In this issue of Blood, De Matteis et al1 describe the expansion of recently activated CD8 T cells in patients with macrophage activation syndrome (MAS) or secondary hemophagocytic lymphohistiocytosis (HLH), demonstrating important immunologic similarities to familial HLH. They also identified a potentially useful new marker of severe disease.
Many hematologists would agree that patients with HLH are among the most memorable ones they have cared for. Although HLH is an inflammatory syndrome with diverse etiologies, is there a distinctive underlying immune profile shared among these patients? Building on prior reports, the data presented by De Matteis et al in this issue clearly say yes, there is a common immune profile among genetic and some secondary forms of HLH. They found that patients with MAS (mostly developing as a complication of juvenile idiopathic arthritis), as well as other secondary forms of HLH, have a clear peripheral blood profile of acute CD8 T-cell activation. This study builds on prior studies involving familial (primary) HLH in 2 important ways. First, it describes increased CD38bright, HLA-DR+ CD8 T cells in patients with MAS, which are essentially identical to those seen in familial HLH.2-4 Similar to familial HLH, the finding of even relatively low frequencies of these cells can efficiently distinguish patients with MAS from those with the same underlying condition without MAS, in this case juvenile idiopathic arthritis. Second, they identify dim expression of CD4 on a fraction of activated (CD38bright, HLA-DR+) CD8+ T cells as a potentially useful marker of MAS disease severity.
What do these findings tell us more generally about HLH? First, they demonstrate a unified immune profile among both familial forms of HLH and some forms of secondary HLH in humans. Of note, the series described by De Matteis et al included 30 patients with MAS or other secondary HLH, but only 2 had malignancies and none with iatrogenic forms of HLH (eg, recipients of CAR T cells or T-cell–activating agents). However, despite the limited scope of the cohort, this finding suggests that similar immune pathogenesis among diverse patient groups underlies their clinical similarities.
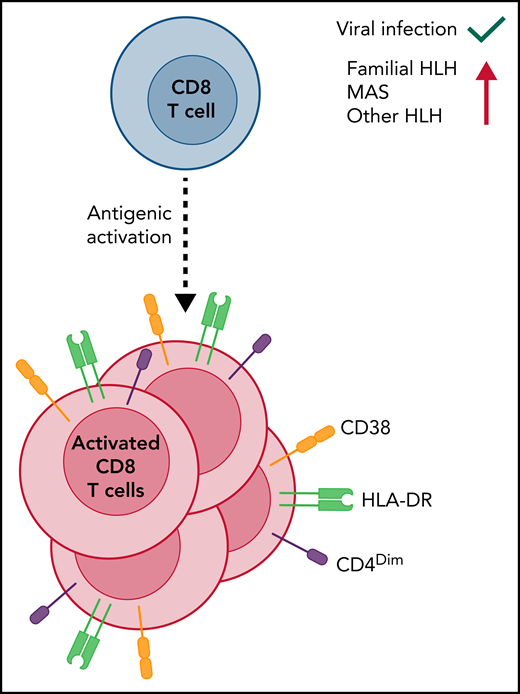
Second, these findings reinforce that HLH is fundamentally a disorder of unusual and harmful acute CD8 T-cell activation. Experimental animal models of HLH have demonstrated that HLH is driven by excessive antigen-driven activation of CD8 T cells, which are otherwise appropriately triggered and directed.5 This contrasts with the pathology of autoimmune disorders (misdirected toward self), autoinflammatory disorders (inappropriately triggered), or lymphoproliferative disorders (where toxicity of T-cell activation is unclear). However, what do recently activated CD8 T cells look like in humans (see figure)? Miller et al assessed T-cell responses to vaccination and found that dual expression of CD38 and HLA-DR by CD8 T cells is an efficient way to identify antigen-specific T cells, in other words, those T cells that have recently been activated by antigen presentation and are responding to the infection.6 This CD38/HLA-DR profile is also seen after natural viral infection,7 so it is not necessarily specific for disease states. However, De Matteis et al and Chaturvedi et al both showed that it is highly sensitive for MAS or HLH and can be quite specific when attempting to distinguish MAS/HLH from other clinically relevant disorders (active juvenile idiopathic arthritis or sepsis, respectively).2 Assessment of CD8 T-cell activation with these markers is quite simple for most clinical laboratories and appears to have diagnostic value, although this will require further study.
CD8 T-cell activation as a key measure in various forms of HLH, including MAS. When CD8 T cells are activated by encounter with cognate antigens, they divide and differentiate. Some cell surface markers, including high levels of CD38, HLA-DR, and low levels of CD4, have been demonstrated in humans to signify recent antigenic activation. Although this process is a normal part of T-cell responses, experimental models of HLH demonstrate that CD8 T-cell activation is greatly heightened and drives disease pathogenesis. Similarly, patients with familial HLH display distinctive and high levels of recently activated CD8 T cells. De Matteis et al have now extended this finding to patients with MAS and other secondary HLH and identify dim CD4 expression as correlating with MAS severity.
CD8 T-cell activation as a key measure in various forms of HLH, including MAS. When CD8 T cells are activated by encounter with cognate antigens, they divide and differentiate. Some cell surface markers, including high levels of CD38, HLA-DR, and low levels of CD4, have been demonstrated in humans to signify recent antigenic activation. Although this process is a normal part of T-cell responses, experimental models of HLH demonstrate that CD8 T-cell activation is greatly heightened and drives disease pathogenesis. Similarly, patients with familial HLH display distinctive and high levels of recently activated CD8 T cells. De Matteis et al have now extended this finding to patients with MAS and other secondary HLH and identify dim CD4 expression as correlating with MAS severity.
Third, the current finding of dim CD4 expression on CD8 T cells in patients with MAS or secondary HLH extends the T-cell activation phenotype described in prior studies with familial HLH. Low-level CD4 expression on CD8 T cells has been described as a marker of T-cell activation for decades (at least in vitro8) but has not been previously tied to a particular disease state. Its correlation with disease severity deserves further study, especially in comparison with currently used markers, such as sCD25. Although a prior report demonstrated good correlation between T-cell activation and other disease-relevant markers of inflammation,2 whether CD4 expression (or CD38/HLA-DR) on CD8 T cells has prognostic value in HLH or MAS remains to be determined.
As the previously mysterious syndromes of HLH and MAS reveal their secrets, it is increasingly obvious that they share a common pathophysiology involving T-cell hyperactivation and likely share common therapeutic targets. Revealing this similarity reminds us that, like birds of a feather, they flock together.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal