Abstract
Infection with the SARS-CoV-2 virus, resulting in COVID-19 disease, has presented a unique scenario associated with high rates of thrombosis. The risk of venous thrombosis is some three- to sixfold higher than for patients admitted to a hospital for other indications, and for patients who have thrombosis, mortality appears to increase. Thrombosis may be a presenting feature of COVID-19. Pulmonary thrombi are the most frequent events, some related to deep vein thrombosis, but also to in situ microvascular and macrovascular thrombosis. Other venous thromboses include catheter- and circuit-associated in patients requiring hemofiltration and extracorporeal membrane oxygenation. Arterial thrombosis is less commonly documented, with 3% of patients in intensive care units having major arterial strokes and up to 9% having myocardial infarction, both of which are most likely multifactorial. Risk factors for thrombosis above those already documented in hospital settings include duration of COVID-19 symptoms before admission to the hospital. Laboratory parameters associated with higher risk of thrombosis include higher D-dimer, low fibrinogen, and low lymphocyte count, with higher factor VIII and von Willebrand factor levels indicative of more severe COVID-19 infection. All patients should receive thromboprophylaxis when admitted with COVID-19 infection, but the dose and length of treatment are still debated. Thrombosis continues to be treated according to standard VTE guidelines, but adjustments may be needed depending on other factors relevant to the patient’s admission.
Introduction
COVID-19 disease is the result of infection with the SARS-CoV-2 virus.1 Many individuals have mild disease or may be asymptomatic, but ∼20% of cases of severe or moderate disease require hospital admission. Definitions have been published, with severe COVID-19 infection identified as SpO2 <94% on room air at sea level, the ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2) <300 mm Hg, a respiratory rate >30 breaths per minute, or lung infiltrates >50%.2
COVID-19 infection is a thromboinflammatory disorder, and the primary prothrombotic features are termed “COVID-19-associated coagulopathy.”3 Evidence suggests that this is linked to the inflammatory response against SARS-CoV-2, with complex interactions between the viral, immune, and inflammatory systems and coagulation pathways at local and systemic levels, and the inflammatory response and endothelial injury involved in development of microvascular and macrovascular thrombosis. COVID-19 has been associated with increased rates of venous thromboembolism (VTE), including pulmonary embolism (PE) and deep vein thrombosis (DVT), and arterial thromboses, including acute ischemic stroke and peripheral arterial thrombosis. Concurrent venous and arterial thromboses have been reported.4 In severe COVID-19 disease, there is an increased risk of bleeding.5
Laboratory features of coagulopathy
Initial studies from Wuhan reported abnormalities in clotting parameters in COVID-19, including elevated D-dimer levels, and it was rapidly recognized that these abnormal parameters were associated with prognosis. Early studies showed an association between D-dimer level and survival,6 and elevated D-dimer continues to be a consistent marker of poor outcome.7 COVID-19–associated coagulopathy3 has been described as a unique syndrome distinct from bacterial sepsis–induced coagulopathy and disseminated intravascular coagulation (DIC), with increased fibrinogen and D-dimer levels in COVID-19–associated coagulopathy, but initially, only minimal changes in platelet count and prothrombin time.3 Comparably, the levels of natural anticoagulants (protein C and antithrombin) are in the normal range and levels of protein S are reduced.8 Other features include marked elevation of factor VIII and von Willebrand factor (VWF). VWF antigen/ADAMTS-13 activity ratio has been associated with the degree of severity of COVID-19 infection.9
The platelet count is generally well preserved in COVID-19 disease but, in a large retrospective study, 20% patients had platelets <150 × 109/L.10 Mortality rates were higher with severe thrombocytopenia with relative risk of death at 3.42 (95% confidence interval [CI], 2.36-4.96), for platelet count 100 × 109/L to 150 ×109/L; 9.99 (95% CI, 7.16-13.94) for platelets 50 × 109/L to 100 × 109/L and 13.68 (95% CI, 9.89-18.92) for platelets <50 × 109/L.10 The incidence of DIC is low in COVID-19, reported at <1%, even in severe cases,4,11 and is often a preterminal event.12 The higher rate of 8.7% (16 of 183) cases meeting International Society on Thrombosis and Haemostasis criteria for diagnosis of DIC, is still below that seen in sepsis, where DIC occurs in ∼30% of cases.12 The progressive consumptive coagulopathy in the later phases of some COVID-19 cases may be due to superimposed bacterial infection.3 Thus, clinically relevant thrombocytopenia, reduced fibrinogen, and DIC are rare in patients with COVID-19, but have been associated with significant manifestations of bleeding.13
Autopsy studies
Pulmonary microthrombi are commonly found at the postmortem examination of patients with COVID-19, in addition to the major pulmonary thrombosis reported as a common fatal complication in autopsy series.14 One study compared lung autopsies from patients with COVID-19 and H1N1 influenza, and alveolar capillary microthrombi were 9 times more common in patients with COVID-19, consistent with the clinical increase in thrombosis compared with many other viral pneumonias.15 Microthrombi have been identified at autopsy in some extrapulmonary organs, but less consistently across studies and at significantly lower rates.16,17
VTE in COVID-19
Incidence of VTE
Patients admitted to a hospital with COVID-19 infection are at high risk for venous thrombosis, but accurate assessment of the true incidence has been challenging. Reported rates vary, not only with study quality, but with stage of disease and where the patient is treated (medical unit vs intensive care unit [ICU]). These factors, in turn, may also affect the intensity of anticoagulation used as thromboprophylaxis, which may affect the likelihood of development of thrombosis. Prophylactic anticoagulation is not routinely used as the standard of care in hospitalized patients in some countries (eg, China), thus the high incidence of DVT in early Chinese studies could be due to prolonged hospital stays and immobility without thromboprophylaxis.18 Other factors affecting thrombosis rates include proactive screening for DVT using Doppler ultrasonography (US), or PE with computed tomographic pulmonary angiography. Interpretation is further complicated because (1) the criteria for hospitalization with COVID-19 and thresholds for ICU admission differ between institutions, (2) the difference in comorbidities may also influence thrombosis rates, and (3) the treatments have improved over time.19
Meta-analyses have estimated overall VTE rates of 12% to 31% in COVID-19 inpatients, with lower VTE rates in more recent metanalyses20-30 (Table 1).
Selected systematic reviews and meta-analyses of VTE rates in COVID-19 inpatients
| Meta-analysis . | Data cutoff . | Studies included, n . | Patients, n . | Overall VTE prevalence % . | VTE prevalence screened . | VTE not screened . | VTE prevalence ICU, % . | VTE Non-ICU, % . | PE, % . | DVT, % . |
|---|---|---|---|---|---|---|---|---|---|---|
| Chi et al21 | May 2020 | 11 | ∼2 000 | 23.9 | — | — | 30.4 | 13 | 5.6 non-ICU; 15.7 ICU | 13.6 non-ICU; 10.6 ICU |
| Porfidia et al26 | Jun 2020 | 30 | 3 500? | 26 | 13 | 6 | 24 | 9 | 12 | 14 |
| Jiménez et al28 | July 2020 | 49 | >18 000 | 17 | 33.1% | 9.8% | 27.9 | 7.1 | 12.1 | 7.1 |
| Nopp et al20 | August 2020 | 66 | >28 000 | 14.1 | 40.3% | 9.5% | 22.7 | 7.9 | 3.5 non-ICU 13.7 ICU | — |
| Tan et al30 | September 2020 | 102 | 64 000 | 14.7 | 25.2% | 12.4% | 23.2 | 9 | 7.8 | 11.2 |
| Mansory et al29 | December 2020 | 91 | 35 000 | 12.8 | — | — | 24.1 | 7.7 | — | — |
| Meta-analysis . | Data cutoff . | Studies included, n . | Patients, n . | Overall VTE prevalence % . | VTE prevalence screened . | VTE not screened . | VTE prevalence ICU, % . | VTE Non-ICU, % . | PE, % . | DVT, % . |
|---|---|---|---|---|---|---|---|---|---|---|
| Chi et al21 | May 2020 | 11 | ∼2 000 | 23.9 | — | — | 30.4 | 13 | 5.6 non-ICU; 15.7 ICU | 13.6 non-ICU; 10.6 ICU |
| Porfidia et al26 | Jun 2020 | 30 | 3 500? | 26 | 13 | 6 | 24 | 9 | 12 | 14 |
| Jiménez et al28 | July 2020 | 49 | >18 000 | 17 | 33.1% | 9.8% | 27.9 | 7.1 | 12.1 | 7.1 |
| Nopp et al20 | August 2020 | 66 | >28 000 | 14.1 | 40.3% | 9.5% | 22.7 | 7.9 | 3.5 non-ICU 13.7 ICU | — |
| Tan et al30 | September 2020 | 102 | 64 000 | 14.7 | 25.2% | 12.4% | 23.2 | 9 | 7.8 | 11.2 |
| Mansory et al29 | December 2020 | 91 | 35 000 | 12.8 | — | — | 24.1 | 7.7 | — | — |
In the systematic review and meta-analysis of 49 studies and >18 000 patients by Jiménez et al, pooled incidence for VTE in patients with COVID-19 was 17.0%.28 Incidence of DVT was 12.1% and PE was 7.1%. In subgroup meta-analyses, VTE incidence was higher when identified by screening (33.1%) than when identified by clinical diagnosis (9.8%), in patients in ICU (27.9%) vs those in medical units (7.1%), in prospective studies (25.5%), compared with retrospective studies (12.4%) and when catheter-associated thrombosis/isolated distal DVTs and isolated subsegmental PEs were included.28
VTE rates compared with other diseases
Critically ill patients exhibit a VTE rate of 5% to 10%, despite thromboprophylaxis.31 VTE rates in patients with COVID-19 appear greater still, with 3 to 6 times the expected rates of thrombosis in COVID-19 populations vs retrospective comparator non-COVID-19 ICU populations.4,32 COVID-19 has an increased PE rate, compared with severe acute respiratory syndrome and Middle East respiratory syndrome or H1N1 influenza.33 However, the high VTE rates in critically ill patients with COVID-19 may simply be consistent with a particularly sick ICU subpopulation (eg, in patients without COVID-19 with severe sepsis and septic shock, VTE incidence was 37% with systematic screening, despite patients receiving thromboprophylaxis34).
Timing of VTE
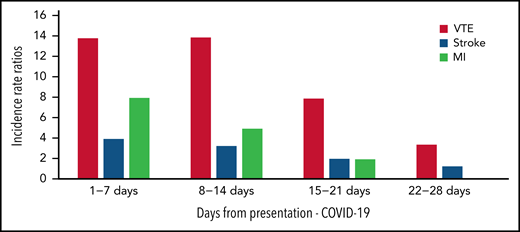
Thrombosis may be the presenting feature of COVID-19,35,36 and a significant proportion of events are diagnosed soon after hospital admission. In an Italian study, ∼50% of patients were diagnosed with thrombosis within 24 hours of hospital admission.37 National data from the United Kingdom on COVID-19 vaccination, SARS-CoV-2–positive test data and hospital admissions to the National Health Service describes thrombosis risk and timing of thrombotic events38 (Figure 1) and showed highest incidence rate ratios of VTE on days 1 to 7 (13.78 [12.66 to 14.99]) and days 8 to 14 (13.86 [12.76 to 15.05]) after a positive SARS-CoV-2 test result.38
Incidence rate ratios of VTE, stroke, and myocardial infarction in COVID-19 infection. Timing of venous and arterial event from presentation of COVID-19 infection to day 28.38
Incidence rate ratios of VTE, stroke, and myocardial infarction in COVID-19 infection. Timing of venous and arterial event from presentation of COVID-19 infection to day 28.38
A VTE incidence of 2.6% in the first 90 days after discharge in patients without thromboprophylaxis has been documented.39 However, in a prospective multicenter study of >1 800 discharged patients, 6-week VTE rate was 4.8 of 1000 discharges, similar to baseline rates in the previous year of 3.1 of 1000 discharges.40 In a prospective, observational, single-center study of 485 consecutive patients with systematic screening for VTE, VTE incidence was low at 6 weeks after COVID-19 hospitalization, with 1 DVT (0.7%) and 1 symptomatic PE (0.7%) detected. Postdischarge thromboprophylaxis was prescribed in 28%.41
Sites of VTE: PE and DVT
Classic DVT embolizing to PE may be relatively less common in COVID-19,42 and a high incidence of pulmonary thrombosis without DVT has been described, with DVT present in only 42% patients with COVID-19 with PE in 1 meta-analysis,27 lower than the usual prevalence of 60%.43 One reason could be that the thrombus has completely embolized, but another explanation is that it supports the increased incidence of in situ thrombosis and microvascular thrombosis identified at postmortem examination in patients with COVID-19.33,44 The contribution of in situ thrombosis and thrombosis in smaller vessels is further reinforced by the high incidence of peripheral rather than central pulmonary emboli.45 Dual-energy computed tomographic imaging has been used to study lung perfusion in COVID-19, and scan appearances also appear more in keeping with pulmonary thrombosis in situ than embolism.46 The mechanism of in situ thrombosis most likely still reflects Virchow’s triad with endothelial inflammation and systemic and local prothrombotic changes and altered local pulmonary blood flow in response to lung parenchymal processes.42 Further confirmation of this theory was based on histological analysis.15
Cerebral venous sinus thrombosis
Patients with COVID-19 may present with central venous sinus thrombosis (CVST).47,48 A multinational retrospective study reported on all cases of CVST with COVID-19 infection up to June 2020 and compared 13 patients to historic patients with CVST from the same centers. Patients with COVID-19 tended to be older and had fewer standard CVST risk factors and poorer outcomes.49 A recent multicenter cohort study of 13 500 consecutive patients with COVID-19 over a 3-month period reported an image-proven CVST incidence of 8.8 per 10 000 cases, much higher than the expected rate of 5 per million per year.50 A retrospective cohort study based on electronic health records of >500 000 patients with COVID-19 found the incidence of CVST in the 2 weeks after a COVID-19 diagnosis was 42.8 per million people.38 The mortality rate in 1 study was 25%, despite the standard management of anticoagulation, endovascular thrombectomy, and surgical hematoma evacuation.50 Variable increases in inflammatory and hypercoagulability markers were found, but it is unclear whether monitoring them has any value for predicting risk or severity of sinus thrombosis.51
Other venous thrombotic complications
Other thrombotic complications are well described in COVID-19 and include central venous catheter–associated thrombosis and thrombosis in venous-venous extracorporeal membrane oxygenation circuits.13,36 Very high rates of thrombosis in extracorporeal circuits (up to 8%) have been reported.4 In an international cohort study from the Extracorporeal Life Support Organization (ELSO) of extracorporeal membrane oxygenation support in COVID-19, circuit changes were required in 148 (15%) of 983 patients,52 and hemodialysis circuits have been observed to clot at unusually high frequency (28 of 29 patients on renal replacement therapy in 1 cohort).4
Impact of VTE on prognosis
Thrombotic complications in COVID-19 have been associated with increased mortality in several studies.36,53 In a meta-analysis of 42 studies including >8000 patients with COVID-19, a thrombotic complication was associated with 74% increase in odds of overall mortality (23% vs 13%).54 It is unknown if thrombosis is a direct cause of the worse outcomes or a marker of disease severity. A further meta-analysis showed no difference in mortality between hospitalized patients with COVID-19, with and without VTE, although patients with VTE were more likely to be admitted to ICU and mechanically ventilated.55
The long-term impact of COVID-19–related VTE is unclear. The usual incidence of chronic thromboembolic pulmonary hypertension after acute PE is 2% to 3%,56 but persistent respiratory symptoms will be difficult to disentangle initially from post-ICU morbidity and long COVID.
Predicting VTE: risk factors for thrombosis
Early investigators identified standard VTE risk factors, such as age, obesity, smoking, immobilization, previous VTE, and comorbidities,57 as well as ICU-associated risk factors58 that contribute to venous thrombosis risk in COVID-19. Laboratory parameters associated with higher risk for VTE include higher D-dimer, lower lymphocyte count, or higher neutrophil/lymphocyte ratio and prolongation of clotting times, with D-dimer being the strongest predictor.53,59 Ethnicity (Black race) was also identified as a thrombosis risk factor in 1 study.60 Li et al developed the “Wuhan score” for thrombosis prediction with risk factors comprising age, cancer, longer duration of symptoms before admission, lower fibrinogen, higher D-dimer, and D-dimer increment. D-dimer increment ≥1.5-fold had the most significant association with VTE in hospitalized patients with COVID-19 in their cohort.61 Hospitalized patients with cancer and COVID-19 have elevated risk of thrombosis, and a specific VTE risk assessment model has been developed for this group, including cancer subtype, VTE history, ICU admission, D-dimer, recent systemic anticancer therapy, and non-Hispanic ethnicity.62
Diagnosing VTE
COVID-19 symptoms may overlap with symptoms associated with presentation of PE.63 In severe cases, COVID-19 pneumonitis and PE often coexist. Clinical guidelines on management of patients with COVID-19 recommend imaging for PE only when it is clinically suspected and not to apply routine screening,64 with computed tomographic pulmonary angiography as the first-line imaging method because of its high degree of accuracy and ability to identify coexisting lung pathologies. Particular challenges include unstable patients who cannot be transferred or patients with acute kidney injury where contrast may be an issue. Compression US can assess for DVT, although absence of DVT does not imply the absence of pulmonary thrombosis, particularly because of the potential for in situ pulmonary thrombosis in COVID-19.65 Echocardiography may suggest PE with a clot in the right side of the heart or new right heart strain, but has a more limited diagnostic role.65
Treating VTE
Current guidelines recommend management as for non-COVID VTE, with anticoagulation being the cornerstone of therapy, as there is a paucity of COVID-19–specific evidence in this area.64 Risk stratification remains central to identifying patients at increased risk of early death who may benefit from reperfusion therapy, mechanical circulatory support, or both.65 The inclusion of troponin in the definition of intermediate-risk PE (right ventricular strain on echocardiogram or imaging, raised troponin, and elevated pulmonary embolism severity index score) is confounded by the fact that troponin levels are frequently increased in COVID-19.65,66
Parenteral anticoagulation is preferred in critically ill patients with evolving practice worldwide toward low-molecular-weight heparin (LMWH) becoming the standard of care for prevention and treatment of thromboembolism compared with unfractionated heparin (UFH), due to better bioavailability, fixed dosing, and decreased risk of heparin-induced thrombocytopenia.67 The duration of anticoagulation on discharge follows the standard guidance for anticoagulation for non-COVID VTE.64
Arterial thromboembolism
We have less data to inform us about rates of arterial thromboembolism (ATE) in COVID-19, but increased arterial events in patients with COVID-19 have been reported to affect up to 3% of patients in ICU (95% CI, 2-5).36
Acute ischemic stroke
Acute cerebrovascular events such as stroke may occur in relation to COVID-19 infection,68,69 with an incidence of up to 3%,36,37 and a propensity toward occlusion of large vessels, multiterritory vessels, and uncommon vessels (eg, pericallosal artery).70 In 2 retrospective studies, stroke symptoms developed later during the hospital admission, a median of 10 days after symptom onset71,72; however, diagnosing stroke in sedated, ventilated patients may be challenging. In a proportion of patients with COVID-19 presenting with stroke, diagnosis of the viral infection was made after admission,51 and guidelines therefore recommend appropriate personal protective equipment for acute stroke teams.
Stroke patients with COVID-19 infection have a higher severity score (National Institutes of Health Stroke Scale) at admission73,74 and a more severe disease course.71,72 More recently, a case series reported large-vessel stroke as a presenting feature of COVID-19 in the young (<50 years).75 The role of mechanical thrombectomy in patients who have an acute ischemic stroke with COVID-19 is yet to be determined.76 Evidence from current studies indicates a negative impact of COVID-19 on outcomes in stroke patients who undergo mechanical thrombectomy, irrespective of timely, successful angiographic recanalization.76
Cardiac thrombosis
In >3000 hospitalized patients with COVID-19, myocardial infarction was the commonest arterial thrombotic complication, occurring in 8.9% of patients.16 Some cardiac complications in COVID-19 may be related directly to thrombosis, but elevated myocardial injury markers may be seen in patients with COVID-19 without macrovascular occlusion.6 Alternative mechanisms include direct viral myocyte invasion, myocarditis, endothelial damage, type 2 myocardial infarction, and stress cardiomyopathy, and hence cardiac involvement is likely to be multifactorial.77,78
Preventing thrombosis and reducing mortality?
Given the increased risk of both macrovascular and microvascular thrombosis in patients with COVID-19, anticoagulation therapy has been explored from the early stages of the pandemic. Early data on admitted patients who were receiving long-term anticoagulation suggested a reduced thrombosis rate but no effect on mortality,36 but results from differing cohorts were inconsistent. However, in propensity score–matched cohorts, no significant difference was found in mortality, time until ventilation, or length of stay.84,85
There have been >75 clinical trials registered investigating different primary thromboprophylactic strategies in COVID-19,86 most studying heparin therapies. Table 2 summarizes the design and outcomes of the major recent randomized clinical trial (RCT) and international multiplatform studies.
Design and outcomes of the major RCTs and international multiplatform studies of thromboprophylaxis in COVID-19 disease
| Study . | Study type . | Comparator . | Population . | Sample size, N . | Primary outcome . | Bleeding . | Comment . |
|---|---|---|---|---|---|---|---|
| Sadeghipour et al (INSPIRATION)87 | RCT | Prophylactic vs intermediate intensity enoxaparin (1 mg/kg daily) | ICU | 562 Iran | No significant difference in primary composite outcome (venous or arterial thrombosis, ECMO, or 30-d mortality) | Bleeding events rare. Major bleeding and CRNMB, 6.2% intermediate-dose vs 3.1% prophylactic; P = NS. | Not critically ill ICU population; only 50% of each cohort had invasive or NIV. Median number of days was 7 in both groups |
| Perpepu et al113 | RCT | Prophylactic vs intermediate intensity enoxaparin | ICU or coagulopathy D-dimer ≥1.00 ug/mL | 156 United States | No significant difference in preventing death or thrombosis at 30 d. | Major bleeding occurred in 2% of patients in each arm | ICU and non-ICU patients included |
| Goliger et al (International multiplatform trial)88 | Combined 1 RCT (ACTIV-4a) + 2 studies with response-adaptive randomization (ATTACC and REMAP-CAP) | Prophylactic heparin vs therapeutic dose UFH or LMWH | Critically ill | 1098 | No difference in primary outcome of days free from organ support or survival until hospital discharge, despite decreasing major thrombotic events | Major bleeding, 3.8% therapeutic, 2.3% thromboprophylaxis; not significantly different | Seemingly contradictory outcomes in a non–critically ill population (see text). |
| Lawler et al (International multiplatform trial)92 | Combined 1 RCT (ACTIV-4a) + 2 studies with response-adaptive randomization (ATTACC and REMAP-CAP) | Prophylactic heparin vs therapeutic dose UFH or LMWH | Non–critically ill | 2219 | Reduced organ support–free days to day 21 (primary outcome) | Major bleeding, 1.9% therapeutic, 0.9% thromboprophylaxis | Treatment benefit independent of D-dimer level, although treatment effect greater in patients with higher D-dimer |
| Lopes et al (ACTION)90 | Open-label RCT | Therapeutic Anticoagulation: rivaroxaban 20 mg or enoxaparin 1 mg/kg twice daily for clinically unstable patients (defined similarly to critically unwell/ICU patients in heparin studies) vs SOC enoxaparin or UFH prophylaxis | COVID-19 inpatients D-dimer >ULN 90% non–critically ill | 615 Brazil | No difference in primary outcome of time to death, duration of hospitalization and supplemental oxygen to day 30. No difference in thrombotic outcomes. | Increased major bleeding/CRNMB in 8% vs 2% (RR, 3.43; 95% CI, 1.61-8.27; P = 0.001) with 1 fatal ICH in a clinically unstable patient on therapeutic enoxaparin. | — |
| Sholzberg et al (RAPID)91 | RCT | Prophylactic heparin vs therapeutic dose UFH or LMWH | Non–critically ill D-dimer >ULN | 465 Brazil, Canada, Ireland, Saudi Arabia, UAE and United States | No difference in primary composite outcome (invasive ventilation, ICU, or death). Therapeutic heparin decreased odds of death at 28 d (odds ratio, 0.22; 95% CI, 0.07-0.65) | Major bleeding, 0.9%, therapeutic heparin and 1.7%, prophylactic heparin; P = NS | Underpowered for the analysis of the primary outcome. |
| Spyropoulos AC et al (HEP-COVID)89 | RCT | Prophylactic or intermediate dose UFH or LMWH vs therapeutic dose enoxaparin | COVID-19 inpatients D-dimer >4 × ULN or sepsis induced coagulopathy score ≥4; 67.2% noncritically ill | 253 United States | Reduction in primary composite outcome (VTE, ATE, death) 41.9% “standard-dose” heparins vs 28.7% therapeutic LMWH (RR, 0.68; 95% CI, 0.49-0.96; P = .03). Reduction in thromboembolism 29.0% vs 10.9%; RR, 0.37; 95% CI, 0.21-0.66; P <.001. | Major bleeding 1.6% with standard-dose vs 4.7% therapeutic-dose heparins; P = NS | Treatment effect was not seen in patients in ICU. |
| Marcos-Jubilar et al (BEMICOP)96 | RCT | Prophylactic vs therapeutic dose bemiparin for 10 d | Non–critically ill D-dimer >ULN | 65 Spain | No difference in primary composite outcome (death, ICU, invasive ventilation mod/severe ARDS, VTE or ATE within 10 d). | No major bleeding event, but short duration of study treatment period. | Prespecified interim analysis performed at 40% recruitment; trial halted due to slow recruitment/futility. |
| X-COVID-19114 | RCT | Prophylactic vs intermediate intensity enoxaparin (40 mg twice daily) | Noncritically ill | 189 Italy | Primary efficacy outcome (VTE) occurred in 6 of 92 (6.5%, 0 DVT, 6 PE) once daily dose group and 0 in twice daily. Group. | Two major bleeding events in each arm (1.1%). | Trial halted due to slow recruitment. 189 of 2712 recruited; underpowered and few events. |
| Study . | Study type . | Comparator . | Population . | Sample size, N . | Primary outcome . | Bleeding . | Comment . |
|---|---|---|---|---|---|---|---|
| Sadeghipour et al (INSPIRATION)87 | RCT | Prophylactic vs intermediate intensity enoxaparin (1 mg/kg daily) | ICU | 562 Iran | No significant difference in primary composite outcome (venous or arterial thrombosis, ECMO, or 30-d mortality) | Bleeding events rare. Major bleeding and CRNMB, 6.2% intermediate-dose vs 3.1% prophylactic; P = NS. | Not critically ill ICU population; only 50% of each cohort had invasive or NIV. Median number of days was 7 in both groups |
| Perpepu et al113 | RCT | Prophylactic vs intermediate intensity enoxaparin | ICU or coagulopathy D-dimer ≥1.00 ug/mL | 156 United States | No significant difference in preventing death or thrombosis at 30 d. | Major bleeding occurred in 2% of patients in each arm | ICU and non-ICU patients included |
| Goliger et al (International multiplatform trial)88 | Combined 1 RCT (ACTIV-4a) + 2 studies with response-adaptive randomization (ATTACC and REMAP-CAP) | Prophylactic heparin vs therapeutic dose UFH or LMWH | Critically ill | 1098 | No difference in primary outcome of days free from organ support or survival until hospital discharge, despite decreasing major thrombotic events | Major bleeding, 3.8% therapeutic, 2.3% thromboprophylaxis; not significantly different | Seemingly contradictory outcomes in a non–critically ill population (see text). |
| Lawler et al (International multiplatform trial)92 | Combined 1 RCT (ACTIV-4a) + 2 studies with response-adaptive randomization (ATTACC and REMAP-CAP) | Prophylactic heparin vs therapeutic dose UFH or LMWH | Non–critically ill | 2219 | Reduced organ support–free days to day 21 (primary outcome) | Major bleeding, 1.9% therapeutic, 0.9% thromboprophylaxis | Treatment benefit independent of D-dimer level, although treatment effect greater in patients with higher D-dimer |
| Lopes et al (ACTION)90 | Open-label RCT | Therapeutic Anticoagulation: rivaroxaban 20 mg or enoxaparin 1 mg/kg twice daily for clinically unstable patients (defined similarly to critically unwell/ICU patients in heparin studies) vs SOC enoxaparin or UFH prophylaxis | COVID-19 inpatients D-dimer >ULN 90% non–critically ill | 615 Brazil | No difference in primary outcome of time to death, duration of hospitalization and supplemental oxygen to day 30. No difference in thrombotic outcomes. | Increased major bleeding/CRNMB in 8% vs 2% (RR, 3.43; 95% CI, 1.61-8.27; P = 0.001) with 1 fatal ICH in a clinically unstable patient on therapeutic enoxaparin. | — |
| Sholzberg et al (RAPID)91 | RCT | Prophylactic heparin vs therapeutic dose UFH or LMWH | Non–critically ill D-dimer >ULN | 465 Brazil, Canada, Ireland, Saudi Arabia, UAE and United States | No difference in primary composite outcome (invasive ventilation, ICU, or death). Therapeutic heparin decreased odds of death at 28 d (odds ratio, 0.22; 95% CI, 0.07-0.65) | Major bleeding, 0.9%, therapeutic heparin and 1.7%, prophylactic heparin; P = NS | Underpowered for the analysis of the primary outcome. |
| Spyropoulos AC et al (HEP-COVID)89 | RCT | Prophylactic or intermediate dose UFH or LMWH vs therapeutic dose enoxaparin | COVID-19 inpatients D-dimer >4 × ULN or sepsis induced coagulopathy score ≥4; 67.2% noncritically ill | 253 United States | Reduction in primary composite outcome (VTE, ATE, death) 41.9% “standard-dose” heparins vs 28.7% therapeutic LMWH (RR, 0.68; 95% CI, 0.49-0.96; P = .03). Reduction in thromboembolism 29.0% vs 10.9%; RR, 0.37; 95% CI, 0.21-0.66; P <.001. | Major bleeding 1.6% with standard-dose vs 4.7% therapeutic-dose heparins; P = NS | Treatment effect was not seen in patients in ICU. |
| Marcos-Jubilar et al (BEMICOP)96 | RCT | Prophylactic vs therapeutic dose bemiparin for 10 d | Non–critically ill D-dimer >ULN | 65 Spain | No difference in primary composite outcome (death, ICU, invasive ventilation mod/severe ARDS, VTE or ATE within 10 d). | No major bleeding event, but short duration of study treatment period. | Prespecified interim analysis performed at 40% recruitment; trial halted due to slow recruitment/futility. |
| X-COVID-19114 | RCT | Prophylactic vs intermediate intensity enoxaparin (40 mg twice daily) | Noncritically ill | 189 Italy | Primary efficacy outcome (VTE) occurred in 6 of 92 (6.5%, 0 DVT, 6 PE) once daily dose group and 0 in twice daily. Group. | Two major bleeding events in each arm (1.1%). | Trial halted due to slow recruitment. 189 of 2712 recruited; underpowered and few events. |
ARDS, acute respiratory distress syndrome; CRNMB, clinically relevant non–major bleeding; ECMO, extracorporeal membrane oxygenation; ICH, intracranial hemorrhage; RR, relative risk; SOC, standard of care; ULN upper limit of normal.
Patients with severe COVID-19 do not appear to benefit from therapeutic anticoagulation according to the results of the INSPIRATION RCT, the multiplatform studies (ACTIV-4a, ATTAC, and REMAP-CAP), and the subgroup analysis of HEP-COVID RCT, which included the approximately one-third of the patients who were in ICU.87-89
In hospitalized patients with moderate COVID-19, the data are mixed. In the ACTION RCT, therapeutic rivaroxaban did not improve survival or duration of hospitalization compared with standard heparin thromboprophylaxis.90 In the RAPID RCT, there was no significant difference between therapeutic or prophylactic dose heparin in the composite outcome of ICU admission, need for ventilation, or death. However, therapeutic dose anticoagulation was associated with a decrease in the secondary outcome of death at 28 days.91 In contrast, in the noncritically ill patients in the multiplatform study, therapeutic anticoagulation was associated with a 4.6% increased probability of survival until hospital discharge without requirement for organ support, although there was no effect on in-hospital mortality (7.3% vs 8.2%).92 In the HEP-COVID RCT, therapeutic dose anticoagulation in patients not admitted to the ICU (two-thirds of the patient cohort) significantly reduced the composite outcome of VTE, ATE, and mortality.89
The 2 findings from the international multiplatform trial appear to be contradictory, with benefits of primary thromboprophylaxis with treatment-dose anticoagulation in patients with moderate COVID-19 disease, but not in those with severe disease. This difference in benefit may be due to several different factors. The first could be the pathophysiology of COVID-19 at different stages of the disease: in critically ill patients, the thrombotic and inflammatory changes may have progressed too far to be affected by higher doses of anticoagulation.93 However, other explanations may be related to the inherent study design and other confounding factors. Critically, a platform study does not equal a RCT, as if patients did not undergo randomization concurrently, it cannot be guaranteed that the trial groups have (on average) equivalent patient populations.94
More important, “standard” heparin dosing was not standardized in these trials. Dosing for “usual-care pharmacological thromboprophylaxis” was at the treating physician’s discretion, leading to a combination of conventional prophylaxis doses and intermediate doses being used in the platform studies. In critically ill patients, 51.7% of those in the control group received intermediate dose LMWH and 22.4% in the therapeutic dose arm did not receive therapeutic intensity anticoagulation.93 In the noncritically ill study, 26.5% of the control group received an intermediate dose and 20.4% of the therapeutic-dose group did not receive therapeutic anticoagulation.93 Finally, different populations were investigated in the 2 studies, with most patients recruited in the United Kingdom for the study of the critically ill, but in the United States/Brazil for the moderately ill, meaning a different ethnic mix, as well as other variables are likely.93
Given that the ACTION study included mainly stable non-ICU patients (90%),90 it is important to consider why therapeutic anticoagulation with rivaroxaban did not improve outcomes in the medical floor setting, in contrast to the results with therapeutic LMWH from the international multiplatform trial and HEP-COVID.89,92 The investigators postulate that it may be because rivaroxaban does not share the other anti-inflammatory properties of heparin, may not affect microvascular thrombosis, or simply because oral anticoagulants may not be well absorbed in hospitalized patients with erratic eating habits (rivaroxaban is dependent on food for absorption).90
There are differences across the studies as a whole that need to be considered, such as definitions of critically ill patients, different composite primary outcomes and cutoffs for an elevated D-dimer level.95 In the anticoagulation trials in patients with COVID-19, many screened patients were not deemed eligible or did not consent to participate. The findings therefore may not be generalizable to all moderately ill patients with COVID-19 admitted to medical floors.90 Safety data appear much more consistent, with bleeding risk increasing with increased intensity anticoagulation in most studies. In the BEMICOP study, there were no major bleeding events, most likely because of the short duration of the treatment period.96
Several meta-analyses of anticoagulation in patients with COVID-19 have already been published.97-99 Limitations are present as well in the meta-analyses, such as combining different anticoagulants, combining patients admitted and not admitted to the ICU, and combining different doses of anticoagulants. A meta-analysis by Reis et al, which included 8 RCTs and 5580 patients concluded that moderately affected patients with COVID-19 may benefit from therapeutic-dose anticoagulation, but the risk for bleeding is increased. Intermediate-dose anticoagulation may have little or no effect on thrombotic events or death, but may increase major bleeding in patients with moderate to severe COVID-19. However, it acknowledged that the certainty of evidence is still low.97
When considering thromboprophylaxis in the COVID-19 outpatient setting, it seems likely that there would be a net benefit to patients at low bleeding risk if risk of symptomatic VTE is >1.8% at 35 to 42 days after discharge, extrapolating from the RCT that assessed extended thromboprophylaxis in general medical patients.64 However, overall VTE rates after discharge appear to be lower than this, as described earlier. It may be that selectively using thromboprophylaxis in high-risk patients is the best strategy, but the question remains how to risk stratify. Prospectively validated risk assessment models to estimate thrombosis and bleeding risk of patients with COVID-19 after hospital discharge are not available.100
In an open-label, multicenter RCT in Brazil, 320 patients hospitalized with COVID-19 at increased risk for VTE (International Medical Prevention Registry on VTE [IMPROVE] score of ≥4, or 2-3 with a D-dimer >500 ng/mL) were randomized to treatment with rivaroxaban or no anticoagulation for 35 days on discharge. The primary composite outcome (symptomatic, fatal, or asymptomatic VTE; symptomatic ATE; and cardiovascular death at day 35) occurred in 5 of 159 (3%) patients assigned to rivaroxaban and 15 of 159 (9%) patients assigned to no anticoagulation (relative risk, 0.33; 95% CI, 0.12-0.90; P = .0293). No major bleeding occurred in either study group.101 Patients should continue to be offered enrollment into clinical trials where possible. National and international guidelines have been developed by ASH102 and NICE (National Institute of Clinical Effectiveness).103
Currently, there are no guidelines supporting routine use of aspirin in the primary prevention of arterial thrombosis in patients with COVID-19, but trials with antiplatelet agents are ongoing (registered at www.clinicaltrials.gov as NCT0465309). Data on the efficacy of LMWH on preventing specific arterial thromboembolic complications are also limited.
Bleeding
Bleeding is a less prominent phenotype in the patient with COVID-19, but can cause significant morbidity and is a potential cause of death in a subset of patients.104 However, one needs to unpick the contribution from the COVID-19 disease itself and iatrogenic factors, such as anticoagulation, procedures, steroids, and other ulcerogenic agents.
A multicenter retrospective study of 400 hospitalized patients reported an overall bleeding rate of 4.84% (95% CI, 2.9-7.3) with a major bleeding rate of 2.3% (95% CI, 1.0-4.2). Elevated D-dimer at presentation was predictive, not only of thrombosis, but of bleeding (adjusted OR for bleeding, 3.56; 95% CI, 1.1-12.66).13 Hemorrhage was reported as a cause of death in 6% of patients with COVID-19 in 1 series.105 In a recent systematic review and meta-analysis of 49 studies and >18 000 patients by Jiménez et al, there was a pooled incidence of 7.8% (95% CI, 2.6-15.3) for bleeding and 3.9% (95% CI, 1.2-7.9) for major bleeding.28 The highest incidence of bleeding was reported in patients receiving intermediate- or full-dose anticoagulation (21.4%), and the lowest in the only prospective study that assessed bleeding events (2.7%).28
Gastrointestinal bleeding
Gastrointestinal (GI) bleeding has been described in 4% to 13.7% of severely affected patients, 40% of whom had stool that was PCR positive for the SARS-CoV-2 virus.106,107 It has been hypothesized that prolonged hypoxia may lead to cell necrosis and mucosal damage, leading to GI ulceration.5 In a recent meta-analysis of acute GI bleeding in 127 patients with COVID-19, 59% responded to conservative measures. The mortality rate associated with GI bleeding was low (pooled mortality secondary to GI bleeding, 3.5% (95% CI, 1.3% to 9.1%), and there was a low risk of rebleeding of 11.3% (95% CI, 6.8-18.4).108
Intracranial hemorrhage
Case series have suggested that there are distinct patterns to intracerebral hemorrhage in COVID-19–positive cases compared with patients without COVID-19: namely, hemorrhage severity, mortality, and younger age.109 This is also described in aneurysmal subarachnoid hemorrhage (high frequency of small aneurysms, dissecting pseudoaneurysms, and younger age in COVID-19–positive cases).110 However, a recent large study from 62 centers in the United States did not suggest that rates of intracerebral hemorrhage were higher in patients with COVID-19, occurring in 0.2% (154 of 85 645) of patients with COVID-19 and 0.3% (667 of 197 073) of patients without the virus.111 The in-hospital mortality among patients with intracerebral hemorrhage and COVID-19 was significantly higher than in those without (40.3% vs 19%; P < .0001). This result is thought to be mediated by the higher frequency of comorbidities and adverse in-hospital events.111
Management of bleeding
Management of bleeding complications follows the same principles as in nonpatients with COVID-19 with surgical control of bleeding lesions and use of the antifibrinolytic agent tranexamic acid. Vitamin K status should be optimized. Coagulation factor replacement with fresh frozen plasma or 4-factor prothrombin complex concentrate, fibrinogen replacement with cryoprecipitate or fibrinogen concentrate, and platelet transfusion should follow the usual guidance for patients without COVID-19 and be individualized to meet the clinical needs and procedural requirements of the patient.112
Conclusion
The disease course of COVID-19 in hospitalized patients is often complicated by thrombosis, and pulmonary thrombosis and VTE are more common than arterial events. Some cases of pulmonary thrombosis are secondary to embolic deep vein thrombi from the legs, but in situ microvascular and macrovascular thromboses appear more common in COVID-19 infection. Bleeding is rarer than thrombosis and is potentially associated with escalated doses of anticoagulants and other iatrogenic factors. Reported rates of both thrombosis and bleeding are sensitive to the population studied and nature of data collection. Treatment of thrombotic and bleeding complications follow the same principles as in patients without COVID-19, but the optimal thromboprophylactic strategy for different disease stages is still under debate. Evidence from platform studies and smaller RCT is moving the debate forward, but we do not yet have the full answer on how to protect our patients from thrombosis and potentially improve mortality.
Authorship
Contribution: M.S. and M.R.T. equally designed the review, reviewed the literature, and contributed to the final content of the manuscript.
Conflict-of-interest disclosure: M.S. has received speaker’s fees and served on advisory boards for Takeda, Sanofi, Alexion, Octapharma, and Novartis; and has received research grants from Shire and Alexion. M.R.T. has received speaker’s fees and has served on advisory boards for Sanofi and Bayer.
Correspondence: Marie Scully, University College London Hospital, 250 Euston Rd, London, NW1 2PG, United Kingdom; email: m.scully@ucl.ac.uk.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal