TO THE EDITOR:
Alloimmune platelet transfusion refractoriness (alloPTR) is a serious clinical issue present in ∼5% to 15% of patients who undergo platelet transfusion owing to sensitization against alloantigens such as HLA class I and human platelet antigens (HPAs).1 To provide transfusable platelets for a patient suffering from alloPTR but with no compatible registered donor, we developed an induced pluripotent stem cell–derived platelet product (iPSC-PLT). We have previously established immortalized megakaryocyte progenitor cell lines (imMKCLs) by introducing doxycycline-inducible c-MYC, BMI1, and BCL-XL overexpression during differentiation from iPSCs.2 The imMKCLs expand by the expression of 3 transgenes, and thereafter, they mature and generate iPSC-PLTs. We further produced clinically required, 1011-scale competent iPSC-PLTs ex vivo by developing a turbulent flow–mediated VerMES Bioreactor, based on the discovery that turbulence regulates thrombopoiesis, and using several novel drugs:3 a thrombopoietin mimetic, TA-316;4 a Rho-associated kinase inhibitor, Y-39983 and an aryl hydrocarbon receptor antagonist, GNF-316, together enables adhesion-independent manufacturing;3 and an ADAM17 inhibitor, KP-457, which blocks glycoprotein Ibα (CD42b) shedding.5 (Figure 1A; supplemental Tables 1-8, available on the Blood website).
Production of iPSC-PLTs from imMKCL MCB and their functional properties. (A) Somatic cells (peripheral blood mononuclear cells) from an aplastic anemia patient with alloPTR owing to anti–HPA-1a, were reprogrammed using episomal vectors. iPSCs were differentiated to hematopoietic progenitor cells using the revised pluripotent stem cell (PSC)-sac method. Then, under megakaryocyte differentiating condition, c-MYC, BMI-1, and BCL-XL were sequentially transduced by lentiviral vectors to establish imMKCLs. A single imMKCL clone was selected based on expandability and platelet production and stocked as a master cell bank (MCB) in liquid nitrogen. From the MCB, iPSC-PLTs were produced under good manufacturing practice (GMP)–based conditions: an MCB vial was thawed, cultured with a thrombopoietin mimetic (TA-316), stem cell factor and doxycycline, and expanded for 23 days. After expansion, the cells were transferred to medium without doxycycline but with a Rho-associated kinase inhibitor (Y-39983), an aryl hydrocarbon receptor antagonist (GNF-316), and an ADAM17 inhibitor (KP-457). The 6-day maturation culture was done in 4 vessels of 8 L-scale VerMES, which are turbulent flow bioreactors. iPSC-PLTs released into the media were concentrated, separated, and washed using hollow fibers and an ACP215 centrifugation system with low and high settings and depleted for nucleated cells using leukocyte depleting filters. Approximately 10 × 1011 iPSC-PLTs were resuspended in 200 mL bicarbonate linger solution with 20% acid citrate dextrose and 2.5% human serum albumin and packaged into a blood bag. The final package included ∼107 imMKCLs but was gamma-ray irradiated with 25 Gy to eliminate tumorigenicity. (B) Sizes and percentages of the large immature platelet fraction (IPF) corresponding with iPSC-PLTs (batch 18) and blood donor–derived Japanese Red Cross product platelets (JRC-PLTs) at different storage days. Representative data of 3 batches are shown. (C) Representative transmission electron micrograph images of iPSC-PLTs (batch 17) and JRC-PLTs. Scale bars, 1 μm. (D) In vitro functional assay of iPSC-PLTs: (i) representative flow cytometric analysis data for iPSC-PLT samples before processing, after filtration, and after a second ACP215 centrifugation for washing and after irradiation and (ii) aggregation assay. iPSC-PLTs were stimulated with 50 μM adenosine diphosphate (ADP) or 5, 10, and 20 μg/mL collagen. (E) In vivo circulation in thrombocytopenic rabbit models for 6 batches of iPSC-PLTs (3 batches per rabbit, 2 rabbits) by measuring the ratio of human and rabbit platelets in peripheral blood using flow cytometry. (F) In vivo hemostasis in thrombocytopenic rabbit models for iPSC-PLTs by measuring the bleeding time of the ear incision before and after the transfusion of human iPSC-PLTs (batch 09). The maximum measuring time was 600 seconds. (G) imMKCLs or iPSC-PLTs introduced with Akaluc expression vectors were injected into NOD/Shi-scid,IL-2RγKO (NOG) mice. Phosphate-buffered saline (PBS) was injected for the control group. Results of in vivo imaging after 2, 6, and 48 hours are shown. CCI, corrected count increment; max, maximum; min, minimum.
Production of iPSC-PLTs from imMKCL MCB and their functional properties. (A) Somatic cells (peripheral blood mononuclear cells) from an aplastic anemia patient with alloPTR owing to anti–HPA-1a, were reprogrammed using episomal vectors. iPSCs were differentiated to hematopoietic progenitor cells using the revised pluripotent stem cell (PSC)-sac method. Then, under megakaryocyte differentiating condition, c-MYC, BMI-1, and BCL-XL were sequentially transduced by lentiviral vectors to establish imMKCLs. A single imMKCL clone was selected based on expandability and platelet production and stocked as a master cell bank (MCB) in liquid nitrogen. From the MCB, iPSC-PLTs were produced under good manufacturing practice (GMP)–based conditions: an MCB vial was thawed, cultured with a thrombopoietin mimetic (TA-316), stem cell factor and doxycycline, and expanded for 23 days. After expansion, the cells were transferred to medium without doxycycline but with a Rho-associated kinase inhibitor (Y-39983), an aryl hydrocarbon receptor antagonist (GNF-316), and an ADAM17 inhibitor (KP-457). The 6-day maturation culture was done in 4 vessels of 8 L-scale VerMES, which are turbulent flow bioreactors. iPSC-PLTs released into the media were concentrated, separated, and washed using hollow fibers and an ACP215 centrifugation system with low and high settings and depleted for nucleated cells using leukocyte depleting filters. Approximately 10 × 1011 iPSC-PLTs were resuspended in 200 mL bicarbonate linger solution with 20% acid citrate dextrose and 2.5% human serum albumin and packaged into a blood bag. The final package included ∼107 imMKCLs but was gamma-ray irradiated with 25 Gy to eliminate tumorigenicity. (B) Sizes and percentages of the large immature platelet fraction (IPF) corresponding with iPSC-PLTs (batch 18) and blood donor–derived Japanese Red Cross product platelets (JRC-PLTs) at different storage days. Representative data of 3 batches are shown. (C) Representative transmission electron micrograph images of iPSC-PLTs (batch 17) and JRC-PLTs. Scale bars, 1 μm. (D) In vitro functional assay of iPSC-PLTs: (i) representative flow cytometric analysis data for iPSC-PLT samples before processing, after filtration, and after a second ACP215 centrifugation for washing and after irradiation and (ii) aggregation assay. iPSC-PLTs were stimulated with 50 μM adenosine diphosphate (ADP) or 5, 10, and 20 μg/mL collagen. (E) In vivo circulation in thrombocytopenic rabbit models for 6 batches of iPSC-PLTs (3 batches per rabbit, 2 rabbits) by measuring the ratio of human and rabbit platelets in peripheral blood using flow cytometry. (F) In vivo hemostasis in thrombocytopenic rabbit models for iPSC-PLTs by measuring the bleeding time of the ear incision before and after the transfusion of human iPSC-PLTs (batch 09). The maximum measuring time was 600 seconds. (G) imMKCLs or iPSC-PLTs introduced with Akaluc expression vectors were injected into NOD/Shi-scid,IL-2RγKO (NOG) mice. Phosphate-buffered saline (PBS) was injected for the control group. Results of in vivo imaging after 2, 6, and 48 hours are shown. CCI, corrected count increment; max, maximum; min, minimum.
The iPSC-PLTs were produced based on GMP using newly established, patient-specific imMKCLs as the MCB and gamma-ray irradiated (25 Gy) to eliminate the tumorigenicity potential of iPSC-PLTs, which still included imMKCLs. Compared with donor platelets available from the JRC-PLTs, iPSC-PLTs were larger (approximate diameter, 2.5 μm vs 3.5-4 μm; Figure 1B), but the ultrastructures were comparable (Figure 1C), and in vitro quality and function were sufficient within 5 days (Figure 1D). Consistent with results using JRC-PLTs and iPSC-PLTs of a laboratory clone in rabbit models,6 the patient’s iPSC-PLTs showed stable circulation in vivo from 2 to 7 hours (Figure 1E) and 100% success in hemostasis (Figure 1F). Later tests using in vivo imaging system confirmed systemic circulation and distribution in the lung, liver, and spleen (Figure 1G), which resembles patterns reported with general platelet transfusions.7 In contrast, imMKCLs were mostly restricted to the lungs. Further details regarding the production and nonclinical tests confirming the quality and safety of the iPSC-PLTs are published in Blood Advances.
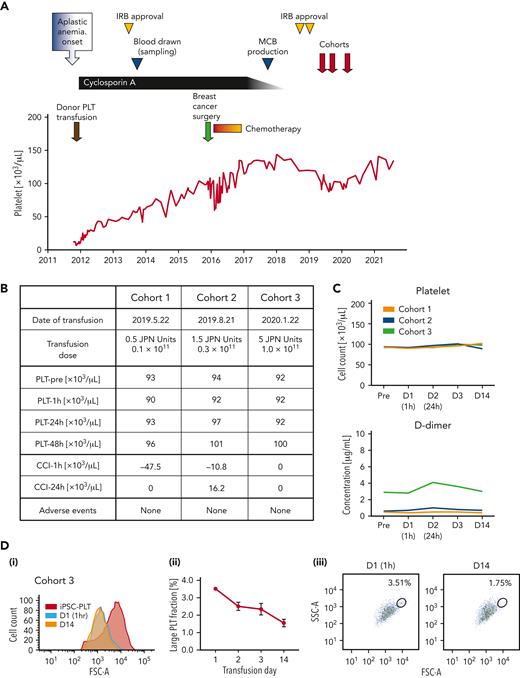
The clinical trial of an autologous transfusion of patient-derived iPSC-PLTs, the iPLAT1 study, was approved by Kyoto University and the Ministry of Health, Labour and Welfare, and this trial was registered at the Japan Registry of Clinical Trials, https://jrct.niph.go.jp/latestdetail/jRCTa050190117, as #jRCTa050190117 (supplemental Figure 1 and protocol). The enrolled patient was a 55-year-old Japanese woman with a history of 2 pregnancies and was diagnosed with severe aplastic anemia at the age of 47. After the first platelet transfusion as a pretreatment to the antithymocyte globulin administration, she developed a fever of 39.8°C and a generalized swollen rash, and no platelet increase was observed, followed by the diagnosis of alloPTR owing to anti–HPA-1a antibodies. However, the frequency of the patient’s HPA-1b/1b phenotype in Japan is estimated to be <0.002%,8 and such blood donors were not available in the JRC repository. Fortunately, cyclosporine monotherapy was effective and eventually completed at the age of 54. She also underwent a mastectomy with lymph node dissection for breast cancer at the age of 51. Postoperative chemotherapy was reduced to monotherapy with dose reduction to avoid serious cytopenia (Figure 2A).
Clinical trial assured safety and circulation of autologous iPSC-PLTs. (A) Clinical course of the participant from the onset of aplastic anemia. Institutional review board (IRB) approval by Kyoto University was received to establish the imMKCLs and produce the iPSC-PLTs. Then, the clinical trial for administering the iPSC-PLTs was approved by Kyoto University and by the Ministry of Health, Labour and Welfare, Japan, based on the Act on Safety of Regenerative Medicine. (B) iPSC-PLTs were administered in a dose-escalating manner in 3 dose cohorts: 0.5, 1.5, and 5 Japanese units, which correspond to 0.1 × 1011, 0.3 × 1011, and 1.0 × 1011 platelets, respectively. No significant adverse event was observed. (C) Peripheral blood platelet count and D-dimer of the patient collected preinfusion on days 1 (D1, 1 hour after transfusion), 2, 3, and 14 for the 3 dose cohorts are shown. (D) Flow cytometry analysis of the patient peripheral blood PLT fraction in cohort 3. (i) An overlaid histogram of the forward scatter (FSC-A) of iPSC-PLTs before infusion (iPSC-PLT) and peripheral blood platelets on days 1 and 14. (ii) Percentage of the gated large PLT fraction on days 1, 2, 3, and 14 of the iPSC-PLT transfusion. Average of triplicates with standard deviation are shown. (iii) Representative scatter plots of the FSC-A and side scatters (SSC-A) with gating of the large platelet fraction on days 1 and 14.
Clinical trial assured safety and circulation of autologous iPSC-PLTs. (A) Clinical course of the participant from the onset of aplastic anemia. Institutional review board (IRB) approval by Kyoto University was received to establish the imMKCLs and produce the iPSC-PLTs. Then, the clinical trial for administering the iPSC-PLTs was approved by Kyoto University and by the Ministry of Health, Labour and Welfare, Japan, based on the Act on Safety of Regenerative Medicine. (B) iPSC-PLTs were administered in a dose-escalating manner in 3 dose cohorts: 0.5, 1.5, and 5 Japanese units, which correspond to 0.1 × 1011, 0.3 × 1011, and 1.0 × 1011 platelets, respectively. No significant adverse event was observed. (C) Peripheral blood platelet count and D-dimer of the patient collected preinfusion on days 1 (D1, 1 hour after transfusion), 2, 3, and 14 for the 3 dose cohorts are shown. (D) Flow cytometry analysis of the patient peripheral blood PLT fraction in cohort 3. (i) An overlaid histogram of the forward scatter (FSC-A) of iPSC-PLTs before infusion (iPSC-PLT) and peripheral blood platelets on days 1 and 14. (ii) Percentage of the gated large PLT fraction on days 1, 2, 3, and 14 of the iPSC-PLT transfusion. Average of triplicates with standard deviation are shown. (iii) Representative scatter plots of the FSC-A and side scatters (SSC-A) with gating of the large platelet fraction on days 1 and 14.
The iPSC-PLTs, which were compatible for specifications (supplemental Figure 2; supplemental Tables 9 and 10) and later confirmed for ultrastuctures (supplemental Figure 3), residual additive concentration, and sterility (supplemental Table 11), were administered sequentially for 3 escalating doses of 1 × 1010, 3 × 1010, and 1 × 1011 (1/20, 3/20, and 1/2 the dose of standard transfusions in Japan, respectively; Figure 2A-B). As for the primary endpoint of frequency and extent of adverse events, the patient did not present any significant clinical symptoms or signs throughout the study period (Figure 2B; supplemental Table 12). Laboratory data showed no remarkable change, but the level of a coagulation marker, D-dimer, and white blood cell count were slightly increased 24 hours after the transfusion in cohorts 2 and 3 (Figure 2C; supplemental Table 12). Nevertheless, lower extremity ultrasonography identified no deep vein thrombosis, and the D-dimer concentration spontaneously decreased. As for the secondary endpoint of CCI, the CCI at 1 hour and 24 hours were determined to be 0 even in cohort 3 (Figure 2B-C). However, a flow cytometry analysis of peripheral blood showed the existence of larger platelets after the transfusion, which gradually decreased (Figure 2D). The external Efficacy and Safety Assessment Committee confirmed the safety of the transfusion, approved transition to the next cohorts after the 28-day follow-up for cohorts 1 and 2, and concluded that the autologous transfusion of iPSC-PLTs is safe for this patient after the observation period of 1 year following cohort 3 (supplemental Figure 1).
The study outcome is of great significance given that anti–HPA-1a antibody could cause posttransfusion purpura, which even decreases platelet count after transfusion. The lack of an increase in CCI could be attributed to the difficulty in detecting the relatively small increase compared with the high pretransfusion platelet count and the common blood count device used, which could ignore large-sized iPSC-PLTs (Figure 1B). However, we observed populations of larger platelets in posttransfusion samples by flow cytometry, suggesting their circulation. Moreover, the CCI could have peaked at 2 to 6 hours later according to the kinetics in the rabbit study (Figure 1E), thus missing the optimal time point in this trial. The delayed peaking can be because of the fragmentation of large-sized iPSC-PLTs, as suggested in a previous study using mouse models.3
Meanwhile, the slight increase in D-dimer concentration after transfusion may indicate coagulation by iPSC-PLTs soon after the transfusion. The concurrent slight white blood cell increase may further indicate enhanced inflammation. Although the number of imMKCLs infused was small (<107), such reactions may have been caused by the immune properties of megakaryocytes. However, only megakaryocytes in the lung parenchyma are reported to have the immune phenotype,9,10 whereas the infused imMKCLs in this study were probably trapped in pulmonary circulation (Figure 1G). In addition, the compromised circulation could be owing to mild activation caused by irradiation (Figure 1D) or the aberrant status of glycosylation and sialylation,11,12 which reflects the possible embryonic/fetal nature of iPSC-PLTs, as seen with erythrocytes.13,14 We plan to assess these possibilities thoroughly in future studies.
In summary, we succeeded in the clinical scale manufacturing of GMP-based, 1011-scale autologous iPSC-PLTs for a patient who did not have a compatible platelet donor and completed the first-in-human clinical trial of iPSC-PLTs, which assured the safety of the product through the dose-escalation study. At the same time, the study revealed a discrepancy in the circulation between animal models and the human participant.
Significant improvements in the efficacy and cost of producing iPSC-PLTs could realize the practical personalized medicine of autologous platelet products, which are essentially nonrejectable. Furthermore, the production system and the observed findings in this study should contribute to the clinical application of allogeneic iPSC-PLTs for off-the-shelf use. Given that the main causative antigen of alloPTR is HLA class I, iPSC-PLTs genetically depleted of HLA class I could serve as an optimal product for mass production,15-17 with the additional genetic conversion of HPA being an option.18 These advancements will allow many patients with thrombocytopenia, especially those with alloPTR, to benefit from this combined modality of transfusion medicine and regenerative medicine.
Acknowledgments
The authors thank the people of the Eto laboratory (Center for iPS Cell Research and Application [CiRA], Kyoto University) and the CiRA Foundation for the iPSC-PLT production and evaluation; staffs at Institute for Advancement of Clinical and Translational Science (iACT) (Kyoto University Hospital) for the clinical trial operation; Peter Karagiannis (CiRA) for proofreading this article; Misaki Ouchida (CiRA) for providing the graphical figure; the Japanese Red Cross Society for providing donor–derived human platelets and plasma; and Akira Shimizu (Kyoto University), Tadaaki Hanatani, and Yuji Arakawa (CiRA) for helpful comments and support.
This work was supported by grants from the Japan Agency for Medical Research and Development: The Highway Program for Realization of Regenerative Medicine (grant JP17bm0504008 (K.E.), The Research Project for Practical Applications of Regenerative Medicine (grant JP17bk0104039) (K.E.), and Core Center for iPS Cell Research (JP17bm0104001) (N.S., S.N., K.E.); and a grant-in-aid for scientific research (S) (21H05047, K.E.) from the Japan Society for the Promotion of Science (JSPS).
All the interests were reviewed and are managed by Kyoto University in accordance with its conflict-of-interest policies.
Authorship
Contribution: N.S. designed and performed the research, analyzed the data, and wrote the paper; J.K. designed and performed the clinical trial and wrote the paper; S.N. designed and performed the experiments, produced the product, and analyzed the data; T. Kitano, M. Hishizawa, and T. Kondo designed the clinical trial; S.S. and A. Shigemasa designed and performed the research, produced the product, and analyzed the data; H.H. and Y.A. designed and performed the clinical trial; M.M. and H.T. designed the clinical trial and analyzed the data; D.M. and K.-R.K. performed clinical care; M.N. and N.W. designed and performed the experiments and analyzed the data; S.O. and M. Handa supervised the experiments; A. Sawaguchi designed and performed the experiments and analyzed the data; N.M., M.T., T.H., and A.F. designed and performed the experiments and analyzed the data; Y.T. supervised the experiments; A.T.-K. designed and performed the research and wrote the manuscript; and K.E. designed and performed the research and wrote the manuscript.
Conflict-of-interest disclosure: S.N. and K.E. have applied for patents related to this manuscript. N.S. serves as a consultant for Megakaryon Co. J.K. serves as a consultant for Astellas Pharma, an adviser for Daiichi Sankyo Co, Janssen Pharmaceutical, Megakaryon Co, SymBio Pharmaceuticals, and Takeda Pharmaceutical, and receives research funding from Eisai Co. S.S. is employed at Megakaryon. A.T.-K. serves as an adviser for Megakaryon and receives research funding from Ono Pharmaceutical. K.E. is a founder of Megakaryon and a member of its scientific advisory board without salary and receives research funding from Megakaryon, Otsuka Pharmaceutical, and Kyoto Manufacturing Co. The remaining authors declare no competing financial interests.
Correspondence: Koji Eto, Department of Clinical Application, CiRA, Kyoto University, 53 Kawaharacho, Shogoin, Sakyoku, Kyoto 606-8507, Japan; e-mail: kojieto@cira.kyoto-u.ac.jp; and Akifumi Takaori-Kondo, Department of Hematology, Kyoto University Hospital, 54 Kawaharacho, Shogoin, Sakyoku, Kyoto 606-8507, Japan; e-mail: atakaori@kuhp.kyoto-u.ac.jp.
References
Author notes
∗N.S. and J.K. contributed equally to this study.
Protocols are provided as a supplement. Data are available on request from the corresponding authors, Koji Eto (kojieto@cira.kyoto-u.ac.jp) and Akifumi Takaori-Kondo (atakaori@kuhp.kyoto-u.ac.jp).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal