after LPS. Symbols used: **** indicates p≤0.0001.
Quercetin inhibits IL-1β, IL-18, and TNF-α following in vivo LPS challenge. XIAP-deficient or WT mice were fed mouse chow with or without quercetin (50 mg/kg per day exposure) for 7 days and, on day 7, were treated with LPS. Blood cytokines were measured at 4 hours after LPS. Symbols used: **** indicates p≤0.0001.
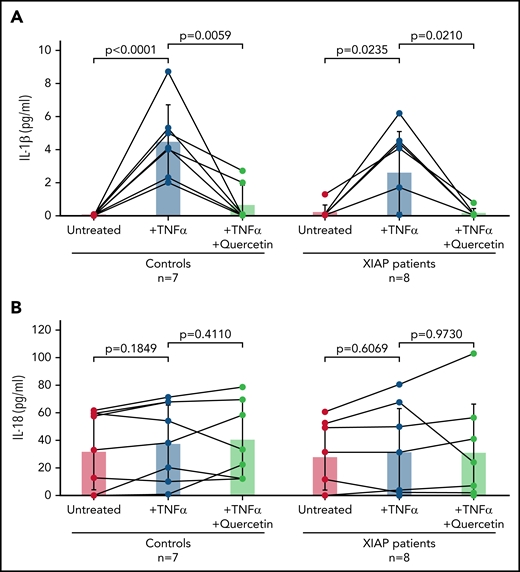
Finally, we obtained blood samples from 8 patients with known XIAP deficiency (2 patients were related) and 7 healthy individuals. Pathogenic variants included XIAP c.389_392delACAG (p.D130fsX140), c.1189delA (p.I397fs), c.1141C>T (p.R381*), c.997_1001del (p.Gln333fs), c.225_226insA (p.Gly76Argfs*24), c.60del (p.Glu21Lysfs*4), and c.664C>T (p.R222*). IL-1β was secreted upon treatment with TNF-α in both control subjects and patients, as human peripheral blood monocytes are known to have constitutively activated caspase-1 and inflammasome function.39 IL-1β production was attenuated by prior quercetin treatment in both control subjects and patients (Figure 7A). We also examined IL-18 levels with no difference observed between conditions (Figure 7B), which was expected given the short length of time of the experiment. Of note, 2 patients receiving clinical treatment with TNF-α inhibitors, 1 on etanercept and 1 on infliximab, produced little to no IL-1β upon TNF-α stimulation, confirming these agents strongly inhibited TNF-α.
Quercetin attenuates IL-1β secretion by human monocytes in vitro. Blood from healthy control subjects or XIAP-deficient patients was ficolled using lymphocyte separation media, and the resulting peripheral blood mononuclear cells were cultured in 6 well plates to allow monocyte attachment to the plates. Control and XIAP-deficient patient monocytes were stimulated as indicated, and supernatants were assayed for (A) IL-1β and (B) IL-18. Bars indicate the mean, whereas lines indicate standard deviation. Wilcoxon matched-pair test was used. Exact P values noted.
Quercetin attenuates IL-1β secretion by human monocytes in vitro. Blood from healthy control subjects or XIAP-deficient patients was ficolled using lymphocyte separation media, and the resulting peripheral blood mononuclear cells were cultured in 6 well plates to allow monocyte attachment to the plates. Control and XIAP-deficient patient monocytes were stimulated as indicated, and supernatants were assayed for (A) IL-1β and (B) IL-18. Bars indicate the mean, whereas lines indicate standard deviation. Wilcoxon matched-pair test was used. Exact P values noted.
Discussion
The NLRP3 inflammasome complex is the most studied of all inflammasomes and has been associated with a large number of autoimmune and inflammatory diseases, including Alzheimer's disease, cancer, colitis, COVID-19, metabolic syndromes, multiple sclerosis, skin diseases, and type 2 diabetes.40-43 The inflammation-inducing cytokines IL-1β and IL-18 arise from the direct conversion of pro–IL-1β and pro–IL-18 by active inflammasome complexes.44 Knowing that XIAP-deficient patients constitutively express high levels of IL-18 and that inflammasome function is dysregulated downstream of TNF-receptor signaling, we postulated that the NLRP3 complex might represent a crucial and targetable contributor to XIAP deficiency-associated hyperinflammation.11,12,15-18 Our work presented here confirms that the NLRP3 inflammasome plays a critical role in XIAP deficiency-associated hyperinflammation and that targeting the NLRP3 inflammasome represents a viable therapeutic strategy.
Currently, the only definitive cure for XIAP deficiency is allogeneic hematopoietic cell transplantation (HCT). HCT is a high-risk procedure, particularly for XIAP-deficient patients. XIAP-deficient patients appear prone to particularly high mortality with traditional fully myeloablative conditioning regimens.21 Deficiency of XIAP in recipient tissues also conveys an increased risk of severe and fatal GVHD in murine models,22,23 and patients suffer poor outcomes related to GVHD.24 In light of these data, investigations into possible alternative life-long therapies are a high priority.
We observed that several indirect or direct NLRP3 inflammasome inhibitors have therapeutic potential for patients with XIAP deficiency. Quercetin is an exceptionally promising agent given that it is a naturally occurring ROS scavenger, and it profoundly inhibits NLRP3 inflammasome priming. It also inhibited TNF-α secretion, which is beneficial in the setting of XIAP deficiency. Dietary quercetin supplementation of XIAP-deficient mice attenuated in vivo hyperinflammation associated with LPS challenge, providing proof of concept that daily enteral supplementation can offer therapeutic benefit. The pharmacokinetics of quercetin in adult human subjects has been described in detail. In one study by Moon and colleagues, the average peak quercetin concentration was 463 ng/mL 3.5 hours after 500 mg of quercetin (total of 1500 mg/day dosing).45 In our experience in a pediatric Fanconi anemia (FA) phase 1 study, similar exposure was achieved with allometrically scaled weight-based dosing (unpublished). The lowest concentration used in our in vitro experiments presented here as well as preclinical in vitro FA animal studies and patient sample studies was 50 µM, which equals 15 111 ng/mL, roughly 30 times higher than peak levels observed by Moon and colleagues. However, we were able to achieve biologically relevant blood levels with ROS reduction in FA patients and were able to recommend optimized dosing for pediatric patients. Careful correlation of human pharmacokinetic studies with early pharmacodynamic readouts such as peripheral blood ROS levels and IL-18 levels will be needed to ensure that in vivo levels of quercetin in humans result in a meaningful physiologic effect in XIAP patients also.
Quercetin may represent a benign alternative to therapeutics currently used for or being studied for the treatment of XIAP deficiency, or it may offer a simple way to boost additive or synergistic effect in combination with other targeted approaches. Agents directed against IL-1β may be effective in some patients with XIAP deficiency,46 and treatment with IL-18–targeted therapy is currently being tested in a human trial for patients with XIAP deficiency and with activating NLRC4 mutations (https://clinicaltrials.gov/ct2/show/NCT03113760). Anti-TNF approaches are often clinically used with varying results. The ability of quercetin to simultaneously prevent TNF-α, IL-18, and IL-1β secretion may offer obvious advantages in terms of potential therapeutic benefit in the setting of XIAP deficiency either instead of or in combination with other agents. We are not aware that quercetin has been trialed in any inherited inflammasome dysfunction disorders, but quercetin was shown to inhibit IL-1β secretion and ASC oligomerization in BMDMs derived from mice used to model cryopyrin-associated periodic syndromes with an activating NLRP3 A350V mutation.37
Another benefit of quercetin is that it is a naturally occurring substance. It is a flavonoid found in many fruits, vegetables, and nuts and is already sold as a dietary supplement; hence it is a likely benign and safe agent with far fewer side effects compared with the other synthesized NLRP3-blocking compounds.35 Indeed, an ongoing pilot study of quercetin use in patients with FA has been associated with no serious adverse events related to quercetin.47
In addition to XIAP deficiency, our work has more broad implications. NLRP3 inhibitors are of significant interest and are under a great deal of investigation by multiple pharma companies for the treatment of a wide variety of common diseases.48 Quercetin may offer a benign treatment approach to other conditions besides XIAP deficiency where NLRP3 dysregulation has been observed.40,41,43 Our data provide the rationale to conduct a human trial of quercetin for patients with XIAP deficiency in hopes of finding an effective long-term therapy, and such efforts may lead to widespread investigations into common disorders.
Acknowledgments
The authors thank Colin Duckett for gifting XIAP-deficient mice.
This work was funded by the Matthew and Andrew Akin Foundation, Histio UK, and Mason Spencer Lives On Fund at Illinois Prairie Community Foundation.
Authorship
Contribution: S.C.C.C. created figures and wrote the manuscript; E.O. performed experiments, organized data, and edited the manuscript; N.P., N.A., V.C., and C.E.T. performed experiments; P.A.M. and S.M.D. contributed valuable quercetin expertise and edited the manuscript; M.B.J. and C.B. contributed critical expertise and edited the manuscript; and R.A.M. directed experiments and figure generation and wrote and edited the manuscript.
Conflict-of-interest disclosure: S.M.D. receives research funding from Alexion and is a consultant with Novartis Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Rebecca A. Marsh, Cincinnati Children's Hospital Medical Center, 3333 Burnet Ave, Cincinnati, OH 45229; e-mail: rebecca.marsh@cchmc.org.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
REFERENCES
Author notes
S.C.C.C. and E.O. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal