Key Points
Mnk1 regulates mRNA translational events in megakaryocytes and platelets.
Mnk1 contributes to megakaryopoiesis and platelet production, cPLA2 activity, and thrombosis.
Abstract
The MAPK-interacting kinase (Mnk) family includes Mnk1 and Mnk2, which are phosphorylated and activated in response to extracellular stimuli. Mnk1 contributes to cellular responses by regulating messenger RNA (mRNA) translation, and mRNA translation influences platelet production and function. However, the role of Mnk1 in megakaryocytes and platelets has not previously been studied. The present study investigated Mnk1 in megakaryocytes and platelets using both pharmacological and genetic approaches. We demonstrate that Mnk1, but not Mnk2, is expressed and active in human and murine megakaryocytes and platelets. Stimulating human and murine megakaryocytes and platelets induced Mnk1 activation and phosphorylation of eIF4E, a downstream target of activated Mnk1 that triggers mRNA translation. Mnk1 inhibition or deletion significantly diminished protein synthesis in megakaryocytes as measured by polysome profiling and [35S]-methionine incorporation assays. Depletion of Mnk1 also reduced megakaryocyte ploidy and proplatelet forming megakaryocytes in vitro and resulted in thrombocytopenia. However, Mnk1 deletion did not affect the half-life of circulating platelets. Platelets from Mnk1 knockout mice exhibited reduced platelet aggregation, α granule secretion, and integrin αIIbβ3 activation. Ribosomal footprint sequencing indicated that Mnk1 regulates the translation of Pla2g4a mRNA (which encodes cPLA2) in megakaryocytes. Consistent with this, Mnk1 ablation reduced cPLA2 activity and thromboxane generation in platelets and megakaryocytes. In vivo, Mnk1 ablation protected against platelet-dependent thromboembolism. These results provide previously unrecognized evidence that Mnk1 regulates mRNA translation and cellular activation in platelets and megakaryocytes, endomitosis and thrombopoiesis, and thrombosis.
Introduction
Megakaryocytes produce ∼1 × 1011 platelets in adults daily under physiological conditions1 by projecting proplatelets into bone marrow sinusoidal vessels,2-4 regulating hemostasis and thrombosis.5-7 Platelet activation is associated with a change in platelet shape, secretion of granule contents, activation of the fibrinogen receptor, and generation of the lipid mediator thromboxane A2 (TxA2).8-10
MAPK-interacting kinase 1 (Mnk1) belongs to a group of serine/threonine kinases called MAPK interacting protein kinases.11,12 In mammalian cells, there are only 2 Mnk family members: Mnk1 and Mnk2. Both Mnk1 and Mnk2 can be activated by either Erk or p38 MAPK.11 In other cells, Mnk1 regulates the translation of inflammatory genes while also driving receptor tyrosine kinase activity (Spry2).13-16 Mnks mediate the pathophysiology of malignant and thrombo-inflammatory diseases,17-20 but Mnk knockout (KO) mice develop normally, suggesting Mnks may be a safe therapeutic target. Drugs targeting Mnks are in clinical development.21
Translation of messenger RNAs (mRNAs) is a critical step in proplatelet formation and platelet function.22,23 Translation is generally divided into 3 steps: initiation, elongation, and termination, and initiation is often the rate-limiting step. Initiation begins when eukaryotic initiation factor 4F (eIF4F) binds to the 5′ cap of mRNAs. The eIF4F protein complex comprises 3 subunits: eIF4A, which processes ATPase and RNA helicase activities; the 5′ mRNA cap-binding protein eIF4E; and the scaffolding protein eIF4G, which recruits other translation factors and ribosomes to the 5′ end of mRNAs. In nucleated cells, the activity of eIF4E is regulated via its phosphorylation by Mnks and binding to eIF4E-binding protein repressor proteins.13,24 Mnks are the only known kinases that phosphorylate eIF4E, and eIF4E is the only known Mnk substrate validated in vivo.25 Mnk1 drives the inducible phosphorylation of eIF4E, whereas Mnk2 primarily drives the basal constitutive phosphorylation of eIF4E.25
eIF4E is present and active in human platelets and is regulated by outside-in signals delivered by integrins.26 However, the expression and function of Mnk1 in megakaryocytes and platelets remain largely unknown. In this study, we sought to elucidate the role of Mnk1 in human and murine megakaryocytes and platelets.
Methods
Platelets and murine megakaryocytes were prepared from healthy donors, C57BL/6 wild-type (WT) or global Mnk1 KO mice.25,27-33 Human megakaryocytes were transfected with MKNK1 clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9) (Alt-R CRISPR/Cas9 RNAs with >65% on-target score or nontargeting control) on culture day 5.34
For polyploidy assays, bone-marrow murine megakaryocytes (culture day 5) were isolated, stained with CD41 antibody, fixed (paraformaldehyde), and incubated with propidium iodide (50 μg/mL, 1 hour) and RNAase A (300 μg/mL), and flow cytometry was performed.
For ribosome footprint profiling, megakaryocytes were treated with cycloheximide to preserve ribosomes natively attached to mRNAs (ARTseq-Ribosome Profiling Kit). Sequencing was done using Illumina HiSeq. Significantly differentially expressed ribosome-protected RNAs (RPRs) were those with a false discovery rate < 0.05 and a log2 fold change ≥ 1.5 in Mnk1 KO mice.
Platelet aggregation to various agonists was measured using a lumi-aggregometer (37°C stirring). In select experiments, platelets were preincubated with CGP 57380, a Mnk inhibitor. cPLA2 activity was assessed in murine platelets using the Cytosolic Phospholipase A2 assay kit (Abcam; ab133090). Levels of TxB2 were determined using a Correlate-EIA thromboxane B2 enzyme immunoassay kit (Assay Designs, Inc., Ann Arbor, MI).
The inferior vena cava (IVC) venous thrombosis stasis model was performed as described.35,36 Following sterile laparotomy, the IVC was dissected bluntly, and side branches were ligated with 8-0 Prolenesuture, and lumbar branches were closed. The IVC was separated from the aorta and completely ligated with 8-0 Prolene suture. After 48 hours, thrombi were collected and weighed.
For the pulmonary embolism model, collagen (0.2 mg/kg) and epinephrine (15 mg/kg) were administered through retro-orbital injection, and time to cessation of respiration was recorded.
For polysome profiling assays, sucrose gradients (2-6x, 10%-50%, 12%-50%, or 15%-50%) were prepared, and cells were pretreated with 100 μg/mL cycloheximide (5 minutes). Cells were collected by centrifugation for 2 minutes at 2000g, and the cell pellet was washed with 1x phosphate-buffered saline with 100 μg/mL cycloheximide, collected by centrifugation (2 minutes, 2000g), and resuspended in laemmlie sample buffer supplemented with ribonuclease and cycloheximide. Gradients were analyzed on a UV reader and collected into tubes containing TRIzol LS.
For the [35S]-Methionine incorporation assay, megakaryocytes (culture day 5) were suspended in Dulbecco’s modified Eagle medium that lacked methionine and cysteine. EasyTag EXPRESS [35S] protein labeling mix was added to the megakaryocytes (15 minutes). Megakaryocytes were adhered on a fibrinogen-coated plate (10 mg/mL). The cell pellets and supernatants were collected, washed, and lysed in aadioimmunoprecipitation assay buffer, precipitated (20% trichloroacetic acid), loaded onto a Whatman grade GF/C glass microfiber filter, and read using a liquid scintillation counter.
Each experiment was repeated at least 3 independent times. Data were analyzed using Prism software. Significant differences were determined using Student t test, analysis of variance, and Kaplan-Meier survival analyses as appropriate. A 2-tailed P value < .05 was considered significant.
Please also see supplemental Materials (available on the Blood website).
Results
Mnk1 is functionally active in megakaryocytes and platelets
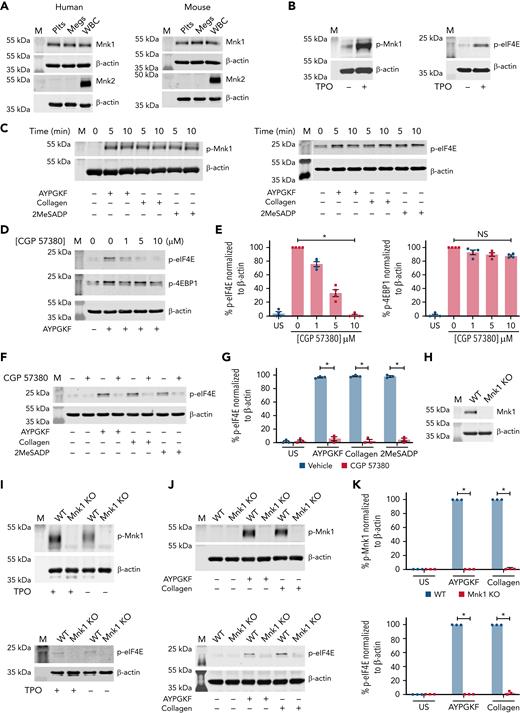
Mnk1 protein, but not Mnk2, is basally expressed in both human and mouse megakaryocytes and platelets comparable to white blood cells (Figure 1A). Mnk1 is rapidly phosphorylated in activated megakaryocytes and platelets (Figure 1B-C). One agonist that induced phosphorylation of Mnk1 was thrombopoietin (TPO), which also regulates platelet production. Agonists that phosphorylated Mnk1 also phosphorylated eIF4E (Figure 1B-C).
Megakaryocytes and platelets express Mnk1 protein. (A) Washed platelets (Plts), megakaryocytes (Megs), and white blood cells (as a control) from both healthy human donors and WT C57Bl/6 mice were lysed and analyzed for total Mnk1 and Mnk2 protein expression by western blotting. β-Actin was used as a loading control. Images representative of n ≥ 3 independent experiments. (B) CD34+ megakaryocytes derived and cultured from human cord blood were lysed after stimulation with or without TPO (100 ng/mL) on culture day 13 for 15 minutes. Lysates were analyzed for phosphorylated Mnk1 (p-Mnk1, left) and phosphorylated eIF4E (p-eIF4E, right) by western blotting. β-Actin was used as a loading control. Images representative of n ≥ 3 independent experiments. (C) Washed human platelets were left alone (0 minutes) or stimulated with AYPGKF (100 μM), collagen (5 μg/mL), or 2MeSADP (50 nM) for 5 and 10 minutes. Samples were analyzed for p-Mnk1 (left) and p-EIF4E (right) by western blotting. β-Actin was used as a loading control. Images representative of n ≥ 3 independent experiments. (D-E) Washed human platelets were left unstimulated (US) in DMSO vehicle control or treated with different concentrations of the Mnk1 inhibitor CGP 57380 for 5 minutes. Then, platelets were stimulated with AYPGKF (100 μM, 5 minutes). Cells were then lysed and analyzed for p-eIF4E and p-4EBP1 by western blotting. β-Actin was used as a loading control. Quantitative analysis of p-eIF4E and p-4EBP1 normalized to β-actin are shown in the bar graphs to the right. Western blot images are representative of n ≥ 3 independent experiments. (F-G) Washed human platelets were left US or treated with CGP 57380 (10 μM) for 5 minutes. The cells were then left alone in DMSO (Veh) or stimulated with AYPGKF (100 μM), collagen (5 μg/mL), or 2MeSADP (50 nM) for 5 minutes and probed for p-eIF4E by western blotting. β-Actin was used as a loading control. Quantitative analysis of p-eIF4E normalized to β-actin is shown in the bar graph to the right (∗P < .05). Western blot images are representative of n ≥ 3 independent experiments. (H) The expression of total Mnk1 protein in platelets from either WT or Mnk1 KO mice was analyzed by western blotting. β-Actin was used as a loading control. Western blot images are representative of n ≥ 3 independent experiments. (I) Cultured bone marrow megakaryocytes from either WT or Mnk1 KO mice were left alone or stimulated with TPO (100 μM) for 15 minutes and then analyzed for p-Mnk1 (top) and p-eIF4E (bottom) by western blotting. β-Actin was used as a loading control. Western blot images are representative of n ≥ 3 independent experiments. (J) Washed platelets from either WT or Mnk1 KO mice were left unstimulated or stimulated with AYPGKF (100 μM) or collagen (5 μg/mL) for 5 and 10 minutes. Platelets were analyzed for p-Mnk1 (top) and p-eIF4E (bottom) by western blotting. β-Actin was used as a loading control. Images are representative of n ≥ 3 independent experiments. (K) Quantitative analyses of p-Mnk1 (top) and p-EIF4E (bottom) normalized to β-actin (∗P < .05). M, marker; NS, not significant.
Megakaryocytes and platelets express Mnk1 protein. (A) Washed platelets (Plts), megakaryocytes (Megs), and white blood cells (as a control) from both healthy human donors and WT C57Bl/6 mice were lysed and analyzed for total Mnk1 and Mnk2 protein expression by western blotting. β-Actin was used as a loading control. Images representative of n ≥ 3 independent experiments. (B) CD34+ megakaryocytes derived and cultured from human cord blood were lysed after stimulation with or without TPO (100 ng/mL) on culture day 13 for 15 minutes. Lysates were analyzed for phosphorylated Mnk1 (p-Mnk1, left) and phosphorylated eIF4E (p-eIF4E, right) by western blotting. β-Actin was used as a loading control. Images representative of n ≥ 3 independent experiments. (C) Washed human platelets were left alone (0 minutes) or stimulated with AYPGKF (100 μM), collagen (5 μg/mL), or 2MeSADP (50 nM) for 5 and 10 minutes. Samples were analyzed for p-Mnk1 (left) and p-EIF4E (right) by western blotting. β-Actin was used as a loading control. Images representative of n ≥ 3 independent experiments. (D-E) Washed human platelets were left unstimulated (US) in DMSO vehicle control or treated with different concentrations of the Mnk1 inhibitor CGP 57380 for 5 minutes. Then, platelets were stimulated with AYPGKF (100 μM, 5 minutes). Cells were then lysed and analyzed for p-eIF4E and p-4EBP1 by western blotting. β-Actin was used as a loading control. Quantitative analysis of p-eIF4E and p-4EBP1 normalized to β-actin are shown in the bar graphs to the right. Western blot images are representative of n ≥ 3 independent experiments. (F-G) Washed human platelets were left US or treated with CGP 57380 (10 μM) for 5 minutes. The cells were then left alone in DMSO (Veh) or stimulated with AYPGKF (100 μM), collagen (5 μg/mL), or 2MeSADP (50 nM) for 5 minutes and probed for p-eIF4E by western blotting. β-Actin was used as a loading control. Quantitative analysis of p-eIF4E normalized to β-actin is shown in the bar graph to the right (∗P < .05). Western blot images are representative of n ≥ 3 independent experiments. (H) The expression of total Mnk1 protein in platelets from either WT or Mnk1 KO mice was analyzed by western blotting. β-Actin was used as a loading control. Western blot images are representative of n ≥ 3 independent experiments. (I) Cultured bone marrow megakaryocytes from either WT or Mnk1 KO mice were left alone or stimulated with TPO (100 μM) for 15 minutes and then analyzed for p-Mnk1 (top) and p-eIF4E (bottom) by western blotting. β-Actin was used as a loading control. Western blot images are representative of n ≥ 3 independent experiments. (J) Washed platelets from either WT or Mnk1 KO mice were left unstimulated or stimulated with AYPGKF (100 μM) or collagen (5 μg/mL) for 5 and 10 minutes. Platelets were analyzed for p-Mnk1 (top) and p-eIF4E (bottom) by western blotting. β-Actin was used as a loading control. Images are representative of n ≥ 3 independent experiments. (K) Quantitative analyses of p-Mnk1 (top) and p-EIF4E (bottom) normalized to β-actin (∗P < .05). M, marker; NS, not significant.
To determine whether Mnk1 was necessary for eIF4E phosphorylation, we adopted complementary pharmacological and genetic approaches in human and murine platelets. An inhibitor of Mnk1, CGP 57380, abolished agonist-stimulated phosphorylation of eIF4E in platelets in a dose-dependent fashion and without significant off-target effects (Figure 1D-E and not shown). Similar results were observed in platelets activated with collagen and 2MesADP (Figure 1F-G). As expected, CGP 57380 had no significant effect on phosphorylation of eIF4E-binding protein 1 (Figure 1D), which is upstream of Mnk1.
We next used mice where MKNK1 (the gene name for Mnk1) was globally ablated. We confirmed that in these mice (termed Mnk1 KO mice herein), Mnk1 protein is absent in platelets and megakaryocytes (Figure 1H and not shown). Deletion of MKNK1 eliminated phosphorylation of Mnk1 and eIF4E in activated murine megakaryocytes and platelets (Figure 1I-K). Total eIF4E protein levels were not significantly altered in Mnk1 KO mice (supplemental Figure 1A-B). These results show that Mnk1 is expressed and activatable in megakaryocytes and platelets and, once activated, has the potential to associate with downstream eIF4E phosphorylation.
Mnk1 regulates mRNA translation in megakaryocytes
eIF4E initiates mRNA translation, and, in other cells, Mnk1 regulates mRNA translation.37-39 Whether Mnk1 regulates mRNA translation in megakaryocytes and platelets, however, has not been previously established. Therefore, we next evaluated the role of Mnk1 on mRNA translation and de novo protein synthesis in CD34+, cord-blood derived, cultured human megakaryocytes by using polysome profiling and [35S]-methionine incorporation assays. In the polysome profiling assay, megakaryocytes are activated on adherent fibrinogen and then separated on sucrose gradients and fractionated to identify peaks corresponding to ribosomal monosomes (subunits 40S, 60S, and 80S) and polysomes. A shift into the monosome fraction with an accompanying decrease in the polysome fraction is consistent with repressed mRNA translation. As shown in Figure 2A, inhibiting Mnk1 with CGP 57380 resulted in a higher monosome peak and lower polysome peak, suggesting Mnk1 regulates mRNA translation in megakaryocytes.
Mnk1 regulates mRNA translation and de novo protein synthesis in human and murine megakaryocytes. (A) Human, cord blood–derived, CD34+ megakaryocytes were left alone with vehicle control (DMSO, blue line) or treated with CGP 57380 (10 μM, red line) on culture day 13 and then allowed to adhere on fibrinogen-coated plates for 2 hours. Megakaryocytes were then lysed and sedimented by centrifugation on a 5% to 50% sucrose gradient. Isolated monosome and polysome fractions are indicated. Graphs are representative of n = 3 independent experiments. (B) Human, cord blood–derived, CD34+ megakaryocytes were cultured in the presence of CGP 57380 (10 μM) or vehicle control (DMSO) on culture day 13. Megakaryocytes were then resuspended in [35S]-methionine media and allowed to adhere on fibrinogen-coated plates for 2 hours. Protein synthesis was quantified using a scintillation counter (∗P < .05; n = 5 independent experiments). (C) Bone marrow–derived megakaryocytes from either WT or Mnk1 KO mice were resuspended in [35S]-methionine media and allowed to adhere on fibrinogen-coated plates for 2 hours. Protein synthesis was quantified using a scintillation counter (∗P < .05; n = 6 independent experiments).
Mnk1 regulates mRNA translation and de novo protein synthesis in human and murine megakaryocytes. (A) Human, cord blood–derived, CD34+ megakaryocytes were left alone with vehicle control (DMSO, blue line) or treated with CGP 57380 (10 μM, red line) on culture day 13 and then allowed to adhere on fibrinogen-coated plates for 2 hours. Megakaryocytes were then lysed and sedimented by centrifugation on a 5% to 50% sucrose gradient. Isolated monosome and polysome fractions are indicated. Graphs are representative of n = 3 independent experiments. (B) Human, cord blood–derived, CD34+ megakaryocytes were cultured in the presence of CGP 57380 (10 μM) or vehicle control (DMSO) on culture day 13. Megakaryocytes were then resuspended in [35S]-methionine media and allowed to adhere on fibrinogen-coated plates for 2 hours. Protein synthesis was quantified using a scintillation counter (∗P < .05; n = 5 independent experiments). (C) Bone marrow–derived megakaryocytes from either WT or Mnk1 KO mice were resuspended in [35S]-methionine media and allowed to adhere on fibrinogen-coated plates for 2 hours. Protein synthesis was quantified using a scintillation counter (∗P < .05; n = 6 independent experiments).
We next performed an [35S]-methionine incorporation assay in human megakaryocytes to quantify changes in mRNA translation. Inhibiting Mnk1 with CGP 57380 modestly, but significantly, reduced the incorporation of [35S]-methionine into newly synthesized proteins (Figure 2B). We observed a similar reduction in protein synthesis in fibrinogen-activated megakaryocytes from Mnk1 KO mice (Figure 2C). These results indicate that Mnk1 regulates mRNA translation and de novo protein synthesis in activated human and murine megakaryocytes.
Mnk1 regulates megakaryocyte endomitosis and platelet production
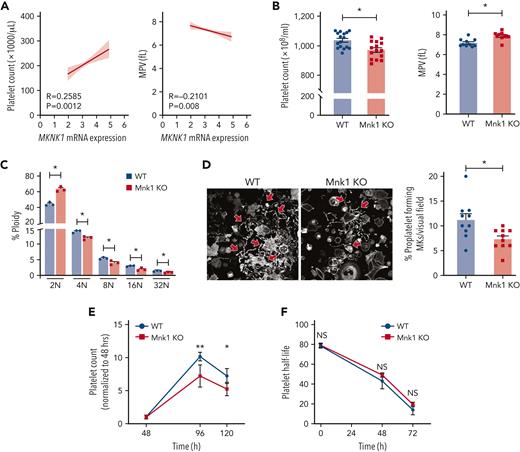
Previous studies have shown that Mnk1 is vital for cell development.39-41 It remains unknown whether Mnk1 regulates megakaryopoiesis and thrombopoiesis. We leveraged data from PRAX1,42 which includes 154 healthy donors, to evaluate any association between Mnk1 RNA expression and platelet count or size. We observed a significant positive association between platelet Mnk1 RNA expression and platelet counts and a significant negative association between Mnk1 RNA expression and mean platelet volume (MPV; Figure 3A). In contrast, Mnk1 RNA expression did not correlate with leukocyte counts (supplemental Figure 2A). Consistent with this human associative data, platelet counts were significantly decreased and the MPV significantly increased in Mnk1 KO mice compared with WT controls (Figure 3B). There were no differences in leukocyte counts in Mnk1 KO mice (supplemental Figure 2B-C).
Mnk1 regulates megakaryocyte endomitosis and platelet production. (A) Pearson correlation analyses of the association between platelet counts (left) and MPV (right) with platelet MKNK1 mRNA expression (n = 154 healthy human donors). Light gray lines represent 95% confidence intervals. (B) Platelet counts (left) and mean platelet volume (right) in WT or Mnk1 KO mice (n > 10 mice per group, ∗P < .05). (C) Bone marrow–derived megakaryocytes from WT or Mnk1 KO mice were collected at culture day 5, and ploidy distribution of megakaryocytes was quantified (n = 3 independent experiments), ∗PANOVA < .05). (D) Bone marrow–derived megakaryocytes from WT or Mnk1 KO mice were collected at culture day 5. Megakaryocytes were then allowed to adhere to fibrinogen-coated plates and incubated overnight at 37°C. Megakaryocytes were then stained with phalloidin, and proplatelet formation was assessed by confocal microscopy. The right bar graph shows the number of proplatelet forming megakaryocytes (∗P < .05, n = 3 independent experiments). (E-F) Platelets were depleted from WT or Mnk1 KO mice by a single IV injection of an anti-GPIbα antibody (Emfret Analytics), leading to near-complete platelet clearance by 48 hours. Platelet counts were measured over 120 hours (eg, 5 days) by Hemavet. The (E) percentage of platelet count recovery was calculated from the platelet nadir at 48 hours, and (F) platelet clearance was calculated from the time of injection of the anti-GPIbα antibody (n = 5 mice per group, ∗P < .05; ∗∗P < .01). ANOVA, analysis of variance; NS, not significant.
Mnk1 regulates megakaryocyte endomitosis and platelet production. (A) Pearson correlation analyses of the association between platelet counts (left) and MPV (right) with platelet MKNK1 mRNA expression (n = 154 healthy human donors). Light gray lines represent 95% confidence intervals. (B) Platelet counts (left) and mean platelet volume (right) in WT or Mnk1 KO mice (n > 10 mice per group, ∗P < .05). (C) Bone marrow–derived megakaryocytes from WT or Mnk1 KO mice were collected at culture day 5, and ploidy distribution of megakaryocytes was quantified (n = 3 independent experiments), ∗PANOVA < .05). (D) Bone marrow–derived megakaryocytes from WT or Mnk1 KO mice were collected at culture day 5. Megakaryocytes were then allowed to adhere to fibrinogen-coated plates and incubated overnight at 37°C. Megakaryocytes were then stained with phalloidin, and proplatelet formation was assessed by confocal microscopy. The right bar graph shows the number of proplatelet forming megakaryocytes (∗P < .05, n = 3 independent experiments). (E-F) Platelets were depleted from WT or Mnk1 KO mice by a single IV injection of an anti-GPIbα antibody (Emfret Analytics), leading to near-complete platelet clearance by 48 hours. Platelet counts were measured over 120 hours (eg, 5 days) by Hemavet. The (E) percentage of platelet count recovery was calculated from the platelet nadir at 48 hours, and (F) platelet clearance was calculated from the time of injection of the anti-GPIbα antibody (n = 5 mice per group, ∗P < .05; ∗∗P < .01). ANOVA, analysis of variance; NS, not significant.
We next evaluated whether the reduced platelet count in Mnk1 KO mice was due to defects in platelet production or clearance. We first examined whether megakaryopoiesis was altered in Mnk1 KO mice. Megakaryocytes progress through development to an endomitotic phase, where cytokinesis is arrested and DNA accumulates in a single polylobed nucleus before a final maturation state that is characterized by proplatelet formation and release.43 Accordingly, we examined endomitosis (polyploidization) in megakaryocytes from Mnk1 KO mice and WT controls. The absence of Mnk1 significantly increased the proportion of 2N megakaryocytes and decreased the proportion of 4N-32N megakaryocytes (Figure 3C). Megakaryocyte maturation as assessed by surface expression markers (eg, CD41) was not significantly altered in Mnk1 KO mice (data not shown).
We next examined proplatelet formation by megakaryocytes. In Mnk1 KO mice, in vitro proplatelet production by megakaryocytes was significantly reduced (Figure 3D). We injected mice with an anti-GP1bα antibody, resulting in near-identical platelet nadirs in WT and Mnk1 KO mice by 48 hours postinjection (Figure 3E; supplemental Figure 3A). Consistent with impaired in vitro platelet production by Mnk1 KO megakaryocytes, in vivo platelet production (measured as the percentage of platelet recovery following platelet nadir at 48 hours) was also significantly lower in Mnk1 KO mice (Figure 3E). Reduced platelet production in Mnk1 KO mice was accompanied by increased platelet size (supplemental Figure 3B). In comparison, platelet half-life did not significantly differ between Mnk1 KO mice and WT control mice (Figure 3F). Collectively, these data indicate that Mnk1 regulates megakaryopoiesis, platelet production, and circulating platelet counts.
To establish whether Mnk1 translational regulation controls platelet production in human cells, we used CRISPR/Cas9 to selectively delete the MKNK1 gene from CD34+, cord-blood derived, cultured human megakaryocytes (supplemental Figure 4A). Similar to our findings in murine bone marrow megakaryocytes (Figure 3D), ablating Mnk1 in human cultured megakaryocytes significantly reduced proplatelet formation (supplemental Figure 4B-C).
Mnk1 alters platelet activation responses
mRNA translation impacts platelet functional responses.26,44,45 In platelets and megakaryocytes, cellular activation signals may induce mRNA translation.26,46,47 Moreover, impaired megakaryopoiesis and thrombopoiesis can alter platelet activation. Therefore, we next examined whether Mnk1 regulates platelet functional responses. Platelets from Mnk1 KO mice stimulated with submaximal thrombin, collagen, and 2MeSADP displayed reduced aggregation, integrin αIIbβ3 activation, and surface P-selectin responses (Figure 4A-D). In activated, human-cultured megakaryocytes where Mnk1 was ablated using CRISPR/Cas9, surface P-selectin was also significantly reduced (supplemental Figure 4A,D). Endogenous surface expression of major platelet receptors CD41a, CD42d, and GPVI were similar between WT and Mnk1 KO mice (supplemental Figure 5A-C).
Mnk1 deficiency reduces platelet activation. (A-B) Washed platelets from WT and Mnk1 KO mice were left unstimulated (US) or were stimulated with thrombin (0.05 and 0.5 U), collagen (2 and 10 μg/mL), and 2MeSADP (2 and 25 nM). The curves are representative of n = 3 independent experiments. The bar graphs on the right show quantitation of platelet aggregation (n = 3 mice per group; ∗P < .05). (C-D) Flow cytometric analyses of JON/A binding (for activated integrin αIIbβ3) or P-selectin surface expression on washed platelets from WT and Mnk1 KO mice following stimulation with thrombin (0.05 and 0.5 U), collagen (2 and 10 μg/mL), and 2MeSADP (2 and 25 nM). Graphs show data from n = 3 mice per group (∗P < .05). NS, not significant.
Mnk1 deficiency reduces platelet activation. (A-B) Washed platelets from WT and Mnk1 KO mice were left unstimulated (US) or were stimulated with thrombin (0.05 and 0.5 U), collagen (2 and 10 μg/mL), and 2MeSADP (2 and 25 nM). The curves are representative of n = 3 independent experiments. The bar graphs on the right show quantitation of platelet aggregation (n = 3 mice per group; ∗P < .05). (C-D) Flow cytometric analyses of JON/A binding (for activated integrin αIIbβ3) or P-selectin surface expression on washed platelets from WT and Mnk1 KO mice following stimulation with thrombin (0.05 and 0.5 U), collagen (2 and 10 μg/mL), and 2MeSADP (2 and 25 nM). Graphs show data from n = 3 mice per group (∗P < .05). NS, not significant.
Mnk1 controls the translation of the PLA2G4A gene
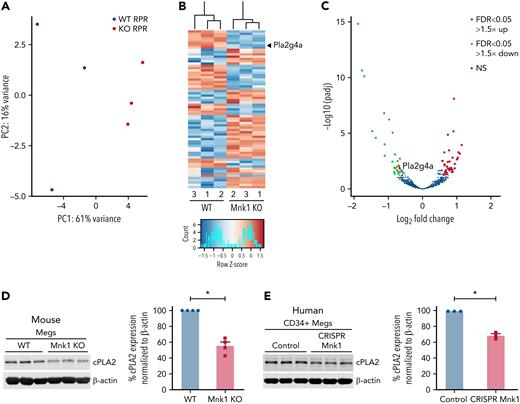
In megakaryocytes and platelets, mRNA is translated and proteins are synthesized in regulated, signal-dependent mechanisms.48,49 Our data suggest that Mnk1 controls activation of eIF4E (Figure 1), which initiates mRNA translation in eukaryotic cells. Therefore, to identify which mRNAs are translationally regulated by Mnk1, we employed ribosome footprint profiling of primary murine bone marrow megakaryocytes adherent to fibrinogen in vitro from WT and Mnk1 KO mice. Ribosome footprint profiling enables the global quantification of RNAs with ≥1 ribosomes attached (RPRs).47,50 RPRs identify RNAs undergoing active translation. We have successfully used this technique in platelets and megakaryocytes.46,47,51 By performing differential expression analyses of RPRs on megakaryocytes from WT or Mnk1 KO mice, we identified significantly upregulated RPRs (ie, more ribosomes attached to RNAs) and downregulated RPRs (ie, fewer ribosomes attached to RNAs) (Figure 5A-C). There were minimal changes in total RNA expression, as assessed by RNA sequencing, between Mnk1 KO and WT mice (supplemental Figure 6).
Mnk1 regulates the translation of mRNAs, including cPLA2, in megakaryocytes. Bone marrow megakaryocytes were isolated from WT (n = 3) or Mnk1 KO (n = 3) mice and cultured for 5 days. Ribosomal footprint profiling was performed to identify RNAs with ≥1 ribosomes attached (RPRs), suggestive of mRNAs being actively translated. (A-B) Principal component analysis and heat map showing mRNAs with differentially abundant RPRs. The gene Pla2g4a is enlarged and highlighted with a black arrowhead. (C) Volcano plot showing significantly (false discovery rate < 0.05) upregulated (log2 fold change > 1.5, red) and downregulated (log2 fold change < 1.5, green) RPRs in megakaryocytes from WT or Mnk1 KO mice. Blue circles represent RPRs that were not significantly changed in megakaryocytes between WT and Mnk1 KO mice. The gene Pla2g4a is enlarged and highlighted with a red arrow. (D) Bone marrow–derived megakaryocytes from WT and Mnk1 KO mice analyzed for cPLA2 protein expression. β-Actin was used as a loading control. Bar graph shows quantification of total cPLA2 protein in Mnk1 KO megakaryocytes compared with WT megakaryocytes as assessed by densitometry (n = 4 per group, ∗P < .05). (E) Immunoblot of cPLA2 protein after Mnk1 CRISPR-Cas9 (CRISPR Mnk1)-based knockdown in day 13 human CD34+-derived cultured megakaryocytes. Guide RNAs not targeting known genes were used as a negative control (Control). Bar graph shows quantification of total cPLA2 protein expression as assessed by densitometry (n = 3 per group, ∗P < .05). NS, not significant.
Mnk1 regulates the translation of mRNAs, including cPLA2, in megakaryocytes. Bone marrow megakaryocytes were isolated from WT (n = 3) or Mnk1 KO (n = 3) mice and cultured for 5 days. Ribosomal footprint profiling was performed to identify RNAs with ≥1 ribosomes attached (RPRs), suggestive of mRNAs being actively translated. (A-B) Principal component analysis and heat map showing mRNAs with differentially abundant RPRs. The gene Pla2g4a is enlarged and highlighted with a black arrowhead. (C) Volcano plot showing significantly (false discovery rate < 0.05) upregulated (log2 fold change > 1.5, red) and downregulated (log2 fold change < 1.5, green) RPRs in megakaryocytes from WT or Mnk1 KO mice. Blue circles represent RPRs that were not significantly changed in megakaryocytes between WT and Mnk1 KO mice. The gene Pla2g4a is enlarged and highlighted with a red arrow. (D) Bone marrow–derived megakaryocytes from WT and Mnk1 KO mice analyzed for cPLA2 protein expression. β-Actin was used as a loading control. Bar graph shows quantification of total cPLA2 protein in Mnk1 KO megakaryocytes compared with WT megakaryocytes as assessed by densitometry (n = 4 per group, ∗P < .05). (E) Immunoblot of cPLA2 protein after Mnk1 CRISPR-Cas9 (CRISPR Mnk1)-based knockdown in day 13 human CD34+-derived cultured megakaryocytes. Guide RNAs not targeting known genes were used as a negative control (Control). Bar graph shows quantification of total cPLA2 protein expression as assessed by densitometry (n = 3 per group, ∗P < .05). NS, not significant.
Reactome and Wiki pathway analyses suggested that Mnk1 controlled the translation of mRNAs encoding proteins involved in platelet activation and eicosanoid lipid synthesis (supplemental Figure 7A-B). Interferon signaling was also significantly altered (supplemental Figure 7A), consistent with published data demonstrating that in other cells, Mnk kinases control translation of interferon-sensitive genes.52 Indeed, the ribosomal occupancy of a number of interferon-sensitive genes was significantly downregulated in platelets from Mnk1 KO mice, including interferon-inducible transmembrane protein 3 (IFITM3, supplemental Figure 8). We recently described the regulated expression and function of IFITM3 in platelets and megakaryocytes during viral infections,53 and these data suggest that IFITM3 may be partly under Mnk1-dependent translational control in platelets.
In a candidate-gene approach, we noted that RPRs for PLA2G4A (the gene encoding cytosolic phospholipase A1 or cPLA2) were downregulated in megakaryocytes from Mnk1 KO mice (Figure 5B; supplemental Figure 9). cPLA2 is a calcium-dependent enzyme phosphorylated upon platelet activation that releases arachidonic acid, a precursor to the synthesis of eicosanoids, from membrane phospholipids.54 In other cells, Mnk1 activates cPLA214.55 Therefore, we chose to establish whether or not Mnk1 regulates cPLA2 expression.
In an independent set of experiments, we probed isolated bone marrow megakaryocytes from Mnk1 KO mice and WT controls for cPLA2 protein levels. Expression of cPLA2 protein was significantly reduced in megakaryocytes lacking Mnk1 (Figure 5D). Human platelets are not readily amenable to genetic manipulation. To circumvent this limitation, we have used CRISPR/Cas9 to edit genes in human megakaryocytes.34,53,56 Megakaryocytes possess many of the functional responses of platelets, including translational changes47,53 and signal-dependent activation,34,56 and can be used as model cells to study genes regulating platelet responses. Consistent with our findings in murine systems, ablating Mnk1 in human megakaryocytes significantly reduced cPLA2 protein levels (Figure 5E).
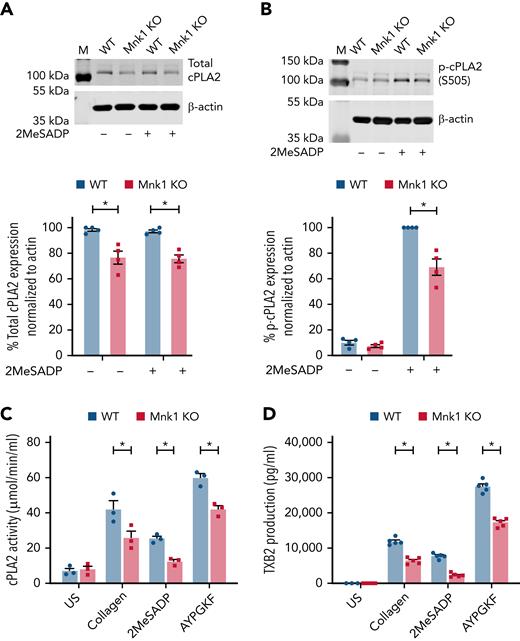
Mnk1 regulates cPLA2 expression and thromboxane production in platelets
Platelet aggregation relies on the generation of secondary mediators such as thromboxane A2 (TxA2), and cPLA2 regulates TxA2 production. In other cells, Mnk1 is known to phosphorylate cPLA2, and our data indicate that Mnk1 regulates PLA2G4A mRNA translation and cPLA2 protein levels in megakaryocytes (Figure 5). Therefore, we next evaluated cPLA2 mRNA translation and protein expression in platelets from Mnk1 KO mice. Consistent with our data in human and murine megakaryocytes, total cPLA2 protein expression was modestly, but significantly, reduced in platelets from Mnk1 KO mice compared with WT littermate controls (Figure 6A). Activation of platelets results in phosphorylation of cPLA2 on Ser505 and Ser727.57 Mnk1 phosphorylates cPLA2 at the Ser727 residue that subsequently regulates arachidonic acid release in CHO cells.58 Therefore, we next tested whether Mnk1 controls cPLA2 activation and TxA2 generation by platelets. As shown in Figure 6B-C; supplemental Figure 10, both cPLA2 phosphorylation and activity were significantly reduced in activated platelets from Mnk1 KO mice. We next evaluated whether Mnk1 controlled the accumulation of TxB2, a stable metabolite of TxA2. In activated platelets from Mnk1 KO mice, TxB2 production was significantly reduced (Figure 6D). Signal transduction pathways downstream of thrombin/PAR4 and 2MesADP/P2Y12 and collagen/GPVI were similar between WT and Mnk1 KO mice (supplemental Figure 11A-D). Stimulating platelets with arachidonic acid, which is downstream of cPLA2, rescued the defects in aggregation in Mnk1 KO mice (Figure 4A-B; supplemental Figure 12A-B).
Mnk1 regulates cPLA2 activity and thromboxane production by platelets. (A-B) Total (A) and phosphorylated (B) cPLA2 protein expression was assessed by immunoblot in unstimulated or 2MeSADP-stimulated (10 nM, 5 minutes) platelets from WT and Mnk1 KO mice. β-Actin was used as a loading control. Immunoblots are representative of n = 3 independent experiments. (C-D) Washed platelets from WT and Mnk1 KO mice were left unstimulated (US) or stimulated with collagen (2 μg/ml), 2MeSADP (2 nM), or AYPGKF (150 μM) 10 minutes. Then, platelet cPLA2 activity (C) and TXB2 production (D) were assessed (n = 3-5 mice per group, ∗P < .05). M, marker.
Mnk1 regulates cPLA2 activity and thromboxane production by platelets. (A-B) Total (A) and phosphorylated (B) cPLA2 protein expression was assessed by immunoblot in unstimulated or 2MeSADP-stimulated (10 nM, 5 minutes) platelets from WT and Mnk1 KO mice. β-Actin was used as a loading control. Immunoblots are representative of n = 3 independent experiments. (C-D) Washed platelets from WT and Mnk1 KO mice were left unstimulated (US) or stimulated with collagen (2 μg/ml), 2MeSADP (2 nM), or AYPGKF (150 μM) 10 minutes. Then, platelet cPLA2 activity (C) and TXB2 production (D) were assessed (n = 3-5 mice per group, ∗P < .05). M, marker.
Mnk1 regulates platelet-dependent thrombosis
Given our findings that Mnk1 regulates platelet activation responses in vitro, we next evaluated the effects of Mnk1 on thrombosis in vivo. In a model of thrombosis due to venous stasis, we found that Mnk1 KO mice develop significantly fewer thrombi and that formed thrombi are significantly smaller (Figure 7A-D). We also examined the effects of Mnk1 deficiency in a collagen-/epinephrine-induced pulmonary thromboembolism model; a thrombosis model in which platelet activation is the most prominent feature.27 Consistent with our ex vivo data, Mnk1 KO mice were significantly protected from death due to pulmonary thromboembolism (Figure 7E). Interestingly, in a cerebral ischemia-reperfusion model, where cerebral injury is due to filament-induced ischemia and subsequent reperfusion, Mnk1 deficiency did not reduce infarct size or outcomes (supplemental Figure 13A-D).
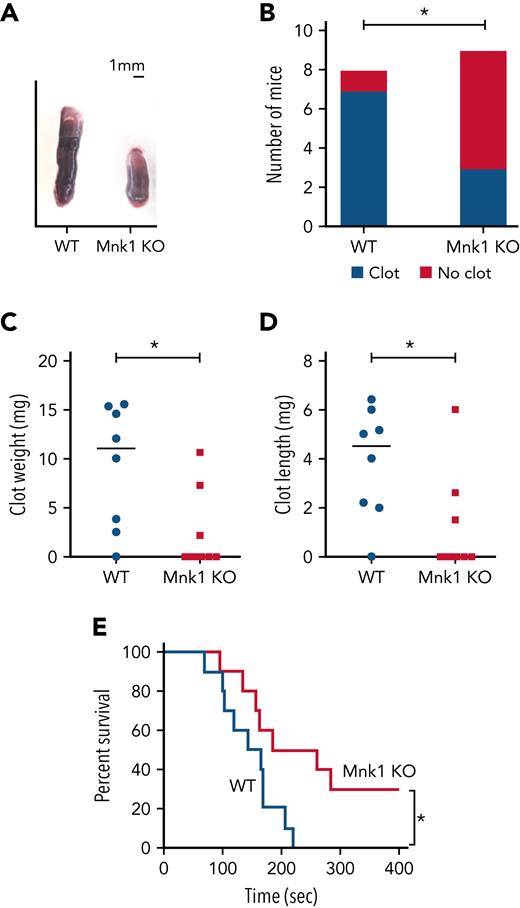
Mnk1 regulates thrombus formation in vitro and in vivo. (A-D) WT (n = 8) or Mnk1 KO (n = 9) mice were subject to a venous stasis model of thrombosis. (A) Representative clots from WT or Mnk1 KO mice. (B) Number of mice in each group with thrombosis (∗P < .05). (C-D) Clots were removed, measured, and weighed (∗P < .05). (E) Survival proportions in WT and Mnk1 KO mice after induction of pulmonary thromboembolism by injecting a collagen/epinephrine mixture through retro-orbital injection (n = 10 mice per group, ∗P < .05).
Mnk1 regulates thrombus formation in vitro and in vivo. (A-D) WT (n = 8) or Mnk1 KO (n = 9) mice were subject to a venous stasis model of thrombosis. (A) Representative clots from WT or Mnk1 KO mice. (B) Number of mice in each group with thrombosis (∗P < .05). (C-D) Clots were removed, measured, and weighed (∗P < .05). (E) Survival proportions in WT and Mnk1 KO mice after induction of pulmonary thromboembolism by injecting a collagen/epinephrine mixture through retro-orbital injection (n = 10 mice per group, ∗P < .05).
Discussion
Regulation of mRNA translation mediates key aspects of megakaryocyte and platelet biology,22,59 although the regulatory steps involved remain largely unknown. Although recent studies have identified Mnk as regulating the translation of a subset of mRNAs in other cells,60-62 the expression and function of Mnk in megakaryocytes and platelets have not previously been reported. In this study, we demonstrate that Mnk1 (but not Mnk2) is selectively expressed and functionally active in human and murine megakaryocytes and platelets to regulate platelet production, platelet activation, and thrombosis. Mnk kinases are active in many cells, especially in cancer and tumor cell lines,63-69 and Mnk1 influences myeloid cell functions.70,71 Prior elegant work dissecting phosphorylation pathways of cPLA2 also found that Mnk1 was present and functionally active in human platelets,58 consistent with our current study. These authors also demonstrated that Mnk1 phosphorylates cPLA2 at Ser727. Our findings suggest that in platelets, Mnk1 also phosphorylates cPLA2 at Ser505.
We also observed that stimulating megakaryocytes and platelets with a variety of agonists (eg, collagen, 2MeSADP, AYPGKF, TPO) activated Mnk1 and triggered the phosphorylation of its dedicated substrate eIF4E. Ablating Mnk1 in platelets and megakaryocytes completely blocked signal-dependent phosphorylation of eIF4E. In other cells, eIF4E is phosphorylated at a single site, Ser209,72 upon stimulation,68,73 and Mnk1 is the only serine/threonine kinase that phosphorylates eIF4E in mice.25 Interestingly, in some settings, eIF4E may act as a protooncogene.74,75
Our studies build upon and extend these observations by showing that eIF4E phosphorylation in activated megakaryocytes and platelets is also controlled by Mnk1. Interestingly, prior studies in murine embryonic fibroblasts suggest that deletion of Mnk1 does not impair cap-dependent mRNA translation or general protein synthesis.25 In contrast, we observed that de novo protein synthesis was significantly reduced in human and murine megakaryocytes where Mnk1 was either pharmacologically inhibited or genetically ablated. This suggests that Mnk1-dependent translational control may differ in megakaryocytes relative to other cells.
Translation of mRNAs is regulated by multiple checkpoints that allow cells to control protein synthesis. Eukaryotic initiation factors regulate the association of ribosomes with mRNAs and subsequent scanning to find the start codon. eIF4E forms a translation initiation complex with eIF4G that also includes Mnk1 and, thus, regulates the initial steps of mRNA translation.24,76,77 Functionally inactive eIF4E or genetic deletion of eIF4E stalls mRNA translation in other nucleated cells.77-80 Regulated mRNA translation orchestrates platelet production and function.22,81,82 However, the checkpoints that regulate mRNA translation remain poorly understood. Using polysome profiling and [35S]-methionine incorporation assays, we found that Mnk1 promotes the translation of a subset of RNAs. One of these identified by ribosomal footprint profiling was activating transcription factor 4 (supplemental Figures 8B and 14), a widely expressed transcription factor previously linked to Mnk1 signaling in cancer cells.83 Interestingly, interferon-sensitive genes, including IFITM3, also appeared to be under Mnk1-dependent translational control in platelets (supplemental Figure 8A). Further investigation of Mnk1 as a translational checkpoint in megakaryocytes and platelets is warranted.
To decipher the role of Mnk1 in megakaryocyte and platelet development, we employed complementary human and murine studies. We leveraged the available PRAX dataset,42,84 which includes transcriptomic, demographic, and platelet phenotyping data on 154 healthy male and female donors from various backgrounds to examine correlations between MKNK1 mRNA expression in platelets and circulating platelet counts and the MPV (an index of platelet size). We observed a modest, but significant, positive association between Mnk1 mRNA expression and platelet counts and a modest, but significant, negative association between Mnk1 mRNA expression and MPV in healthy human donors. Interestingly, the effect size of the association between Mnk1 mRNA expression and platelet counts or MPV in humans appeared to be greater than the effect size observed in Mnk1 KO mice. Although our data support Mnk1 as influencing platelet count and size in humans and mice, we speculate that there may be other factors that influence Mnk1-associated changes in platelet number and size. To mechanistically establish whether Mnk1 regulates platelet production, we employed a mouse model where Mnk1 was globally ablated. Consistent with our associative data in humans, the ablation of Mnk1 in mice significantly reduced circulating platelet counts and increased the platelet MPV. In vitro, megakaryopoiesis and proplatelet production was also impaired in mice lacking platelet Mnk1. This was accompanied by impaired platelet production in vivo without any differences in platelet clearance.
Pathways and proteins regulating platelet production may also control platelet functional responses. In mice lacking Mnk1, we observed reductions in agonist-induced platelet aggregation, integrin αIIbβ3 activation, and α granule release. These changes were similar across various agonists, suggesting a common upstream mediator. Although eIF4E is involved in the process of mRNA translation, there remains uncertainty as to which specific mRNAs are translated when eIF4E is phosphorylated and Mnk1 is activated. mRibosomal footprint profiling enabled us to identify some mRNAs that were under Mnk1-dependent translational control, including mRNAs known to regulate platelet activation. We noted that the eicosanoid lipid synthesis pathway in platelets was altered in the absence of Mnk1. One protein crucial for this pathway is cPLA2, which is also necessary for the generation of TxA2.85-88 TxA2 is produced when arachidonic acid is released from platelet membrane phospholipids via the activation of cPLA2.55,89,90 cPLA2 has a MAPK consensus phosphorylation motif at Ser-505, which is phosphorylated by the MAPK family. ERK and p38 MAPK regulate Ser-505 phosphorylation of cPLA2 in platelets stimulated with thrombin or collagen.57,91 We identified that the expression of cPLA2 protein was significantly reduced in platelets and megakaryocytes lacking Mnk1, and this was accompanied by reduced cPLA2 phosphorylation and activity. Concordantly, in the absence of Mnk1, TxB2 production by platelets was also significantly reduced. To evaluate the functional consequences of Mnk1 deletion, we employed complementary ex vivo and in vivo thrombosis models. Consistent with decreased platelet activation responses, we found that in the absence of platelet Mnk1, venous thrombosis formation and mortality from pulmonary thromboembolism were reduced. We did not observe any protection from Mnk1 deficiency in a stroke model of cerebral injury due to transient ischemia and subsequent reperfusion, suggesting that Mnk1-regulated platelet activation differs in this vascular bed and/or under these experimental conditions.
Although the pharmacologic Mnk1 inhibitor CGP 57380 may have off-target effects in some cells, our observations using CGP 57380 were similar to those made in Mnk1 KO mice and in human megakaryocytes where Mnk1 was selectively ablated by CRISRP/Cas9. Moreover, CGP57380 has not demonstrated any evidence of cellular toxicity, even at high concentrations. Therefore, we do not believe our findings can be attributed to nonspecific pharmacologic effects. Other strengths of our study include the use of human and murine platelets and megakaryocytes and leveraging larger datasets (eg, PRAX1) for associative clinical analyses.
Limitations of our study include use of a mouse model where Mnk1 was globally ablated rather than specifically ablated in platelets and megakaryocytes. As such, we cannot completely exclude the possibility that the absence of Mnk1 in other cells mediates platelet count and size. However, our studies in isolated platelets and megakaryocytes support the role of Mnk1 on platelet production, activation, and thrombosis. We also recognize that although our data indicate that Mnk1 controls platelet count by regulating de novo protein synthesis and endomitosis in megakaryocytes, we have not yet identified the specific molecule(s) that Mnk1 regulates. Finally, although we did not observe differences in signaling pathways downstream of PAR4, P2Y12, or GPVI, we cannot completely exclude the possibility that other signal transduction pathways are altered by Mnk1 deficiency.
In conclusion, we provide new evidence that Mnk1 in platelets and megakaryocytes regulates mRNA translation, cellular development, and function. These findings have implications for clinical arenas where Mnk1 inhibitors are being developed. Further, this study deepens our understanding of the role of translation control pathways in platelets and megakaryocytes in platelet production and thrombosis.
Acknowledgments
The authors thank Diana Lim for her exceptional creativity and experience in figure preparation.
This work was supported by grants from the National Institutes of Health National Institute of Aging (K01AG059892 to R.A.C. and AG048022 to M.T.R.) and the National Heart, Lung, and Blood Institute (HL163019 to R.A.C., HL142804 and HL130541 to M.T.R., and HL145237 to A.S.W.), and an American Heart Association postdoctoral fellowship award (18POST34030020 to B.K.M.). This work was also supported by Merit Review Award Number I01 CX001696 to M.T.R. from the US Department of Veterans Affairs Clinical Sciences R&D (CSRD). This material is the result of work supported with resources and the use of facilities at the George E. Wahlen VA Medical Center, Salt Lake City, Utah. The contents do not represent the views of the US Department of Veterans Affairs or the United States Government.
Authorship
Contribution: B.K.M., R.A.C., A.A., S.B., R.A.C., E.M., P.F.B., F.D., I.P., E.A.M., J.W.R., N.D.T., Y.K., L.G., S.J., A.S.W., and M.T.R. designed and performed experiments; B.K.M., S.B., R.A.C., F.D., and M.T.R. analyzed the results; B.K.M., R.A.C., and M.T.R. wrote the manuscript; and all authors reviewed and critically edited the manuscript.
Conflict-of-interest disclosure: M.T.R. and J.W.R. disclose that they are coinventors on a patent using platelet transcriptomics. The remaining authors declare no competing financial interests.
Correspondence: Matthew T. Rondina, University of Utah, 15 N 2030 E, EIHG 4220, Salt Lake City, UT 84112; e-mail: matthew.rondina@hsc.utah.edu.
References
Author notes
∗B.K.M. and R.A.C. contributed equally to this study.
Data will be shared upon reasonable request. Sequencing data are publicly available (Bioproject PRJNA 854291).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


![Mnk1 regulates mRNA translation and de novo protein synthesis in human and murine megakaryocytes. (A) Human, cord blood–derived, CD34+ megakaryocytes were left alone with vehicle control (DMSO, blue line) or treated with CGP 57380 (10 μM, red line) on culture day 13 and then allowed to adhere on fibrinogen-coated plates for 2 hours. Megakaryocytes were then lysed and sedimented by centrifugation on a 5% to 50% sucrose gradient. Isolated monosome and polysome fractions are indicated. Graphs are representative of n = 3 independent experiments. (B) Human, cord blood–derived, CD34+ megakaryocytes were cultured in the presence of CGP 57380 (10 μM) or vehicle control (DMSO) on culture day 13. Megakaryocytes were then resuspended in [35S]-methionine media and allowed to adhere on fibrinogen-coated plates for 2 hours. Protein synthesis was quantified using a scintillation counter (∗P < .05; n = 5 independent experiments). (C) Bone marrow–derived megakaryocytes from either WT or Mnk1 KO mice were resuspended in [35S]-methionine media and allowed to adhere on fibrinogen-coated plates for 2 hours. Protein synthesis was quantified using a scintillation counter (∗P < .05; n = 6 independent experiments).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/140/23/10.1182_blood.2022015568/4/m_blood_bld-2022-015568-gr2.jpeg?Expires=1764965946&Signature=jY5oR6XAfHaJkTLgfrxWrDaLESL4M9gEGX0~0gYdMO3tGEY7c59YOK3fmbxM~aSTr32FGs5xMt6s9H8F2c-bemPMTuTJJSbeGExSBzDU-jz6ejtV9OJN3~amQ4mVQmruNHgtBoPMPoW1ftIw1jP-A6h1VDFFx1zCgUuz5xKjZsf~2hhgzvpO0aiRNVmWG7VbQEhe4lcuXgDZNPPxPJozuCTg9O6~AEPB5Xjt7E0hn3o~wLWKweIpIXI231xzw-RtIJXRXFKAgH1d~4jBGTs1IeGrsFUn1NaDPWzcEqew-NvjibJtsbxkSgDtjQyJ6RJlhB0Pwen83YvufUdrJGsTjg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal