In this issue of Blood, Sugimoto et al1 report the result of the first clinical trial of an autologous transfusion of induced pluripotent stem cell (iPSC)-derived platelets (iPSC-PLTs) in a patient who had severe aplastic anemia but no compatible platelet donor.
Platelets are abundant, yet short-lived, blood cells. Platelets have multifaceted functions, comprising important physiological processes such as hemostasis, vascular integrity, wound healing, and immunity, as well as pathological roles such as inflammation and tumorigenesis.2,3 Thrombocytopenia is common in many hematological diseases (eg, leukemia, aplastic anemia, and immune thrombocytopenia).3,4 Thrombocytopenia also occurs in many other diseases and conditions such as sepsis, infectious illnesses, cancer chemotherapy, and in the recovery phases of blood stem cell transplantation.3,5 Patients with severe thrombocytopenia, who are at a high risk of bleeding or are bleeding heavily, often require prophylactic or therapeutic platelet transfusion.3 Approximately 2 million units (∼3 × 1011 per unit) of donor-derived platelets are transfused annually in the United States.3 However, there are numerous unsolved limitations with donor-derived platelets, including product unit quality, short shelf life, risks of product bacterial contamination, and transmission of infectious disease.3 In addition, platelet transfusion refractoriness, which is defined as the repeated failure to achieve the desired level of blood platelets in a patient after platelet transfusion from random donors, is also a clinical problem.3 Alloimmune platelet transfusion refractoriness (allo-PTR) occurs in ∼5% to 15% of patients who have undergone platelet transfusion due to sensitization to alloantigens, such as HLA-I and human platelet antigens (HPAs).1 To overcome these problems, there have been many efforts in the past few decades to produce platelets in vitro or ex vivo from precursor cells for clinical application.3,6 However, large-scale manufacturing of good manufacturing practices (GMP)–grade functional platelets ex vivo has remained an elusive goal.
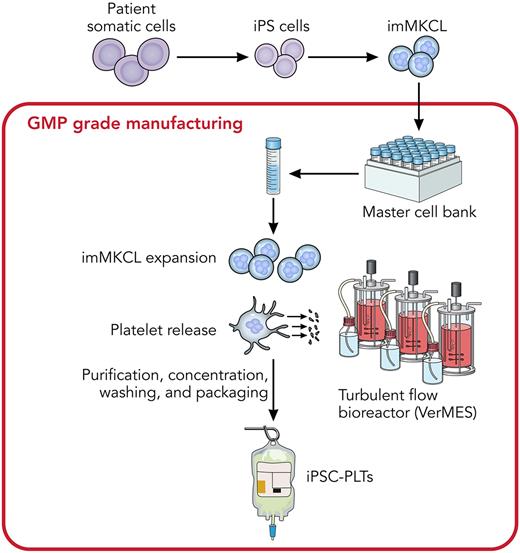
In this study, the Eto group, which is an established team in the field, addresses this goal by focusing on the generation of transfusable platelets for a patient with aplastic anemia with allo-PTR with no compatible registered donor. Based on their previous innovative research,6 the authors established immortalized megakaryocyte progenitor cell lines (imMKCLs) differentiated from induced pluripotent stem cells (iPSCs), which were generated from the patient’s peripheral blood mononuclear cells (see figure).1,7 A competent imMKCL clone was selected for the generation of a master cell bank. After biosafety validation, cells from this master cell bank were used to produce platelets (iPSC-PLTs) using their previously established turbulent flow bioreactors and a mixture of drugs to promote megakaryocyte proliferation/maturation and preserve platelet function. With this system, they successfully achieved clinical-scale manufacturing of GMP-grade autologous iPSC-PLTs for the patient.7 The authors then conducted a first-in-human clinical trial of autologous transfusions (3 sequential escalating doses of 1 × 1010, 3 × 1010, and 1 × 1011) of the iPSC-PLTs into the patient. The transfusion was well tolerated with no significant complications at 1-year follow-up. The absence of side effects is especially encouraging and paves the way for further human studies. Even though these transfused iPSC-PLTs seemed short lived (they only circulated for several hours after transfusions) with untested in vivo functionality in this patient, this study represents a significant step toward the authors’ goal of applying iPSC-PLT technology to clinical use.
Diagram showing the large-scale production of iPSC-PLTs from imMKCLs, derived from a patient with aplastic anemia for autologous transfusion. Professional illustration by Patrick Lane, ScEYEnce Studios.
Diagram showing the large-scale production of iPSC-PLTs from imMKCLs, derived from a patient with aplastic anemia for autologous transfusion. Professional illustration by Patrick Lane, ScEYEnce Studios.
Although the iPSC-PLT technology has great potential, there still is a long path ahead before routine use as a source for platelet transfusion. Long production time and the high cost of producing GMP-grade functional platelets are among the major obstacles that prevent regular clinical use, even for the indication presented in this study. Therefore, the current priority should perhaps be the development of applications that cannot feasibly be achieved with the typical donor-derived platelets; for example, generating genetically modified iPSC-PLTs by deleting the main allo-PRT–causing antigens HLA-I and/or HPA for allogeneic transfusion, as suggested by the authors; generating autologous gene-corrected platelets for patients with inherited platelet disorders; or developing genetically modified autologous iPSC-PLTs to express ectopic therapeutic molecules for targeted delivery to specific sites such as tumor microenvironment for tumor therapy. With further development and suitable indications, the iPSC-PLT technology represents an innovative and promising platform to address many unmet clinical needs.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal