Abstract
Multiple myeloma embodies the paradigm of the deeper the response, the longer the survival. However, results are conflicting regarding achievement of complete remission (CR) and minimal residual disease (MRD) negativity; some patients with persistent M protein have undetectable MRD. We reviewed the frequency of this discordance and outcomes of these patients. We spotlight possible explanations for and consequences of conflicting response criteria and suggest that MRD be assessed in patients achieving very good partial response or better in clinical trials.
Introduction
Assessing response to treatment in multiple myeloma (MM) has 3 main objectives: (1) to identify patients with primary resistance to treatment and greater risk of disease progression, (2) to refine patient prognostication according to the depth of response, and (3) to compare the efficacy of different therapies under investigation in clinical trials. We believe that serologic assessment of tumor burden remains the most effective approach to identify primary resistance. However, in this article, we raise the question of whether embedding the definition of complete remission (CR) within that of minimal residual disease (MRD) negativity is inhibiting better patient prognostication and treatment end points in clinical trials.
Strengths and limitations of CR
In the 2016 consensus criteria of the International Myeloma Working Group for response and MRD assessment, CR was defined by negative immunofixation on serum and urine, disappearance of any soft tissue plasmacytomas, and <5% plasma cells (PCs) in bone marrow (BM) aspirates.1 This definition is nearly identical to that proposed by Blade et al2 in 1998. In the last 25 years, the prognostic value of CR has been shown in meta-analyses of clinical trial data3-5 and confirmed in single-center studies of routine practice.6,7 In parallel, potential caveats to the definition of CR have been identified, namely, the utility of urine immunofixation in patients with or without light-chain MM8,9 and of morphologic assessment of PC infiltrates in BM.10,11 Stringent CR is composed of CR as defined plus normal free light chain ratio and absence of clonal PCs in BM biopsy by immunohistochemistry. Similarly to conventional microscopy, the sensitivity and clinical utility of immunohistochemistry and free light chain assays in patients achieving CR with optimal treatment hhave been questioned,10,12 and the superiority of stringent over conventional CR remains to be confirmed in large prospective studies.
Strengths and limitations of MRD assessment
In the International Myeloma Working Group 2016 consensus criteria, there are 4 definitions of MRD negativity (ie, flow, sequencing, imaging, and sustained), and all of them require confirmation of CR.1 The superior sensitivity and clinical utility of MRD negativity over CR has been shown in clinical trials and routine practice.4,13-16 By contrast, the level of standardization by which CR is assessed and reported has not yet been achieved in MRD testing.17 Another caveat to MRD assessment is the discordance across different methods in use.18 For example, there is a risk of false-negative results using immunophenotypic and molecular methods that analyze a single BM aspirate each time, with limited periodicity because of the invasiveness of the procedure. Accordingly, the prognostic value of MRD has increased in recent years with the combined use of cellular and imaging methods to mitigate false-negative results because of patchy or extramedullary disease,19,20 as well as with the input of sustained MRD negativity over the result from a single time point to identify patients with lower risk of progression.16,21,22 The time points for MRD assessment should be adapted to the design of each clinical trial in order to measure treatment efficacy after certain stages of treatment (eg, induction and intensification) and periodically (eg, every 12 months) during therapy administered for long periods or until disease progression.
The role of MRD negativity in long-term survival outcomes was unequivocally shown in a recent large meta-analysis that included >8,000 patients with MM.23 Overall, the achievement of MRD negativity reduced the risk of progression and/or death in 67%, and this was observed in newly diagnosed transplantation-eligible and -ineligible as well as relapsed/refractory settings, in patients with standard and those with high cytogenetic risk, regardless of the depth of conventional response.23 Indeed, the hazard ratio for progression-free survival (PFS) upon MRD negativity was 0.38 (95% CI, 0.29-0.50; P < .001) in patients achieving CR or better and 0.31 (95% CI, 0.23-0.43; P < .001) in those attaining very good partial response (VGPR) or better.23
Discordance between CR and MRD results
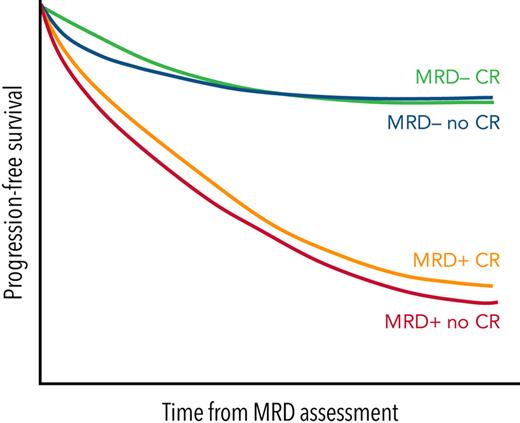
Detecting persistent MRD in patients achieving CR reflects the higher sensitivity of the former and allows the identification of cases with significantly inferior outcomes.4,10,14,24,25 In fact, it has been reported that the PFS of patients in CR but with persistent MRD is similar to that of patients achieving VGPR.4,14 However, the opposite and unexpected discordance has also been described: persistent M protein in patients with undetectable MRD.
In an attempt to minimize missing MRD data, its assessment in many clinical trials has been prespecified at well-defined time points (eg, before or after autologous stem cell transplantation [ASCT]) rather than at achievement of CR. MRD is therefore evaluated in patients with depth of response other than CR, such as VGPR, and a number of studies have reported MRD-negative rates in these cases (Table 1). In protocols using low-sensitivity flow cytometry, ∼25% of patients with undetectable MRD after ASCT showed positive immunofixation.26,27 In the PETHEMA/GEM2005MAS65 clinical trial for transplantation-ineligible patients with MM, 22.5% of patients with undetectable MRD after 6 induction courses had persistent M protein.28 Using polymerase chain reaction, the percentages of patients with undetectable MRD after ASCT showing positive immunofixation were 14% and 27% in 2 Spanish studies, respectively.29,30 Silvennoinen et al31 used both flow cytometry and allele-specific oligonucleotide real-time quantitative polymerase chain reaction and reported 38% of patients with undetectable MRD and positive immunofixation, regardless of the method. Interestingly, discordances remain despite the transition from older, lower-sensitivity methods to next-generation flow (NGF) and sequencing (NGS) technologies. Using NGF, 25% of patients with undetectable MRD after ASCT in the PETHEMA/GEM2012MENOS65 clinical trial had persistent M protein.24 Using NGS, the rates of discordance were 13%, 31%, 38%, and 8% in a pooled analysis of 4 Spanish protocols,32 in the IFM-2009 clinical trial,33 according to the experience of the German-speaking Myeloma Multicenter Group,34 and in a series of Japanese patients.35 In 2 studies conducted in the United States, the percentages of patients with positive immunofixation despite negative NGS were 4% and 9%, respectively.18,36 Therefore, this unexpected discordance is not driven, at least predominantly, by false-negative MRD testing as a result of less sensitive methods. Another possible explanation could result from patchy BM infiltration and/or extramedullary disease, but Hillengass et al37 reported a striking 86% of patients with persistent M protein despite CR on magnetic resonance imaging. Using positron emission tomography/computed tomography, Derman et al18 reported 29% discordance. In a subset of patients in less than CR, these results are inconsistent with a generalized explanation of false-negative MRD resulting from nonrepresentative BM aspirates. Indeed, it could indicate that there may be various possible explanations for positive M protein despite undetectable MRD, instead of a single methodologic aspect or treatment effect.
List of studies reporting rates of MRD negativity in patients with persistent M protein
| Study . | Method . | Sensitivity . | Patients with undetectable MRD . | Outcome . | Reference . | |
|---|---|---|---|---|---|---|
| CR (%) . | Less than CR (%) . | |||||
| GEM2000 | Flow cytometry | 10−4 | 94/125 (75) | 31/125 (25) | Median PFS, 71 vs 65 mo | 26 |
| GEM2005MAS65 | Flow cytometry | 10−4 | 24/31 (77) | 7/31 (23) | Median PFS not reached in either∗ | 28 |
| PETHEMA/GEM† | Flow cytometry | 10−4 to 10−5 | 177/259 (68) | 82/259 (32) | Median PFS, 63 vs 62 mo | 4 |
| MRC Myeloma IX | Flow cytometry | 10−4 | 183/246 (74) | 63/246 (26) | Not reported‡ | 27 |
| NCT00861250 | Flow cytometry | 6 × 10−5 | 56/91 (61.5) | 35/91 (38.5) | Not reported | 31 |
| NCT01402284 | Flow cytometry | 10−5 | 29/34 (85) | 5/34 (25) | Not reported | 36 |
| NCT01816971 | Flow cytometry | 10−4 to 10−5 | 34/45 (75.5) | 11/45 (24.5) | Not reported | 18 |
| GEM2012MENOS65 | NGF | 3 × 10−6 | 182/205 (89) | 23/205 (11) | 4-y rate, 87% vs 78.5%; P = .35 | 40 |
| GMMG-HD6 | NGF | 6 × 10−6 | 37/54 (68.5) | 17/54 (31.5) | Not reported | 34 |
| Tschautscher et al | NGF | 10−5 to 2 × 10−6 | 116/204 (57) | 88/204 (43) | Median PFS not reached in either§ | 39 |
| GEM2000 | F-PCR | Not reported | 19/26 (73) | 7/26 (27) | Not reported | 29 |
| GEM2000 | ASO qRT-PCR | 10−5 | 6/7 (86) | 1/7 (14) | Not reported | 30 |
| NCT00861250 | ASO qRT-PCR | 4 × 10−6 | 37/60 (62) | 23/60 (38) | Not reported | 31 |
| PETHEMA/GEM‖ | NGS | 10−5 | 26/30 (87) | 4/30 (13) | Not reported | 32 |
| NCT01402284 | NGS | Not reported | 22/23 (96) | 1/23 (4) | Not reported | 36 |
| IFM-2009 | NGS | 10−6 | 54/90 (60) | 36/90 (40) | Not reported | 33 |
| GMMG-HD6 | NGS | 2 × 10−6 | 37/60 (62) | 23/60 (38) | Not reported | 34 |
| Takamatsu et al | NGS | 10−6 | 24/26 (92) | 2/26 (8) | Not reported | 35 |
| NCT01816971 | NGS | 10−4 to 10−6 | 35/38 (92) | 3/38 (8) | Not reported | 18 |
| Hillengass et al | MRI | Not reported | 3/23 (13) | 20/23 (87) | Not reported | 37 |
| NCT01816971 | PET/CT | Not reported | 20/28 (71) | 8/28 (29) | Not reported | 18 |
| Study . | Method . | Sensitivity . | Patients with undetectable MRD . | Outcome . | Reference . | |
|---|---|---|---|---|---|---|
| CR (%) . | Less than CR (%) . | |||||
| GEM2000 | Flow cytometry | 10−4 | 94/125 (75) | 31/125 (25) | Median PFS, 71 vs 65 mo | 26 |
| GEM2005MAS65 | Flow cytometry | 10−4 | 24/31 (77) | 7/31 (23) | Median PFS not reached in either∗ | 28 |
| PETHEMA/GEM† | Flow cytometry | 10−4 to 10−5 | 177/259 (68) | 82/259 (32) | Median PFS, 63 vs 62 mo | 4 |
| MRC Myeloma IX | Flow cytometry | 10−4 | 183/246 (74) | 63/246 (26) | Not reported‡ | 27 |
| NCT00861250 | Flow cytometry | 6 × 10−5 | 56/91 (61.5) | 35/91 (38.5) | Not reported | 31 |
| NCT01402284 | Flow cytometry | 10−5 | 29/34 (85) | 5/34 (25) | Not reported | 36 |
| NCT01816971 | Flow cytometry | 10−4 to 10−5 | 34/45 (75.5) | 11/45 (24.5) | Not reported | 18 |
| GEM2012MENOS65 | NGF | 3 × 10−6 | 182/205 (89) | 23/205 (11) | 4-y rate, 87% vs 78.5%; P = .35 | 40 |
| GMMG-HD6 | NGF | 6 × 10−6 | 37/54 (68.5) | 17/54 (31.5) | Not reported | 34 |
| Tschautscher et al | NGF | 10−5 to 2 × 10−6 | 116/204 (57) | 88/204 (43) | Median PFS not reached in either§ | 39 |
| GEM2000 | F-PCR | Not reported | 19/26 (73) | 7/26 (27) | Not reported | 29 |
| GEM2000 | ASO qRT-PCR | 10−5 | 6/7 (86) | 1/7 (14) | Not reported | 30 |
| NCT00861250 | ASO qRT-PCR | 4 × 10−6 | 37/60 (62) | 23/60 (38) | Not reported | 31 |
| PETHEMA/GEM‖ | NGS | 10−5 | 26/30 (87) | 4/30 (13) | Not reported | 32 |
| NCT01402284 | NGS | Not reported | 22/23 (96) | 1/23 (4) | Not reported | 36 |
| IFM-2009 | NGS | 10−6 | 54/90 (60) | 36/90 (40) | Not reported | 33 |
| GMMG-HD6 | NGS | 2 × 10−6 | 37/60 (62) | 23/60 (38) | Not reported | 34 |
| Takamatsu et al | NGS | 10−6 | 24/26 (92) | 2/26 (8) | Not reported | 35 |
| NCT01816971 | NGS | 10−4 to 10−6 | 35/38 (92) | 3/38 (8) | Not reported | 18 |
| Hillengass et al | MRI | Not reported | 3/23 (13) | 20/23 (87) | Not reported | 37 |
| NCT01816971 | PET/CT | Not reported | 20/28 (71) | 8/28 (29) | Not reported | 18 |
ASO, allele-specific oligonucleotide; CT, computed tomography; F-PCR, fluorescence-based PCR; MRI, magnetic resonance imaging; PET, positron emission tomography; qRT-PCR, quantitative real-time PCR.
The outcome comparison reported was between MRD-negative patients in stringent CR vs no stringent CR.
Pooled analysis of the GEM2000, GEM2005MENOS65, and GEM2010MAS65 clinical trials.
Although not reported, it seems that patients with undetectable MRD and less than CR had inferior outcomes when compared with those in CR.
At 1 y, risk of progressive disease was 20% in the CR group compared with 40% in the non-CR group (P = .004 per Wilcoxon test).
Pooled analysis of the GEM2000, GEM2005MENOS65, GEM2005MAS65, and GEM2010MAS65 clinical trials.
The obvious next question is: what is the impact of persistent M protein in otherwise MRD-negative patients? In a pooled analysis of 3 Spanish protocols, patients who were MRD-negative despite persistent M component showed PFS and overall survival similar to those of MRD-negative patients in CR.4 The UK group reported similar findings in the Myeloma IX and PADIMAC clinical trials.27,38 Investigators from the Mayo Clinic retrospectively analyzed the impact of residual serum M protein on outcomes of patients who were MRD negative by NGF in routine practice.39 Up to 43% of patients were immunofixation positive despite being MRD negative by NGF. At 1 year, the risk of progression was 40% in these patients vs 20% in those with negative immunofixation.39 In the PETHEMA/GEM2012MENOS65 clinical trial, 11% of MRD-negative patients by NGF after consolidation showed positive immunofixation and identical PFS to those with negative immunofixation (4-year rate, 87% vs 78.5%; P = .35).40 Therefore, despite the higher sensitivity of NGF and the later time point (ie, consolidation), ∼1 in 10 MRD-negative patients by NGF continued showing positive immunofixation, and their outcomes were as favorable as those of MRD-negative patients in CR.40 Together, these results suggest that patients with positive immunofixation and undetectable MRD are at risk of relapse, but the relative risk is no different from that of patients with negative immunofixation and undetectable MRD.
Possible explanations for positive M protein despite undetectable MRD
We postulate 7 possible explanations for conflicting results: (1) lower sensitivity of some MRD methods,18,31 (2) inadequate sampling of tumor burden in a single BM aspirate, (3) long half-live of immunoglobulins,28,41 (4) oligoclonal responses after treatment,28,42 (5) interference of therapeutic monoclonal antibodies, (6) immature clonotypic cells secreting identical immunoglobulins,40 and (7) patient reclassification as achieving less than CR because of unavailable measurements in clinical trials.8 We believe that the first 2 scenarios may be possible but are unlikely to be true in a majority of patients because (1) discordances persist with high-sensitivity NGF or NGS, (2) survival of patients with negative MRD but persistent M protein is similar to that of the remaining patients with undetectable MRD, and(3) a high rate of false-negative results would be incompatible with MRD being one of the most relevant prognostic factors in MM. Therefore, we reason that the other 5 scenarios that could explain false-positive detection of M proteins are more realistic. The fact that many patients with detectable M protein and negative MRD achieve CR later in time has been well described.24,28,41 Presence of oligoclonal bands in patients achieving CR is associated with favorable outcome.42 Therapeutic immunoglobulin G-κ bands can be misinterpreted as persistent M protein on immunofixation electrophoresis, unless a hydrashift assay or mass spectrometry is performed.43 Recent findings from the PETHEMA (Programa Español de Tratamientos en Hematología)/GEM (Grupo Español de Mieloma) suggest that, at least in some patients, discordances between NGF and immunofixation could be attributed to B cells with a VDJ rearrangement identical to that of tumor cells, which could potentially secrete low amounts of an immunoglobulin similar to the M protein.40 Interestingly, these B cells share random somatic mutations with myeloma cells, but not with driver mutations or copy-number alterations. Therefore, disease relapse could be driven by a pool of B cells from which MM originates rather than a source of cancer stem cells, because primary genetic abnormalities observed at diagnosis are usually present during progression.44-46 Also, the PETHEMA/GEM showed recently that patients meeting all criteria for CR but without urine immunofixation assessment have outcomes comparable to those of patients in CR and superior to those of patients achieving VGPR, and their depth of response should not be downgraded from CR to VGPR in clinical trials.8 Taken together, we believe that the probability of false-positive detection of M protein is greater than that of a false-negative MRD test.
Consequences of conflicting response criteria
In routine practice, contradictory results across laboratory methods assessing depth of response will decrease the level of confidence in their clinical value and may constitute a barrier to broader use of MRD in treatment decisions. Moreover, how will clinicians who are not familiar with the nuances we have enumerated interpret the result of a particular treatment that yields a rate of CR lower than that of MRD negativity? In clinical trials, downgrading MRD-negative patients to other response categories because they do not fulfill CR criteria could have 2 important consequences. First, results of clinical trials using MRD as a randomization or stratification factor could be reported erroneously, before investigation of regimens of different intensity and/or duration. If, for example, there is a high proportion of MRD-negative patients downgraded to an inferior response category because of persistent M protein, and these cases are allocated to a treatment arm of greater intensity and/or duration, it could be misinterpreted that more intensive/longer regimens are able to abrogate the poor prognosis of patients achieving suboptimal responses. This scenario could be particularly worrying during early phases of treatment, where discordance between CR and MRD may be higher because of the long half-life of immunoglobulins. Second, the erroneous estimation of MRD-negative rates across treatment arms could result in MRD becoming accepted as a surrogate end point for accelerated drug approval. For example, in the CASSIOPEIA trial, the proportion of patients who achieved CR or better after consolidation was 39% in the daratumumab plus bortezomib, thalidomide, and dexamethasone (VTd) group vs 26% in the VTd-alone group; surprisingly, the respective MRD-negative rates (at a threshold of 1 tumor cell per 105 white cells) were 64% vs 44%.47 Similarly, in the GMMG-HD7 trial that investigated the addition of isatuximab to lenalidomide, bortezomib, and dexamethasone (RVd) as induction therapy for newly diagnosed transplantation-eligible patients with MM, the rates of CR after isatuximab plus RVd vs RVd induction were 24.2% and 21.6% (P = .46), whereas the rates of MRD negativity were 50.1% vs 35.6% (P < .001), respectively.48 If bound to the achievement of CR, the rate of MRD negativity in the experimental arm of this trial would have been inferior.
Conclusion
The treatment landscape of MM is evolving dramatically. MM is at the forefront of MRD testing in hematology, with an armamentarium of next-generation cellular methods plus imaging techniques and mass spectrometry. By contrast, the definition of CR proposed in 1998 by Blade et al2 remains in force today. A new iteration of CR criteria is outside the scope of this article. However, we propose that ongoing and future clinical trials assess MRD in patients achieving at least VGPR. This would increase the accuracy of patient stratification according to MRD response, as well as the comparison of MRD-negative rates between treatment arms and across clinical trials.
Authorship
Contribution: B.P., J.S.-M., and H.A.-L. designed, wrote, and critically revised the manuscript.
Conflict-of-interest disclosure: B.P. reports honoraria for lectures from and membership on advisory boards of Adaptive, Amgen, Becton Dickinson, Bristol-Myers Squibb/Celgene, Janssen, Kite Pharma, Roche, Sanofi, and Takeda; unrestricted grants from Celgene, EngMab, Roche, Sanofi, and Takeda; and consultancy for Bristol-Myers Squibb/Celgene, Janssen, and Sanofi. J.S.-M. served as consultant for and on advisory boards of Amgen, Bristol-Myers Squibb, Celgene, Janssen, Merck Sharp & Dohme, Novartis, Takeda, Roche, Sanofi, GlaxoSmithKline, AbbVie, and Karyopharm. H.A.-L. reports no competing financial interests.
Correspondence: Hervé Avet-Loiseau, Myeloma Genomics Laboratory, IUCT-Oncopole, 1 ave Irene Joliot-Curie, 31059 Toulouse, France; e-mail: avetloiseau.herve@iuct-oncopole.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal