Abstract
Patients with blood disorders who are immune suppressed are at increased risk for infection with severe acute respiratory syndrome coronavirus 2. Sequelae of infection can include severe respiratory disease and/or prolonged duration of viral shedding. Cellular therapies may protect these vulnerable patients by providing antiviral cellular immunity and/or immune modulation. In this recent review of the field, phase 1/2 trials evaluating adoptive cellular therapies with virus-specific T cells or natural killer cells are described along with trials evaluating the safety, feasibility, and preliminary efficacy of immune modulating cellular therapies including regulatory T cells and mesenchymal stromal cells. In addition, the immunologic basis for these therapies is discussed.
Introduction
Despite the development of highly efficacious vaccines, the COVID-19 pandemic continues to take a massive toll on populations internationally. Multiple studies have demonstrated elevated hospitalization and mortality rates in immunocompromised patients, including those with inborn errors of immunity, cancer, hematopoietic stem cell transplantation (HSCT), or solid organ transplantation.1-4 Registry reports have indicated that mortality rates in immunocompromised patients requiring hospitalization for severe COVID-19 are as high as 20% despite currently available therapies. Although some individuals with underlying immunodeficiency who become infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) will achieve effective viral clearance,5,6 multiple reports have demonstrated a risk of prolonged shedding of viable virus with intrahost accrual of novel viral variants over time.7-11 Studies of immunocompromised populations have shown suboptimal responses to vaccination,12-17 and although booster vaccines in these populations is required, it is unclear how durable their protective immunity will be compared with immune-competent individuals. Disease severity and the potential for new variants highlight the need for new preventative and therapeutic approaches to protect immunocompromised populations from COVID-19. Adoptive cellular therapy has been used in prior studies to prevent or treat viral infections in the setting of inborn errors of immunity or transplantation, with evidence of safety and efficacy against herpesviruses, polyomaviruses, and some respiratory pathogens such as adenovirus.18-23 Accordingly, adoptive T-cell therapy is being explored as a preventative or therapeutic adjunctive therapy against SARS-CoV-2.
Among adults infected with SARS-CoV-2, severe disease is accompanied by characteristic immunologic profiles, including markedly elevated plasma levels of inflammatory cytokines. Several studies have demonstrated patterns of cytokine elevation, including interleukin 6 (IL-6) and IL-10, which correlate with risk of mortality.24-26 Treatment with dexamethasone among patients requiring respiratory support improves outcomes,27 suggesting that anti-inflammatory agents may be beneficial in some patients with COVID-19. Trials of immunosuppressive agents including tocilizumab,28 Janus kinase inhibitors, and Bruton’s tyrosine kinase inhibitors have yielded mixed results.29-33 In this context, regulatory T cells (Tregs) and mesenchymal stromal cells may be beneficial adjunctive treatments with immune modulatory and tissue reparative properties that could improve outcomes among patients with severe COVID-19.
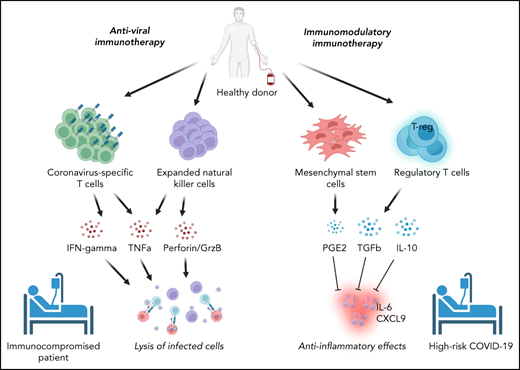
Cellular therapies for SARS-CoV-2 can be conceptually classified based on their intended function as either (1) adoptive immunotherapies designed to enhance viral clearance in patients with ineffective immune responses to SARS-CoV-2 or (2) immune modulatory therapies to correct a dysregulated immune response contributing to clinical deterioration (Figure 1). Within the former category, adoptive immunotherapy may be used for the prevention or treatment of active SARS-CoV-2 infection in immunocompromised patients and includes adoptive immunotherapy with SARS-CoV-2–specific T cells or natural killer (NK) cells. By contrast, immune modulatory cellular therapies include Tregs and mesenchymal stromal cells. Phase 1/2 trials are underway to evaluate these therapies clinically. Hence, this review focuses on the immunologic basis for their development and the results to date.
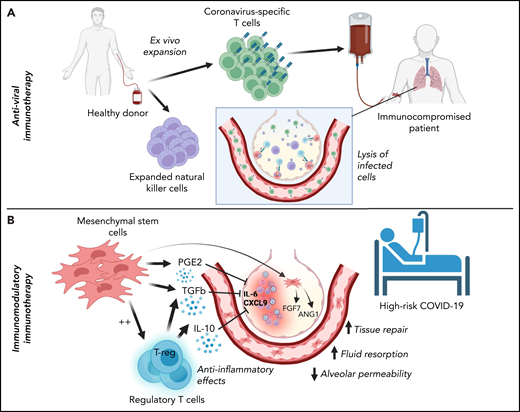
Classification of cellular therapies for SARS-CoV-2 infection. Cellular therapy for SARS-CoV-2 can be classified as adoptive anti-viral immunotherapy with CST or NK cells to aid immunocompromised patients with viral clearance (A) or immunomodulatory immunotherapy with Tregs or MSCs to correct dysregulated immune responses in patients with severe COVID-19 (B).
Classification of cellular therapies for SARS-CoV-2 infection. Cellular therapy for SARS-CoV-2 can be classified as adoptive anti-viral immunotherapy with CST or NK cells to aid immunocompromised patients with viral clearance (A) or immunomodulatory immunotherapy with Tregs or MSCs to correct dysregulated immune responses in patients with severe COVID-19 (B).
T-cell responses to SARS-CoV-2 infection
The adaptive immune response to SARS-CoV-2 has been an area of intense investigation since the onset of the pandemic. Understanding antigen-specific B-cell, CD4+ T-cell, and CD8+ T-cell responses has been essential for developing effective vaccines and defining the immunopathology that contributes to severity of illness caused by SARS-CoV-2. Although maladaptive immune responses to SARS-CoV-2 remain incompletely understood, several studies to date suggest a link between severe COVID-19 and insufficient early innate immune responses to SARS-CoV-2, resulting in weak or delayed adaptive response,24,34 rather than a primary pathologic role of antigen-specific humoral or cell-mediated adaptive immunity.34-39 Indeed, circulating CD4+ SARS-CoV-2–specific T cells (CSTs) are detected in peripheral blood mononuclear cells (PBMCs) from >90% of convalescent individuals, CD8+ CSTs are detected in >70%, and the presence of CSTs is associated with recovery and decreased severity of illness.40-44 Lymphopenia is associated with poorer outcomes in COVID-19, and analysis of bronchoalveolar lavage (BAL) fluid from patients with varying illness severity indicates decreased clonal T-cell expansion, suggesting a suboptimal CST presence in the lungs of patients with severe or fatal disease.37,45 Moreover, in patients with hematologic malignancies, CD8+ T-cell depletion has been associated with poor outcomes, particularly in patients who also have impaired humoral responses to SARS-CoV-2.46 These findings suggest that T-cell responses, particularly CSTs, are important for effective viral clearance and are associated with improved outcomes in patients with acute infection. Thus, adoptive cellular therapy with CSTs and/or NK cells may be an effective strategy for improving outcomes in immunocompromised patients with or at risk for SARS-CoV-2 infection.
The potential application of immunotherapy could be relevant to many patient groups. For example, immunomodulatory immunotherapy could be applicable to any patient at high risk of severe COVID-19. CST or NK therapy would be most applicable to immunocompromised patients with T-cell deficiency or those receiving immunosuppressive therapies. In these patients, early identification and treatment of those at high risk for immune dysregulation because of SARS-CoV-2 might enhance viral clearance and prevent severe disease, because lower initial viral load,47 early bystander CD8+ T-cell activation,48 and adequate early type I interferon response38,39,49 correlate with decreased mortality related to SARS-CoV-2.35,50 For example, in patients with autoantibodies to type I interferon, CST therapy could theoretically bypass early defects in interferon signaling and restore immune responses to SARS-CoV-2. Additionally, analysis of T-cell subsets has shown that Th1-skewed and CD8+ T-cell responses are associated with decreased COVID-19 severity,51,52 suggesting that enhanced cell therapy products might be manufactured to favor protective T-cell subsets.53 Whether adoptive immunotherapy with CSTs and/or NK cells administered later in the disease course worsens ongoing inflammation and immunopathology is not known but will be addressed by ongoing phase 1/2 trials (Tables 1 and 2).
SARS-CoV-2–specific T-cell trials for treatment or prevention of COVID-19
| Trial . | Treatment . | Population . | Study design . | Phase . | Estimated enrollment . | Blood disorder eligibility . |
|---|---|---|---|---|---|---|
| NCT04457726 | Allogeneic CSTs | 1 to 90 y SARS-CoV-2 RT-PCR + within 72 h of enrollment | Arm A: severe COVID-19 Arm B: mild to moderate COVID-19 with high risk of progression to severe disease based on age and/or underlying comorbidity | 1/2 (recruiting) | 18 | Not excluded unless receiving >0.5 mg/kg/d steroid |
| NCT04765449 | Partially HLA-matched banked allogeneic CSTs | ≥18 y SARS-CoV-2 RT-PCR + High risk of severe disease based on age and/or underlying comorbidity No supplemental O2 requirement | Arm A: Treatment with CSTs Arm B: No available HLA-matched product, monitored at home and may receive any standard of care treatment for COVID-19 | 1 (recruiting) | 24 | Included: chemotherapy for malignancy within the prior 24 mo Excluded: prior allogeneic HSCT or solid organ transplant; current chemotherapy, radiation, and/or immunosuppressive drug regimen |
| NCT04742595 | Partially HLA-matched banked allogeneic CSTs | ≥18 y Immunocompromised with cancer SARS-CoV-2 RT-PCR + within 2 wk of enrollment Presence of respiratory symptoms | Patients receive CSTs on day 1 and treatment may repeat every 14 d at investigators' discretion. | 1 (recruiting) | 16 | Yes (required for inclusion) |
| NCT04401410 | Partially HLA-matched banked allogeneic CSTs | ≥18 y SARS-CoV-2 RT-PCR + within 5 d of enrollment Hospitalized for COVID-19 High risk of severe disease based on age and/or underlying comorbidity No supplemental O2 requirement | Phase I: dose finding phase Phase II: randomized pilot study comparing CST treatment at dose determined in phase I to current institutional standard of care treatment for COVID-19 | 1/2 (terminated early) | Final number not yet reported | Yes (inclusion criteria as risk factor for severe disease) |
| NCT04762186 | Allogeneic CSTs | ≥18 y SARS-CoV-2 RT-PCR + within 72 h of enrollment Maximum 14 d between symptom onset and enrollment WHO score 5 or 4 with one additional risk factor for progression | Phase I: dose finding phase Phase II: randomized pilot study comparing CST treatment at dose determined in phase I to current institutional standard of care treatment for COVID-19 | 1/2 (recruiting) | 12 | Yes (inclusion criteria as risk factor for severe disease) |
| NCT04896606 | Family derived HLA-matched allogeneic CSTs | 18 to 65 y SARS-CoV-2 RT-PCR + Hospitalized for mild to moderate COVID-19 disease Risk of progression based on underlying comorbidity HLA-matched family related donor with recent SARS-CoV-2 infection at least 10 d from symptom onset available | Experimental arm: Patients receive family donor derived CSTs up to five times every 2 wk along with standard of care. Active Comparator: Standard of care alone. | 1/2 (recruiting) | 50 | Yes (inclusion criteria as risk factor for severe disease) |
| NCT05141058 | HSCT donor-derived allogeneic CSTs | ≥12 y and <80 y ≥28 d and <4 wk after allogeneic HSCT SARS-CoV-2 RT-PCR negative | Arm A: Adults (≥18 to <80 y) will receive a single infusion of CSTs at escalating doses for prophylaxis against SARS-CoV-2 infection. Arm B: Children (≥12 and <18 y) will receive a single infusion of CSTs at escalating doses for prophylaxis against SARS-CoV-2 infection. | 1 (recruiting) | 24 | Yes (required for inclusion) |
| Trial . | Treatment . | Population . | Study design . | Phase . | Estimated enrollment . | Blood disorder eligibility . |
|---|---|---|---|---|---|---|
| NCT04457726 | Allogeneic CSTs | 1 to 90 y SARS-CoV-2 RT-PCR + within 72 h of enrollment | Arm A: severe COVID-19 Arm B: mild to moderate COVID-19 with high risk of progression to severe disease based on age and/or underlying comorbidity | 1/2 (recruiting) | 18 | Not excluded unless receiving >0.5 mg/kg/d steroid |
| NCT04765449 | Partially HLA-matched banked allogeneic CSTs | ≥18 y SARS-CoV-2 RT-PCR + High risk of severe disease based on age and/or underlying comorbidity No supplemental O2 requirement | Arm A: Treatment with CSTs Arm B: No available HLA-matched product, monitored at home and may receive any standard of care treatment for COVID-19 | 1 (recruiting) | 24 | Included: chemotherapy for malignancy within the prior 24 mo Excluded: prior allogeneic HSCT or solid organ transplant; current chemotherapy, radiation, and/or immunosuppressive drug regimen |
| NCT04742595 | Partially HLA-matched banked allogeneic CSTs | ≥18 y Immunocompromised with cancer SARS-CoV-2 RT-PCR + within 2 wk of enrollment Presence of respiratory symptoms | Patients receive CSTs on day 1 and treatment may repeat every 14 d at investigators' discretion. | 1 (recruiting) | 16 | Yes (required for inclusion) |
| NCT04401410 | Partially HLA-matched banked allogeneic CSTs | ≥18 y SARS-CoV-2 RT-PCR + within 5 d of enrollment Hospitalized for COVID-19 High risk of severe disease based on age and/or underlying comorbidity No supplemental O2 requirement | Phase I: dose finding phase Phase II: randomized pilot study comparing CST treatment at dose determined in phase I to current institutional standard of care treatment for COVID-19 | 1/2 (terminated early) | Final number not yet reported | Yes (inclusion criteria as risk factor for severe disease) |
| NCT04762186 | Allogeneic CSTs | ≥18 y SARS-CoV-2 RT-PCR + within 72 h of enrollment Maximum 14 d between symptom onset and enrollment WHO score 5 or 4 with one additional risk factor for progression | Phase I: dose finding phase Phase II: randomized pilot study comparing CST treatment at dose determined in phase I to current institutional standard of care treatment for COVID-19 | 1/2 (recruiting) | 12 | Yes (inclusion criteria as risk factor for severe disease) |
| NCT04896606 | Family derived HLA-matched allogeneic CSTs | 18 to 65 y SARS-CoV-2 RT-PCR + Hospitalized for mild to moderate COVID-19 disease Risk of progression based on underlying comorbidity HLA-matched family related donor with recent SARS-CoV-2 infection at least 10 d from symptom onset available | Experimental arm: Patients receive family donor derived CSTs up to five times every 2 wk along with standard of care. Active Comparator: Standard of care alone. | 1/2 (recruiting) | 50 | Yes (inclusion criteria as risk factor for severe disease) |
| NCT05141058 | HSCT donor-derived allogeneic CSTs | ≥12 y and <80 y ≥28 d and <4 wk after allogeneic HSCT SARS-CoV-2 RT-PCR negative | Arm A: Adults (≥18 to <80 y) will receive a single infusion of CSTs at escalating doses for prophylaxis against SARS-CoV-2 infection. Arm B: Children (≥12 and <18 y) will receive a single infusion of CSTs at escalating doses for prophylaxis against SARS-CoV-2 infection. | 1 (recruiting) | 24 | Yes (required for inclusion) |
NK-cell immunotherapy trials for COVID-19
| Trial . | Treatment . | Population . | Study design . | Phase . | Estimated enrollment . | Blood disorder eligibility . |
|---|---|---|---|---|---|---|
| NCT04900454 | Allogeneic NK cells derived from CD34+ hematopoietic stem cells (DVX201) | 18 to 80 y SARS-CoV-2 RT-PCR + within 7 d of enrollment Hospitalized for COVID-19 Maximum 4L O2 to maintain SO2 ≥93% Inflammatory markers within specified range | Open label, nonrandomized dose-finding study | 1 (recruiting) | 18 | Excluded if receiving systemic immune suppression |
| NCT04280224 | Allogeneic NK cells | 18 to 65 y SARS-CoV-2 RT-PCR + Radiographic evidence of pneumonia | Experimental arm: participants will receive NK cells twice per week, plus conventional therapy No intervention arm: Participants will receive only current standard of care treatment | 1 (recruiting) | 30 | Excluded for malignancy or other serious systemic disorder |
| NCT04365101 | Allogeneic NK cells expanded from human placental CD34+ cells (CYNK-001) | ≥18 y SARS-CoV-2 + using institutionally approved test SO2 ≥92% on supplemental O2 or ≥88% on room air Experiencing any symptom or radiographic finding associated with COVID-19 | Phase 1: evaluate the safety and efficacy of CYNK-001 given on days 1, 4, and 7 in 14 total patients. Phase 2: randomized, open-label assignment to either CYNK-001 or standard of card alone | 1/2 (active, not recruiting) | 86 (14 in phase 1, up to 72 in phase 2) | Excluded for active malignancy and/or immunosuppression |
| NCT04634370 | Allogeneic NK cells | ≥18 y SARS-CoV-2 + using institutionally approved test White or yellow clinical warning criteria174 | Open label, nonrandomized dose-finding study | 1 (not yet recruiting) | 24 | Excluded for malignant blood-borne diseases |
| NCT04363346 | Induced pluripotent stem cell-derived NK cells transduced with high affinity, ADAM17 non-cleavable Fc receptor (CD16) (FT516) | 18 to 76 y old SARS-CoV-2 + Hospitalized for COVID-19 Maximum supplemental O2 requirement 4 L Radiographic evidence of chest infiltrates Inflammatory markers in specified range | Open label sequential assignment dose-finding study | 1 (active, not recruiting) | 5 | Excluded for any known condition requiring systemic immunosuppression |
| NCT04324996 | Cord-blood derived NKG2D-ACE2 CAR-NK cells secreting IL-15 superagonist and GM-CSF neutralizing scFv | ≥18 y Diagnosed with pneumonitis and new coronavirus infection Within 14 d of illness onset | Randomized, parallel assignment with quadruple masking. Arm A: NK cells secreting IL-15 superagonist Arm B: NKG2D CAR-NK cells Arm C: ACE2 CAR-NK cells Arm D: NKG2D-ACE2 CAR NK cells secreting IL-15 superagonist and GM-CSF neutralizing scFv | 1/2 (recruiting) | 90 | Excluded for long-term anti-rejection or immune modulatory drugs |
| NCT04578210 | Allogeneic memory T cells or allogeneic NK cells from convalescent donors | ≤80 y SARS-CoV-2 RT-PCR + within 72 h of enrollment Onset of symptoms within 10 d and <3 d of hospitalization prior to infusion Requiring hospitalization Radiographic evidence of pneumonia Lymphopenia ≤2.5 L O2 requirement | Arm A: single infusion of memory T cells Arm B: single infusion of NK cells | 1/2 (recruiting) | 58 | Not excluded |
| Trial . | Treatment . | Population . | Study design . | Phase . | Estimated enrollment . | Blood disorder eligibility . |
|---|---|---|---|---|---|---|
| NCT04900454 | Allogeneic NK cells derived from CD34+ hematopoietic stem cells (DVX201) | 18 to 80 y SARS-CoV-2 RT-PCR + within 7 d of enrollment Hospitalized for COVID-19 Maximum 4L O2 to maintain SO2 ≥93% Inflammatory markers within specified range | Open label, nonrandomized dose-finding study | 1 (recruiting) | 18 | Excluded if receiving systemic immune suppression |
| NCT04280224 | Allogeneic NK cells | 18 to 65 y SARS-CoV-2 RT-PCR + Radiographic evidence of pneumonia | Experimental arm: participants will receive NK cells twice per week, plus conventional therapy No intervention arm: Participants will receive only current standard of care treatment | 1 (recruiting) | 30 | Excluded for malignancy or other serious systemic disorder |
| NCT04365101 | Allogeneic NK cells expanded from human placental CD34+ cells (CYNK-001) | ≥18 y SARS-CoV-2 + using institutionally approved test SO2 ≥92% on supplemental O2 or ≥88% on room air Experiencing any symptom or radiographic finding associated with COVID-19 | Phase 1: evaluate the safety and efficacy of CYNK-001 given on days 1, 4, and 7 in 14 total patients. Phase 2: randomized, open-label assignment to either CYNK-001 or standard of card alone | 1/2 (active, not recruiting) | 86 (14 in phase 1, up to 72 in phase 2) | Excluded for active malignancy and/or immunosuppression |
| NCT04634370 | Allogeneic NK cells | ≥18 y SARS-CoV-2 + using institutionally approved test White or yellow clinical warning criteria174 | Open label, nonrandomized dose-finding study | 1 (not yet recruiting) | 24 | Excluded for malignant blood-borne diseases |
| NCT04363346 | Induced pluripotent stem cell-derived NK cells transduced with high affinity, ADAM17 non-cleavable Fc receptor (CD16) (FT516) | 18 to 76 y old SARS-CoV-2 + Hospitalized for COVID-19 Maximum supplemental O2 requirement 4 L Radiographic evidence of chest infiltrates Inflammatory markers in specified range | Open label sequential assignment dose-finding study | 1 (active, not recruiting) | 5 | Excluded for any known condition requiring systemic immunosuppression |
| NCT04324996 | Cord-blood derived NKG2D-ACE2 CAR-NK cells secreting IL-15 superagonist and GM-CSF neutralizing scFv | ≥18 y Diagnosed with pneumonitis and new coronavirus infection Within 14 d of illness onset | Randomized, parallel assignment with quadruple masking. Arm A: NK cells secreting IL-15 superagonist Arm B: NKG2D CAR-NK cells Arm C: ACE2 CAR-NK cells Arm D: NKG2D-ACE2 CAR NK cells secreting IL-15 superagonist and GM-CSF neutralizing scFv | 1/2 (recruiting) | 90 | Excluded for long-term anti-rejection or immune modulatory drugs |
| NCT04578210 | Allogeneic memory T cells or allogeneic NK cells from convalescent donors | ≤80 y SARS-CoV-2 RT-PCR + within 72 h of enrollment Onset of symptoms within 10 d and <3 d of hospitalization prior to infusion Requiring hospitalization Radiographic evidence of pneumonia Lymphopenia ≤2.5 L O2 requirement | Arm A: single infusion of memory T cells Arm B: single infusion of NK cells | 1/2 (recruiting) | 58 | Not excluded |
Adoptive virus-specific T-cell therapy
Adoptive cellular therapy with virus-specific T cells (VSTs) has been used safely and effectively for more than 25 years to treat and prevent viral infections in patients with immune deficiencies, especially in the post-HSCT setting.22,54-57 Manufacture of VSTs for clinical use involves either selection or ex vivo expansion from donor PBMCs. VST selection uses major histocompatibility complex–antigen multimers or cytokine capture technology to isolate VSTs from PBMCs, whereas ex vivo expansion involves PBMC culture in the presence of growth-enhancing cytokines and viral antigens for 10 to 12 days to generate a product enriched in VSTs targeting 1 or more viruses.21,56 In the post-HSCT population, treatment with donor-derived VSTs results in reconstitution of antiviral immunity with persistence of VSTs for years after infusion.58,59 For patients who have not undergone HSCT or for whom a donor is not available, banking of cryopreserved ex vivo expanded VSTs has developed as an effective “off-the-shelf” approach to VST therapy, allowing for rapid treatment of fulminant viral infections with partially HLA-matched VST products.57,60-62 HLA matching strategies vary across protocols and research continues to determine the optimal approach. Recent studies have demonstrated that small banks using strategically chosen donors based on their HLA type and immunity to the target of interest can provide potential treatment to a large number of referred patients.63 Evidence to date points to increased efficacy if at least 1 shared HLA allele is known to mediate antiviral T-cell immunity.57 Therefore, in the setting of SARS-CoV-2 infection, donor-derived CSTs could be an option as treatment or prophylaxis for high-risk patients after HSCT, and third party partially HLA-matched CSTs could be used as an early treatment for COVID-19 infection in high-risk patients with blood disorders in general.
Clinical use of SARS-CoV-2–specific T cells
CSTs can be expanded from the PBMCs of most convalescent donors, along with some individuals who have not been infected with SARS-CoV-2, likely because of cross-reactivity with common cold coronaviruses.40,64-66 Epitope mapping has established immunogenic hot spots within SARS-CoV-2, including multiple regions of the spike and nucleocapsid proteins, as well highly conserved regions of the membrane protein, allowing for design of peptide pools for rapid expansion of CSTs for clinical use.64,65 Convalescent donors who have seroconverted display broader T-cell antigenic responsiveness, but even seronegative convalescent individuals can elicit an expandable CST population.64 Spike-directed T-cell responses have also been demonstrated in individuals who have been vaccinated against SARS-CoV-2, thus providing a large pool of potential donors for the generation of CST banks.67-71 Moreover, these spike-directed T-cell responses elicited from vaccinated but previously uninfected donors against peptide pools created from the Wuhan strain have shown cross reactivity to other variant strains, thereby broadening the applicability of this approach.72
Currently, there are 7 registered phase 1/2 trials evaluating allogeneic CSTs for the treatment or prevention of COVID-19, 4 of which are actively recruiting patients (Table 1). Six trials are designed to test third party allogeneic CSTs for treatment of SARS-CoV-2 infection in high-risk patients (based on illness severity score, underlying comorbidities, and/or age) and 1 (NCT05141058) tests HSCT donor-derived CSTs for prophylaxis against SARS-CoV-2 infection in SARS-CoV-2–negative participants after allogeneic HSCT. Two trials (NCT05141058 and NCV04745295) specifically target immunocompromised patients with blood disorders; 3 others include malignancy and/or immune suppression as risk factors for severe disease, which are required for eligibility (Table 1). To date, data from 1 trial (NCT04401410) have been presented detailing 4 patients who were treated with partially HLA-matched CSTs to treat SARS-CoV-2 infection in high-risk individuals. One patient experienced symptoms consistent with cytokine release syndrome but recovered. Three of the 4 treated patients achieved resolution of their infection after CST infusion,73 but without results from the control group, the extent of the CST effect is unclear. Unfortunately, this trial was terminated early because of feasibility concerns with insufficient numbers of patients meeting inclusion criteria.
As SARS-CoV-2 variants arise, CSTs generated for therapeutic use must be evaluated for cross-reactivity against new circulating viral strains. Studies to date demonstrate moderate to high T-cell cross-reactivity against circulating variants, including the omicron variant,74-76 with strong reactivity particularly in the CD4+ compartment.67,70,72,77-79 Indeed, T-cell epitopes identified in CSTs from convalescent patients span the viral proteome40,50,80-82 and stimulate T-cell responses to viral variants that are partially resistant to vaccine-induced humoral spike-specific responses.83-85 Importantly, high-throughput systematic analysis of CD8+ T-cell activity in response to mutant peptides from SARS-CoV-2 variants of concern, including alpha, beta, and delta variants, has suggested decreased ability of some mutant epitopes to bind to certain HLA,86,87 which highlights the need to ensure that CSTs generated for clinical use maintain activity against circulating strains as new variants arise.72 SARS-CoV-2–specific T cells are long-lasting in convalescent individuals, retaining activation and proliferation capacity ex vivo for at least 10 months,88 and supporting the assertion that CSTs may provide durable protection against severe illness due to SARS-CoV-2, even as humoral immunity wanes.89
Limitations/effect of concomitant steroid treatment on adoptive cellular therapy efficacy
Although many groups have classified T-cell immunity to SARS-CoV-2 and demonstrated the feasibility of CST generation, the hyperinflammatory nature of critical COVID-19 calls into question whether T-cell therapy may be beneficial in the setting of pneumonia or acute respiratory distress syndrome (ARDS). Dexamethasone is standard treatment for hypoxic patients following the demonstration of a survival advantage in randomized controlled trials, and this therapy is highly likely to inactivate both endogenous lymphocytes and any unmodified adoptive cell therapies. Basar et al90 showed that corticosteroid-resistant T cells targeting SARS-CoV-2 could be generated via CRISPR/Cas9 knockout of the glucocorticoid receptor (NR3C1), and the resulting cells maintain viability and effector function in the presence of dexamethasone in vitro. These and similar approaches may facilitate the use of cell therapy even when immunosuppressive pharmacotherapy must be administered simultaneously.
NK-cell immunotherapy for COVID-19
NK cells are an essential component of antiviral defense, as demonstrated by many inborn errors of immunity in which NK cell defects result in viral susceptibility.91,92 Studies of COVID-19 have demonstrated that NK lymphopenia is common in acute illness, with loss of CD56dim effector NK cells in patients with severe cases.93 Reports have revealed inverse correlations between NK counts and serum IL-6 level,94 as well as interleukin-2 receptor alpha.95 Robust NK activation has been observed in both peripheral blood and BAL samples from patients with COVID-19, although elevation of adaptive NK cells (NKG2C+/Ksp37+/perforin+) was exclusively seen in severe disease.96 A recent study suggested that the IL-15–IL-15R axis may be a key driver of immune dysfunction in COVID-19 infection, with excess signaling resulting in NK dysfunction.97 Ma et al98 also demonstrated that NK cells modified with a chimeric antigen receptor (CAR) using a single chain variable fragment (scFv) derived from the spike-targeting S309 monoclonal antibody killed spike-expressing targets and had cross-reactivity with common spike variants.
Presently, there are 7 clinical trials evaluating allogeneic NK cell immunotherapy as adjunctive treatment for COVID-19, as well as 1 trial of NK CAR immunotherapy targeting SARS-CoV-2 (Table 2); none specifically target immunocompromised populations. No study results have been published to date and at least 1 such trial is no longer recruiting (NCT04365101).
Cell therapy targeting immune dysregulation in COVID-19
Although characterization of COVID-19 immunopathology has been complicated by the dynamic nature of the immune response over time, distinct immunologic profiles are associated with progression to severe disease in patients infected with SARS-CoV-2.24,39,99,100 Elevated plasma markers of inflammation, including C-reactive protein (CRP), ferritin, IL-6, IL-8, and tumor necrosis factor-α are associated with risk of progression to severe disease and mortality.26,101-104 Additionally, severe COVID-19 is characterized by circulating T-cell lymphopenia and an activated, exhausted T-cell phenotype,105-108 Treg dysfunction,109 and myeloid cell activation in blood and bronchoalveolar samples.37,45,110-112 Multisystem inflammatory syndrome in children, an inflammatory complication of SARS-CoV-2 infection in children, seems to be associated with increased CST responses compared with children who have uncomplicated SARS-CoV-2 infection113 and has an inflammatory profile unique from severe COVID-19 in adults.114,115 Additionally, this syndrome has been associated with activation of CX3CR1+ vascular patrolling T cells.116 These immunologic signatures may allow for targeting of immune modulatory treatments to the patients most likely to benefit from them and point to the potential utility of immune modulation in treating COVID-19.
Multiple immune modulators, including IL-1 and IL-6 receptor blockers and systemic corticosteroids, have been used to target the hyperinflammatory phenomena in severe COVID-19 and multisystem inflammatory syndrome in children, which have recently been reviewed.117,118 Cellular treatments with ex vivo expanded Tregs and/or mesenchymal stromal cells (MSCs) may offer a targeted and less broadly immunosuppressive approach. In addition, Tregs and MSCs have shown promise in preclinical and early clinical trials for the treatment of ARDS,119-124 a hallmark of severe COVID-19.
Use of Treg and MSC therapies in ARDS
ARDS is a syndrome of lung injury defined by the acute development of hypoxemia resulting from alveolar injury and loss of vectorial fluid transport out of the airspaces. This is accompanied by dysregulated alveolar inflammatory cell infiltration that perpetuates the injury, although the exact immunologic responses are only partially understood. The therapeutic potential of Tregs and MSCs in ARDS centers around their immune modulatory and tissue reparative properties. In preclinical models, Tregs attenuate markers of inflammation, promote tissue repair, and shorten time to recovery from lung injury, although Tregs have not been trialed clinically in ARDS before their use in SARS-CoV-2 infection.120 MSCs attenuate systemic inflammation and lung injury in murine models of ARDS, and phase 1 and 2a trials among adults with ARDS have shown them to be safe and well tolerated.122,125 In viral ARDS specifically, preclinical results with MSC treatment have been mixed.126-128 Whether the therapeutic effect of MSCs changes depending on the viral pathogen remains an open question.
Both MSCs and Tregs have been used safely to treat steroid-refractory graft-versus-host disease (GVHD). In this setting, treatment aims to dampen a damaging graft-versus-host immune response by suppressing cytotoxic T cells along with elements of the innate immune response including dendritic and NK cells.129,130 Similar immune modulatory targets may explain the therapeutic potential of these biologics for the treatment of viral ARDS, which is exacerbated by a damaging inflammatory response.131
Treg treatment for COVID-19
The full phenotype and function of Tregs in vivo is well reviewed elsewhere132-134 and is beyond the scope of this review. In brief, Tregs are characteristically CD4+CD25hiCD127lo T lymphocytes that express the transcription factor Forkhead Box Protein 3 and inhibit the activation and proliferation of inflammatory effector cells, including CD4+ and CD8+ effector T lymphocytes, macrophages, B cells, neutrophils, and dendritic cells.132,135,136 Treg products for cellular therapy may be either autologous or allogeneic depending on the clinical context and have been safely administered to patients for the treatment of GVHD, solid organ transplant rejection, and autoimmune diseases in phase 1/2 clinical trials.137-141
In the setting of an acute and life-threatening viral infection, such as severe COVID-19, rapid availability of an “off-the-shelf” product is needed. Thus, clinical trials of Tregs in COVID-19 (Table 3) are evaluating banked, partially HLA-matched allogeneic Treg products. The manufacture of Tregs for treatment of SARS-CoV-2 infection requires expansion from donor cord blood (CB) or peripheral blood, as Tregs comprise only 5% to 10% of the CD4+ population in these sources.
Treg-cell immunotherapy trials for COVID-19
| Trial . | Treatment . | Population . | Study design . | Phase . | Estimated enrollment . | Blood disorder eligibility . |
|---|---|---|---|---|---|---|
| NCT05027815 | Cryopreserved ex vivo expanded polyclonal CD4+CD127loCD25+ cells | 18 to 70 y SARS-CoV-2 + RT-PCR Diagnosis of ARDS requiring intubation within 72 h of enrollment Chest imaging consistent with COVID-19 pneumonia | Nonrandomized, open label, dose escalation trial at 3 doses (100, 200, 400 × 106 cells) | 1/2 (recruiting) | 18 | Excluded for known or suspected immune suppression and/or a history of bone marrow or stem cell transplantation |
| NCT04468971 | CK0802 | ≥18 y SARS-CoV-2 RT-PCR positive Moderate to severe ARDS Intubated for less than 120 h | Multicenter, double blinded, randomized, placebo-controlled safety and early efficacy trial Arm 1: placebo Arm 2: CK0802 at 1 × 108 cells Arm 3: CK0802 at 3 × 108 cells | 1 (active, not recruiting) | 45 | Not excluded |
| Trial . | Treatment . | Population . | Study design . | Phase . | Estimated enrollment . | Blood disorder eligibility . |
|---|---|---|---|---|---|---|
| NCT05027815 | Cryopreserved ex vivo expanded polyclonal CD4+CD127loCD25+ cells | 18 to 70 y SARS-CoV-2 + RT-PCR Diagnosis of ARDS requiring intubation within 72 h of enrollment Chest imaging consistent with COVID-19 pneumonia | Nonrandomized, open label, dose escalation trial at 3 doses (100, 200, 400 × 106 cells) | 1/2 (recruiting) | 18 | Excluded for known or suspected immune suppression and/or a history of bone marrow or stem cell transplantation |
| NCT04468971 | CK0802 | ≥18 y SARS-CoV-2 RT-PCR positive Moderate to severe ARDS Intubated for less than 120 h | Multicenter, double blinded, randomized, placebo-controlled safety and early efficacy trial Arm 1: placebo Arm 2: CK0802 at 1 × 108 cells Arm 3: CK0802 at 3 × 108 cells | 1 (active, not recruiting) | 45 | Not excluded |
In July 2020, Gladstone et al142 published a case series of 2 adult patients with COVID-19 and ARDS who were treated with 2 to 3 doses of 1 × 108 cells per dose of cryopreserved, allogeneic, HLA-matched Tregs, ex vivo expanded from CB. Neither patient experienced adverse events related to the treatment, and both experienced improvement in symptoms and a decrease in inflammatory markers (including IL-6, IL-12, interferon-γ, tumor necrosis factor-α) that was temporally associated with Treg infusion.142 This CB-derived Treg product, termed CK0802, is now under investigation in a multicenter, double-blinded, placebo-controlled, phase 1 trial (NCT04468971) targeting moderate-to-severe ARDS caused by COVID-19. NCT05027815 is also evaluating treatment with allogeneic ex vivo expanded polyclonal CD4+CD127loCD25hi Tregs for treatment of COVID-19 and ARDS in an open label, nonrandomized dose-escalation study. Results from these trials have not yet been reported.
MSC production for treatment of COVID-19
MSCs currently in use in phase 1/2 clinical trials for the treatment of COVID-19 ARDS are derived from bone marrow, adipose tissue, or umbilical cord and then expanded in vitro for manufacture of a clinical product. Similar products have been used safely and effectively in phase 1, 2, and 3 trials to modulate the graft-versus-host response in acute and chronic GVHD.143-148 Their low MHC-II surface expression allows for allogeneic infusion without HLA matching. Although cell-to-cell contact mediates some aspects of potential therapeutic benefit of MSCs,149-151 their immune modulatory, antimicrobial, and tissue reparative properties are largely mediated by paracrine factors collectively referred to as the MSC “secretome.”152-157 MSC-secreted hepatocyte growth factor,124 keratinocyte growth factor,154,158 and angiopoietin-1155,159 have all been linked to protection against lung injury, as have extracellular vesicle-associated RNAs.157 As such, MSC-derived extracellular vesicles are also under investigation for the treatment of severe COVID-19.
Although MSCs show efficacy in preclinical disease models and safety in early clinical trials, the results of phase 3 trials have been mixed. This is likely in part because of variation in tissue source and manufacturing protocols, including culture conditions and number of passages, which can lead to functional and phenotypic differences in MSCs that might go undetected based on minimal release criteria.160 Furthermore, best methods of isolation (plastic adherence vs selection) and ex vivo expansion are also unclear, as head-to-head studies of clinical outcomes using differing manufacturing methods have not been performed.161
Use of MSCs for treatment of COVID-19
Based on preclinical and early clinical data supporting their use in ARDS and observation of the inflammatory response to SARS-CoV-2, several groups completed pilot studies using MSCs to treat severe COVID-19, with no observed treatment-related adverse events.162-167 These included an open-label, individually randomized pilot study conducted by Shu et al,162 in which 41 patients diagnosed with severe COVID-19 were assigned to treatment with a single IV dose of 2 × 106 cells/kg human umbilical cord (hUC)-MSCs (n = 12) or standard treatment (n = 29), with a decline in CRP and IL-6 and more rapid improvement in hypoxemia in the treatment compared with control group. Leng et al164 treated 7 patients diagnosed with COVID-19 with 1 × 106 cells/kg of hUC-MSCs with subsequent clinical improvement and decreased CRP and confirmed that their hUC-MSC product was ACE2 negative and thus unlikely to be infected by SARS-CoV-2, a finding that has been confirmed elsewhere.164,168,169 Guo et al165 treated 31 patients with severe COVID-19 pneumonia with 1 × 106 cells/kg hUC-MSCs with a trend toward improvement in oxygenation and decreased inflammatory markers (IL-6, CRP, procalcitonin) after treatment. Sengupta et al170 treated patients with severe COVID-19 and moderate-to-severe ARDS (n = 24) with MSC-derived exosomes (ExoFlo) in a nonrandomized, open-label cohort study and found an improvement in oxygenation and a survival rate of 83% after treatment, along with an increase in lymphocyte counts and a decrease in CRP.
Results from early randomized controlled trials of MSCs for treatment of COVID-19 have shown promising preliminary efficacy results, although their samples sizes are small. In a double-blinded, randomized, placebo-controlled trial of 40 critically ill adults with COVID-19, 20 of whom were treated with hUC-MSCs, Dilogo et al171 (NCT04457609) reported a survival rate in patients treated with hUC-MSCs that was 2.5 times higher than those who received placebo (P = .047). Lanzoni et al172 (NCT04355728) also performed a double-blinded, randomized, placebo-controlled phase 1/2a study in which 24 patients with COVID-19 ARDS were allocated 1:1 to either treatment with 2 doses of 100 ± 20 × 106 hUC-MSCs or vehicle solution and found significantly improved patient survival (91% vs 42%, P = .015), serious adverse event-free survival, and time to recovery in the hUC-MSC group. Shi et al173 (NCT04288102) treated 101 patients who had been hospitalized for COVID-19, most of whom were classified as being in the convalescent phase of illness at enrollment, with either hUC-MSCs (n = 66) or placebo (n = 35). This study identified an improvement in a 6-minute walk test and computed tomography lung lesion burden in the MSC group compared with placebo. There are currently more than 30 registered clinical trials evaluating safety, feasibility, and efficacy of MSCs for the treatment of COVID-19.
Limitations and future directions
Although multiple studies of cellular therapy targeting COVID-19 are underway or planned, there remain many potential hurdles for these treatments. Although some of the current studies include control groups, randomized controlled studies of cellular therapies are difficult to carry out, particularly when focused on rare patient populations. Cellular therapies can be costly because of the manufacturing requirements. However, current antiviral cell therapies are roughly on par with the cost of IV antiviral medications, and if effective in reducing hospitalization time, they may be economical. As SARS-CoV-2 infection has the potential to progress rapidly, both antiviral and anti-inflammatory cellular therapies would likely require rapid turnaround (likely on the order of days) to be feasible as treatment options. This would likely eliminate individualized cellular therapies for treatment in most cases. Furthermore, the standard use of immunosuppressive medications including corticosteroids in patients with severe respiratory disease may inactivate infused cellular therapies. Gene-engineered products may overcome this limitation but would add to the cost of product generation. Use of previously generated cell banks from healthy donors could overcome many of these hurdles.
Conclusion
There remains a need for novel therapies to protect vulnerable populations from SARS-CoV-2 and future emerging infectious diseases, and adoptive cellular therapy may play a role in the prevention of disease in patients with blood disorders who are unable to mount a vaccine response. In addition, cell-based therapies are a potential treatment for these patients and high-risk individuals with complicated COVID-19. Randomized controlled trials of antiviral and anti-inflammatory immunotherapy are, however, required to help determine whether these therapies will have an established place in our armamentarium against COVID-19.
Acknowledgments
Funding support for this article was provided by the Leukemia and Lymphoma Society (6632-21) and the Board of Visitors at Children's National Hospital, the Katzen Foundation, and the Connor Family Foundation.
Authorship
Contribution: S.R.C. conducted the literature review and wrote the paper; M.D.K. conducted the literature review and edited the paper; and C.M.B. oversaw the literature review and edited the paper.
Conflict-of-interest disclosure: C.M.B. is a cofounder and on the scientific advisory boards for Catamaran Bio and Mana Therapeutics with stock and/or ownership, is on the board of directors for Caballeta Bio with stock options, and has stock in Neximmune and Repertoire Immune Medicines. M.D.K. is on a scientific advisory panel for Enzyvant. C.M.B. and M.D.K. have filed a patent related to SARS-CoV-2 T cell therapy. S.R.C. declares no competing financial interests.
Correspondence: Catherine M. Bollard, Children’s National Hospital, 111 Michigan Ave NW, Washington, DC 20010; e-mail: cbollard@childrensnational.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal