In this issue of Blood, Bergal et al developed a new method to demonstrate that circulating von Willebrand Factor (VWF) multimers experience less tension and undergo less conformational elongation in shear flow than previously thought.1 VWF is a blood protein that binds to and activates platelets to initiate hemostatic blood clots, particularly in arterial flow. This process must be precisely regulated, or thrombi would form spontaneously. The primary regulatory mechanism in VWF is mechanical tension; therefore, characterizing VWF tension and elongation in shear flow is critical for understanding the regulation of arterial hemostasis and thrombosis. Indeed, devices such as artificial heart valves and left ventricular assist devices, which introduce higher and different fluid stresses, are associated with the hemostatic and thrombotic dysregulation of VWF in many patients.
Using instruments that apply piconewton forces to nanoscale single molecules, critical levels of tension have been shown to activate the binding of platelet receptor glycoprotein Ib (GPIb) to VWF,2,3 explaining why shear stress triggers VWF-mediated platelet activation and aggregation. Moreover, tension in VWF exposes a cleavage site for the metalloprotease ADAMTS13, which breaks up VWF multimers to make them less responsive to flow.4 Together, this process creates a remarkable mechanical feedback loop in which VWF is reduced in size until it does not activate platelets in the prevalent flow conditions, but can still respond to new pathological conditions. However, knowing how VWF responds to tension applied by expensive lab instruments is only helpful for designing better blood-contacting devices, if we also know how much tension is applied to VWF under various flow conditions. Unfortunately, previous studies on flow-induced elongation and tension of free VWF were contradictory, with 1 showing dramatic elongation at high physiological shear flow,5 whereas others showed only minor elongation, even at higher flow rates.6,7 Because GPIb binds to VWF multimers only after dramatic elongation,2 the flow conditions required to activate free VWF remain unclear.
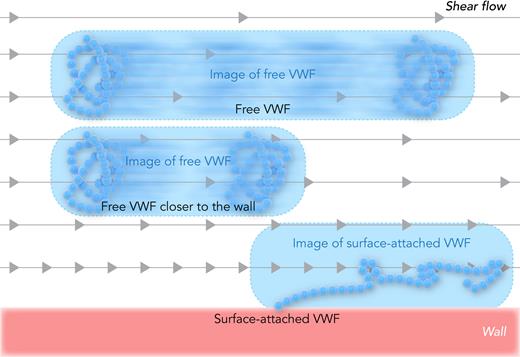
Bergal et al resolved this contradiction by addressing a challenge in imaging technology. At a high shear flow, the VWF moves at a high velocity, and thus blurs into a streak during image acquisition (see figure). A strobing laser can decrease the acquisition time,7 but the blur length should still be measured and subtracted to obtain reliable measurements of the particle length in shear flow. In the past, this required knowledge of the particle velocity from theory, which is complicated in shear flow owing to fluid velocity gradients and flow-induced hydrodynamic lift that moves VWF-sized particles into higher velocity streams.8 Bergal et al developed a clever method called “pulsed laser stroboscopic imaging of single molecules (PULSIS)” that allows calculation of particle and blur lengths without knowing the particle velocity ahead of time. Their accurate and complete measurements confirmed the more conservative prior studies by showing that free VWF elongated too little under physiological shear stress to activate free VWF.
Measuring VWF elongation in shear flow. In shear flow, the fluid farther from the wall moves faster, as illustrated by the gray arrows. Free VWF moves during image acquisition, so the image forms a blur that is longer than the length of the protein, and depends on the distance of VWF from the wall. Surface-attached VWF does not move with the flow, so any elongation in the image reflects actual protein stretching. Professional illustration by Somersault18:24.
Measuring VWF elongation in shear flow. In shear flow, the fluid farther from the wall moves faster, as illustrated by the gray arrows. Free VWF moves during image acquisition, so the image forms a blur that is longer than the length of the protein, and depends on the distance of VWF from the wall. Surface-attached VWF does not move with the flow, so any elongation in the image reflects actual protein stretching. Professional illustration by Somersault18:24.
This study also addressed another critical issue for the development of biomaterials for blood-contacting devices. Surface immobilization activates VWF, contributing to device-dependent hemostatic and thrombotic dysregulation. Some surface chemistries stabilize the active conformation of the GPIb-binding domain of VWF, whereas others do not,9 suggesting that 1 approach to creating safer blood-contacting devices is to control surface chemistry. However, it is also possible that polymers tethered to surfaces stretch more in the flow. Although, in a study by Fu et al,2 the elongation of surface-tethered VWF inflow was intermediate between that of free VWF in previous contradictory studies,5-7 it was significantly more than the elongation of free VWF measured by Bergal et al (see figure). Moreover, a common model for the VWF polymer fits both the Bergal et al–free VWF and Fu et al–tethered VWF extensive data, demonstrating that surface tethering alone is sufficient to increase VWF elongation. Together, this supports the notion that it is important to design blood-contacting devices that limit the shear flow at device surfaces.
Although this study focused on free VWF in shear flow, the methods used here should advance our ability to characterize a range of shear-dependent processes in circulating blood or other fluids. VWF can also bind to other VWF multimers or circulating platelets in flow, which is likely to alter shear-dependent VWF tension and elongation and thus contribute to hemostatic and thrombotic dysregulation in blood-contacting devices. Certain bacteria that cause infective endocarditis bind to immobilized platelets in a shear-dependent manner,10 suggesting that this may also occur in circulating blood, which would likely impact bacterial transport to the endocardium. As illustrated by these examples, binding is a critical factor in many shear-enhanced processes; therefore, the PULSIS method to measure particle size accurately at very high flow rates could be especially powerful if shown to be compatible with dual fluorescent labeling.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal