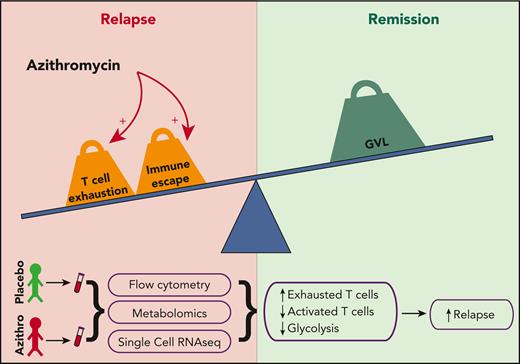
In medicine, actions taken in good faith may lead to manifold unintended consequences, including off-target effects and unexpected toxicities of medications typically considered safe. In this issue of Blood, Vallet et al demonstrate that allogeneic hematopoietic stem cell transplant (HSCT) patients receiving azithromycin have alterations in T cell metabolism and increased T cell exhaustion (see figure), potentially underlying the loss of graft-versus-leukemia (GVL) activity and increased risk of disease relapse observed in the ALLOZITHRO trial.1,2
Azithromycin treatment leads to more T cell exhaustion, which may impair GVL and promote relapse.
Azithromycin treatment leads to more T cell exhaustion, which may impair GVL and promote relapse.
Azithromycin is the second most prescribed antibiotic in the United States.3 In addition to its antibacterial properties, the antiinflammatory functions of azithromycin are commonly used to prevent exacerbations of chronic obstructive pulmonary disease and are associated with improved survival after lung transplantation.4 Chronic graft-versus-host disease (GVHD) of the lung is a debilitating and often fatal complication for HSCT patients. In single-arm studies, combination therapy including azithromycin had shown promise in ameliorating progression of bronchiolitis obliterans syndrome (BOS), a manifestation of chronic lung GVHD.5,6 Limiting inflammation to prevent GVHD after HSCT seems desirable prima facie, particularly with the use of such a common and “safe” medication as azithromycin. However, previous in vitro work implicated azithromycin in inhibiting proliferation and proinflammatory cytokine production of CD4+ T cells.7 The ALLOZITHRO trial evaluating azithromycin for prevention of BOS disappointingly did not demonstrate a reduction in lung GVHD but alarmingly did identify a higher rate of leukemia relapse among patients receiving azithromycin.2 These data are a sobering reminder that seemingly benign interventions may have unwanted and even fatal consequences.
Using patient samples obtained during the ALLOZITHRO trial, Vallet et al sought to understand mechanisms through which azithromycin might promote disease recurrence. The authors performed complementary flow cytometric, mass cytometric, and single-cell transcriptional analyses on peripheral blood, as well as metabolomic analysis, to construct an integrated assessment of cellular composition and pathways affected by azithromycin administration. They found that azithromycin treatment led to an actual reduction in T cell number. Moreover, these T cells were skewed toward increased T regulatory, central and effector memory TH2 CD4+, and exhausted CD8+ central memory (PD-1, TIGIT, and TOX expressing) cells with a concomitant decrease in cytotoxic (Granzyme B expressing) CD8+ central memory cells. Analysis of plasma and intracellular metabolic pathways demonstrated lower glycolytic activity, which is central to T cell activation and effector functions hypothesized to be the basis for GVL. This finding was confirmed in vitro, with inhibition of glycolysis observed in T cells cultured with azithromycin. Additional in vitro work demonstrated that azithromycin treatment directly led to inhibition of T cell proliferation, which was exacerbated by the addition of cyclosporine, suggesting an additive effect of azithromycin with GVHD prophylaxis. These data advance our understanding of possible mechanisms of relapse after HSCT through T cell exhaustion and ineffective cytotoxic function that may be exacerbated by exposure to azithromycin. Future studies should leverage these and other advanced technologies, such as spatial and interactional analysis at single-cell resolution to gain a deeper understanding of intra- and intercellular crosstalk driving both relapse and GVL. Although relapse is likely multifactorial, exhausted or immunoregulatory T cells are likely candidates as drivers. Preclinical studies of graft selection/manipulation to reduce or reverse exhaustion and promote antitumor T cell activity will further elucidate the precise cell subsets and pathways responsible for loss of GVL and identify novel therapeutic targets.
Relapse remains the major cause of failure after allogeneic transplantation for malignant disease.8 Although GVL mediated by donor T cells has been broadly accepted as a primary driver of antitumor activity for decades, the underlying mechanisms remain surprisingly poorly understood.9 GVL and GVHD are often thought of as 2 sides of the same coin: Proinflammatory T cell activity promotes GVL and prevents relapse, but overactivity of donor T cells causes deleterious inflammation leading to GVHD. Various attempts at manipulating HSCT grafts to promote GVL and reduce GVHD have been made but are not yet specific enough to separate these processes. For example, HSCT with in vivo or ex vivo T cell depletion (TCD) has been used successfully for improved GVHD prevention, but some studies of TCD have shown higher relapse rates, particularly for chronic myelogenous leukemia.10 These observations highlight the need for more sophisticated understanding of the precise molecular mechanisms and T cell functional subsets responsible for mediating GVL and GVHD. Integrating next-generation technologies for transcriptional, metabolomic, and pathway analysis improves characterization of the diverse immune subsets at a single-cell level beyond traditional immunophenotyping.
Fine-tuning the balance of pro- and antiinflammatory factors to promote GVL while limiting GVHD is the “holy grail” of HSCT. Altogether, Vallet et al provide compelling evidence for a role of azithromycin in modulating T cell activity after HSCT to promote relapse and underscore how much remains undiscovered in understanding the complex interplay of therapies, cells, and metabolic pathways driving relapse and GVL.
Conflict-of-interest disclosure: R.J.S. is a consultant for Vor Biopharma, Neovii, CSL Behring, Bluesphere Bio, Cugene, and Jasper; on the Data Safety Monitoring Board of Juno Therapeutics/BMS/Celgene USA; and on the Board of Directors of NMPD Be the Match, USA. K.M. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal