In this issue of Blood, Wang et al1 identified a critical role for the tension-sensitive cation channel PIEZO1 in leukocyte transendothelial migration (aka diapedesis or extravasation). Their detailed analysis provides new mechanistic data to fit the PIEZO1-mediated calcium influx into a sequence of endothelial signaling events that controls the extravasation process (see figure).
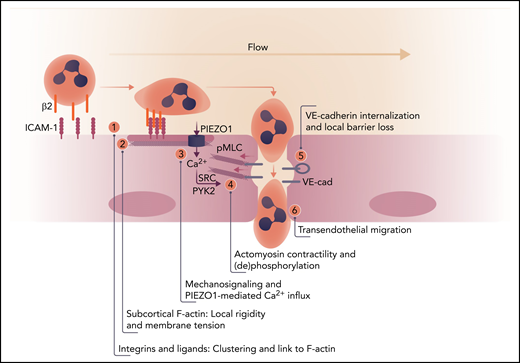
Order of events for endothelial signaling in leukocyte extravasation. (1) Leukocyte firm adhesion mediated by β2-integrins induces clustering of endothelial ICAM-1 and its association with actin-binding proteins (such as α-actinin4 and cortactin1,8). (2) This mediates reorganization of cortical F-actin to promote local stiffening with concomitant increased tension. (3) Flow and local tension cooperate to activate PIEZO1. (4) The increase in intracellular calcium triggers activation of SRC and PYK2 and induces, through phosphorylation of myosin light chain (pMLC), actomyosin contraction, disrupting vascular endothelial (VE)-cadherin complexes in cell-cell contacts. (5) Local loss of VE-cadherin–mediated adhesion drives its internalization and (6) efficient leukocyte migration across the endothelial barrier. Professional illustration by Somersault 18:24.
Order of events for endothelial signaling in leukocyte extravasation. (1) Leukocyte firm adhesion mediated by β2-integrins induces clustering of endothelial ICAM-1 and its association with actin-binding proteins (such as α-actinin4 and cortactin1,8). (2) This mediates reorganization of cortical F-actin to promote local stiffening with concomitant increased tension. (3) Flow and local tension cooperate to activate PIEZO1. (4) The increase in intracellular calcium triggers activation of SRC and PYK2 and induces, through phosphorylation of myosin light chain (pMLC), actomyosin contraction, disrupting vascular endothelial (VE)-cadherin complexes in cell-cell contacts. (5) Local loss of VE-cadherin–mediated adhesion drives its internalization and (6) efficient leukocyte migration across the endothelial barrier. Professional illustration by Somersault 18:24.
Intracellular calcium was recognized as an essential signal for most of cell biology more than 40 years ago. Consequently, in my laboratory, it became common practice to ask at the end of a presentation (irrespective of the topic): “But what about calcium?” And indeed, here we are (again), recognizing the role of endothelial calcium because of PIEZO1, an intriguing piece of the puzzle that illustrates the role of calcium in the process of leukocyte extravasation.
PIEZO1 is a homotrimeric mechanosensitive cation channel that was identified in 2010.2 Conversely, endothelial calcium fluxes were implicated in neutrophil extravasation in the early 1990s.3 However, at the time, the multistep paradigm for leukocyte transendothelial migration had just been proposed,4 and the field focused primarily on leukocyte integrins and their activation by chemokines. In the subsequent 10 to 15 years, increasing evidence has shown that signaling within the endothelium (which is not “a passive bystander”) not only contributed to, but was in fact required for efficient extravasation of various types of immune cell.
These studies implicated endothelial reactive oxygen species, actin-based contractility, kinases, phosphatases, actin-organizing adapter proteins, and internalization and trafficking events in extravasation (reviewed in Nourshargh and Alon5 and in Muller6). However, the relevant surface receptors and upstream signals remained obscure. Antibody-mediated clustering of endothelial adhesion receptors, such as intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1), mimicking their binding to activated leukocyte integrins, revealed their role as signaling molecules. Another important extension of the multistep model came from data showing that mechanosensing and -signaling significantly promoted extravasation. This concept arose from studies that showed that shear forces due to flow7 and local endothelial rigidity due to (ICAM-1–mediated) actin rearrangements8,9 promoted leukocyte transendothelial migration.
In the study by Wang et al, these components come together and expand our mechanistic view of this complex process. The authors combine a series of in vivo mouse inflammation models with in vitro approaches such as electrophysiology to show that PIEZO1 mediates the Ca2+ influx that allows efficient leukocyte breaching of the endothelial barrier. Importantly, firm adhesion of neutrophils did not require PIEZO1, which indicates that the influx of calcium occurs after ICAM-1 clustering. Because the effects downstream of Ca2+, such as SRC and PYK2 activation and actomyosin contractility, are in line with published data, the novelty of the work by Wang et al lies in the identification of the upstream regulation of PIEZO1.
Because PIEZO1 is a force-sensitive channel, the authors measured PIEZO1-mediated Ca2+ fluxes in the presence of laminar flow, but this induced only minimal effects. Similarly, neutrophil firm adhesion in the absence of flow was not very effective. But the increase in intracellular calcium was much more prominent when neutrophils were combined with flow, suggesting a cooperative signal. Neutrophil firm adhesion can be mimicked by antibody-mediated ICAM-1 clustering, which induces local endothelial cell stiffening.8,9 On the basis of these earlier findings, Wang et al decided to analyze changes in membrane tension by using fluorescent sensors. Their elegant studies confirmed the notion that ICAM-1 clustering or neutrophil adhesion cooperates with flow to increase local membrane tension which is sensed by PIEZO1. Intriguingly, the induction of tension was dependent on actin polymerization and myosin activity, suggesting a local positive feedback loop that amplifies endothelial signaling at sites of firm leukocyte adhesion.
In summary, the study by Wang et al underscores the importance of endothelial PIEZO1 for extravasation and identifies its activation by local membrane tension generated by the combined action of flow and ICAM-1 clustering. PIEZO1 is not the only force-sensing ion channel in the microcirculation. For example, in endothelial cells of resistance arteries, flow-sensitive, inwardly rectifying K+ channels contribute to the regulation of blood pressure.10 Throughout the microcirculation, flow speeds and vessel diameters vary considerably, with concomitant effects on local shear forces sensed by the endothelium. The complex regulation of PIEZO1 allows transendothelial migration to be efficient under the appropriate hemodynamic conditions, in this case in postcapillary venules. This concept generates many interesting questions on how the force landscape of the different microvascular beds tunes endothelial responses, for example, in the context of inflammatory or age-related diseases.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal