Key Points
A sequential MRD-guided addition of ibrutinib to venetoclax led to uMRD in 33 (87%) of 38 patients with relapsed/refractory CLL.
This MRD-driven strategy allowed identical depth of response to be reached in each patient with an individualized time-limited approach.
Abstract
Undetectable measurable residual disease (uMRD) is achievable in patients with chronic lymphocytic leukemia (CLL) with the BCL2-inhibitor venetoclax alone or combined with the Bruton’s tyrosine kinase inhibitor ibrutinib. This phase 2, multicenter, MRD-driven study was designed to discontinue treatment upon reaching uMRD4 (<10−4) in patients with relapsed/refractory CLL receiving venetoclax monotherapy or after the addition of ibrutinib. Primary end point of the study was proportion of uMRD4 with venetoclax ± ibrutinib. Secondary end points were overall response rate, partial response, complete response, progression-free survival, duration of response, overall survival, and safety of venetoclax ± ibrutinib. Patients with uMRD4 at Cycle 12 Day 1 discontinued venetoclax. MRD+ patients added ibrutinib and continued both drugs up to Cycle 24 Day 28/uMRD4/progression/toxicity. After Cycle 24 Day 28, MRD+ patients continued ibrutinib. Thirty-eight patients (29% with TP53 aberrations; 79% with unmutated IGHV) started venetoclax. Overall response rate with venetoclax was 36 (95%) of 38 patients (20 complete; 16 partial response). Seventeen patients (45%) with uMRD4 at Cycle 12 Day 1 discontinued venetoclax. Nineteen (55%) MRD+ subjects added ibrutinib. After a median of 7 months (range, 3-10 months) of combined treatment, 16 (84%) of 19 achieved uMRD4, thus stopping both drugs. Two MRD+ patients at Cycle 24 Day 28 continued ibrutinib until progression/toxicity. After a median follow-up of 36.5 months, median progression-free survival was not reached; 10 patients progressed (4 restarted venetoclax, 3 without treatment need, 2 developed Richter transformation, and 1 dropped out). Seven (22%) of 32 patients remain uMRD4 after 3 years of follow-up. Neutropenia was the most frequent grade 3 to 4 adverse event; no grade 5 events occurred on study. This sequential MRD-guided approach led to uMRD4 in 33 (87%) of 38 patients, with venetoclax monotherapy or combined with ibrutinib, delivering treatment combination only in a fraction, and ultimately identifying the few patients benefiting from continuous therapy. This trial was registered at www.clinicaltrials.gov as # NCT04754035.
Introduction
The treatment landscape for patients with chronic lymphocytic leukemia (CLL) has greatly improved in the last decade with the introduction of targeted agents, including Bruton’s tyrosine kinase (ibrutinib, acalabrutinib) and BCL2 (venetoclax) inhibitors. The 2 classes are effectively targeting complementary mechanisms of CLL cell persistence and survival: BTK inhibitors have been shown to inhibit proliferation by blocking the B-cell receptor signaling pathway,1-3 whereas BCL2 inhibitors promote apoptosis by inhibiting BCL2.
As a single agent, venetoclax was able to achieve deep responses in a relevant proportion of patients with CLL, with 5% undetectable measurable residual disease (uMRD) complete responses (CR) in heavily pretreated patients with relapsed/refractory (R/R) CLL,4 and 30% uMRD in peripheral blood (PB) in those carrying TP53 aberrations.5 Of note, uMRD appeared the strongest indicator for response duration when venetoclax was administered in combination with monoclonal antibodies following a fixed-duration schedule.6-9
Continuous treatment with single-agent ibrutinib rarely leads to eradication of detectable CLL cells. Conversely, if combined with CLL cell-depleting agents such as bendamustine and rituximab, ibrutinib has been shown to increase the depth of response as monotherapy after the combination period, allowing uMRD to be achieved in more than one-quarter of patients.10
The combination of BTK inhibitor and BCL2 inhibitor has a strong preclinical rationale11,12 and has been recently tested in patients with treatment-naive and R/R CLL.13-17 These studies applied a one-size-fits-all approach, in which all patients received the combination of the 2 targeted agents for a fixed duration.
Based on this existing knowledge on both single and combination treatments using targeted therapies in CLL, we exploited the analysis of MRD as a biomarker to personalize intensity and duration of treatment in individual patients. This strategy takes into account that some patients already achieve the best response with a single agent, and others require combination strategies, while few might never achieve uMRD and are optimal candidates for continuous treatment to effectively control their disease.
This MRD-driven, phase 2 study “A Multi-Center, Open Label, Uncontrolled, Phase 2a Clinical Trial Evaluating the Safety and Efficacy of the Addition of Ibrutinib to Venetoclax through a MRD-guided Approach in R/R Patients with CLL” (Ibrutinib treatment based on MRD-guided apPROach combined with VEnetoclax, IMPROVE) was designed with the intent of discontinuing treatment upon reaching uMRD in patients with R/R CLL treated with venetoclax monotherapy or by adding ibrutinib to venetoclax in those who did not achieve uMRD with venetoclax alone. Patients with detectable MRD at the end of combination treatment continue ibrutinib monotherapy.
Methods
Study conduct
IMPROVE is an investigator-initiated, single-arm, open-label, phase 2 study in patients with R/R CLL who received ≥1 prior therapy. The study was approved by the Italian national regulatory authority and the ethics committee of participating sites, and it was registered in ClinicalTrials.gov (NCT04754035) (the study protocol is available in the supplemental Material, available on the Blood Web site). It was conducted in accordance with the Declaration of Helsinki and International Conference on Harmonisation Guidelines for Good Clinical Practice. All patients enrolled into the study signed written informed consent. The data lock date was September 19, 2021. All authors had access to primary clinical trial data and contributed to the analysis.
Patients, investigations, and treatment
Key eligibility criteria included: age ≥18 years; diagnosis of CLL according to International Workshop on CLL (iwCLL) criteria18; R/R after ≥1 prior therapy; active disease requiring treatment based on iwCLL criteria18; adequate bone marrow (BM) (absolute neutrophil count ≥1.0 × 109/L, platelets ≥30 × 109/L or ≥20 × 109/L if CLL-related BM involvement, hemoglobin ≥8.0 g/dL), renal (creatinine clearance ≥30 mL/min), and hepatic (aspartate aminotransferase and alanine aminotransferase ≤3× upper normal limit, and bilirubin ≤1.5× upper normal limit) function. Patients previously treated with BCL2 and/or BTK inhibitors, those with active infections (including hepatitis B virus, hepatitis C virus, and HIV), and/or those requiring treatment with warfarin or its derivatives, and/or moderate or strong cytochrome P450 inducers/inhibitors within 3 days before the venetoclax first dose, were excluded. Patients previously treated with phosphatidylinositol 3-kinase inhibitors were eligible.
All patients underwent pretreatment evaluation with physical examination, including vital signs measurement, Cumulative Illness Rating Scale score and Eastern Cooperative Oncology Group performance status, complete blood cell count, blood chemistry, computed tomography (CT) imaging, and BM aspiration and biopsy. At entry, a baseline MRD evaluation was performed on PB and BM samples (the latter only if available). Patients were also assessed for IGHV mutation status, TP53 mutations, and cytogenetic analysis by fluorescence in situ hybridization for 17p13 deletion, 11q22-23 deletion, trisomy 12, and 13q14 deletion before starting treatment. All patients were evaluated for the risk of tumor lysis syndrome (TLS) according to lymph node size on CT imaging and the absolute lymphocyte count (ALC) before starting venetoclax. TLS risk was defined according to venetoclax label as high (lymph nodes ≥10 cm or lymph nodes ≥5 cm and ALC ≥ 25 × 109/L), low (lymph nodes <5 cm and ALC < 25 × 109/L), or intermediate (not fulfilling high or low TLS risk criteria).
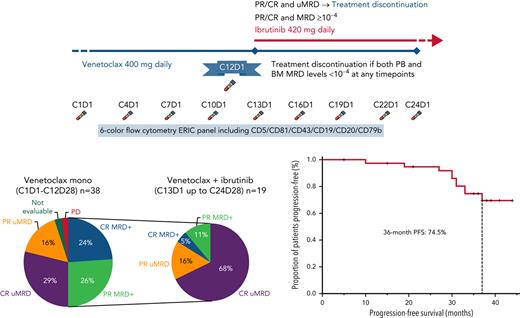
Venetoclax (alone or together with ibrutinib) was administered in 28-day (monthly) cycles from Cycle 1 Day 1 according to the standard 5-week ramp-up schedule (starting from 20 to 400 mg every day) as per label. The 5-week dose-titration phase was designed to gradually reduce tumor burden (debulk) and decrease the risk of TLS. At Cycle 12 Day 1, MRD was assessed in PB by using the European Research Initiative on CLL (ERIC) 6-color flow cytometry panel (CD5/CD81/CD79b/CD19/CD43/CD20).19 Undetectable MRD4 (uMRD4) was defined as <1 CLL cell of 10 000 leukocytes (10−4). Patients with uMRD4 in PB underwent BM evaluation, and those with confirmed uMRD4 in both PB and BM discontinued venetoclax at Cycle 12 Day 28. Patients with detectable MRD (MRD+) added ibrutinib 420 mg every day starting from Cycle 13 Day 1. Patients continued both drugs until Cycle 24 Day 28 or confirmed uMRD or disease progression or unacceptable toxicity, whichever occurred first. MRD status in PB was assessed every 3 cycles: if uMRD4 in PB, MRD was tested in BM and patients with uMRD4 in both PB and BM at any time point discontinued both venetoclax and ibrutinib. Patients MRD+ at Cycle 24 Day 1 discontinued venetoclax at Cycle 24 Day 28 and continued with ibrutinib alone until disease progression or unacceptable toxicity. Laboratory tests, physical examination, and MRD status were assessed every 3 months for 48 months since venetoclax initiation and then every 6 months. Responses with CT scanning (according to iwCLL criteria18) were assessed every 3 months until Cycle 24 Day 1, then every 12 months. Patients who experienced progressive disease requiring treatment after discontinuing venetoclax and ibrutinib were suitable candidates for re-treatment with venetoclax monotherapy according to protocol amendment 1.
Study objectives and end points
The primary objective of the study was to evaluate the efficacy of the addition of ibrutinib to venetoclax in terms of uMRD in patients with R/R CLL. MRD was evaluated in patients with CR or partial response (PR) using the standardized ERIC 6-color flow panel in both PB and BM.19 The primary end point of the study was defined as the proportion of patients achieving uMRD4 (<10−4) during the treatment period. Secondary objectives were to evaluate the efficacy of the ibrutinib and venetoclax combination in terms of overall response rate (ORR), PR rate, and CR rate (based on 2008 revised iwCLL criteria18), progression-free survival (PFS), duration of response, and overall survival (OS), as well as the safety of the combination of the 2 drugs (type, frequency, and severity of adverse events [AEs] and causal relationship). The safety and tolerability of study drug treatments were evaluated by means of AEs, including incidence and severity, as well as performance status, physical examinations, 12-lead electrocardiograms, and laboratory safety evaluations. Laboratory and AE toxicities were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03. The 2008 revised iwCLL criteria18 were used to evaluate hematologic toxicity.
Statistical analysis
The sample size was calculated according to optimal Simon Two-Stage phase 2 design, with a null hypothesis (p0) of uMRD4 CR at 12 months of 5%, and a p1 of uMRD4 CR of 30% in the study. The planned sample size of 29 patients provides 95% power to test a difference of 30% vs 5% in the primary end point, with a standard type I error (α) of 0.05. Considering that 5% of patients are expected to reach a uMRD4 CR with venetoclax monotherapy, and a dropout rate of 10%, the planned sample size was 35.
All primary analyses were based on the intention-to-treat population, consisting of all registered patients, with the exception of subjects who post hoc objectively did not meet the eligibility criteria at enrollment. Safety analysis was based on all enrolled patients who received at least 1 dose of study agent. Categorical variables are reported as numbers and percentages; continuous variables are described by means and range. Time to event variables, including PFS and OS, were calculated by using the Kaplan-Meier method. AEs, including toxicities, were analyzed by calculating the number and percentage of patients with event.
Results
Study population
Between November 2017 and July 2018, a total of 43 patients at 12 Italian participating sites signed the consent form and performed screening procedures. Four patients failed screening and one was excluded from the analysis because of atypical immunophenotype, leading to 38 evaluable patients. Median age was 64 years (range, 47-81 years); 25 (66%) were male; median number of prior treatments was 1 (range, 1-7); and 23 (61%) of 38 had previously received fludarabine, cyclophosphamide, and rituximab, 17 (45%) of 38 bendamustine + rituximab, and 5 (13%) of 38 phosphatidylinositol 3-kinase inhibitors (Table 1; supplemental Table 1). Of note, 27 (79%) of 34 patients had unmutated IGHV, 7 (21%) of 33 carried del(17p), and 9 (29%) of 31 carried the TP53 mutation. TLS risk at the time of venetoclax initiation was high in 14 (37%) of 38 patients, medium in 18 (47%), and low in 6 (16%).
Baseline characteristics (N = 38)
| Characteristic . | Value . |
|---|---|
| Median age, y (range) | 64 (47-81) |
| Male:female | 25:13 |
| Rai stage at screening | |
| 0 | 3 |
| I-II | 21 |
| III-IV | 9 |
| Missing | 5 |
| IGHV, n/N (%) | |
| Unmutated | 27/34 (79) |
| Mutated | 7/34 (21) |
| FISH (according to Döhner hierarchical model), n/N (%) | |
| del(17p) | 7/33 (21) |
| del(11q) | 9/32 (28) |
| Trisomy 12 | 0/33 (0) |
| Normal | 8/32 (25) |
| del(13q) | 9/33 (27) |
| TP53 mutation, n/N (%) | 9/31 (29) |
| Median no. of prior therapies (range) | 1 (1-7) |
| Type of prior therapies, n/N (%) | |
| Fludarabine, cyclophosphamide ± rituximab | 23/38 (60.5) |
| Bendamustine + rituximab | 17/38 (45) |
| Chlorambucil ± anti-CD20 monoclonal antibody | 7/38 8 (18) |
| Phosphatidylinositol 3-kinase inhibitor (idelalisib, duvelisib) | 5/38 (13) |
| TLS risk, n/N (%) | |
| Low | 6/38 (16) |
| Medium | 18/38 (47) |
| High | 14/38 (37) |
| Median ALC count (×109/L) at screening (range) | 97.6 (3.5-208.4) |
| ALC ≥ 25 × 109/L at screening, n/N (%) | 26/38 (68) |
| Lymph node size at screening | |
| ≥5 cm | 16 |
| ≥10 cm | 8 |
| Characteristic . | Value . |
|---|---|
| Median age, y (range) | 64 (47-81) |
| Male:female | 25:13 |
| Rai stage at screening | |
| 0 | 3 |
| I-II | 21 |
| III-IV | 9 |
| Missing | 5 |
| IGHV, n/N (%) | |
| Unmutated | 27/34 (79) |
| Mutated | 7/34 (21) |
| FISH (according to Döhner hierarchical model), n/N (%) | |
| del(17p) | 7/33 (21) |
| del(11q) | 9/32 (28) |
| Trisomy 12 | 0/33 (0) |
| Normal | 8/32 (25) |
| del(13q) | 9/33 (27) |
| TP53 mutation, n/N (%) | 9/31 (29) |
| Median no. of prior therapies (range) | 1 (1-7) |
| Type of prior therapies, n/N (%) | |
| Fludarabine, cyclophosphamide ± rituximab | 23/38 (60.5) |
| Bendamustine + rituximab | 17/38 (45) |
| Chlorambucil ± anti-CD20 monoclonal antibody | 7/38 8 (18) |
| Phosphatidylinositol 3-kinase inhibitor (idelalisib, duvelisib) | 5/38 (13) |
| TLS risk, n/N (%) | |
| Low | 6/38 (16) |
| Medium | 18/38 (47) |
| High | 14/38 (37) |
| Median ALC count (×109/L) at screening (range) | 97.6 (3.5-208.4) |
| ALC ≥ 25 × 109/L at screening, n/N (%) | 26/38 (68) |
| Lymph node size at screening | |
| ≥5 cm | 16 |
| ≥10 cm | 8 |
FISH, fluorescence in situ hybridization.
Efficacy
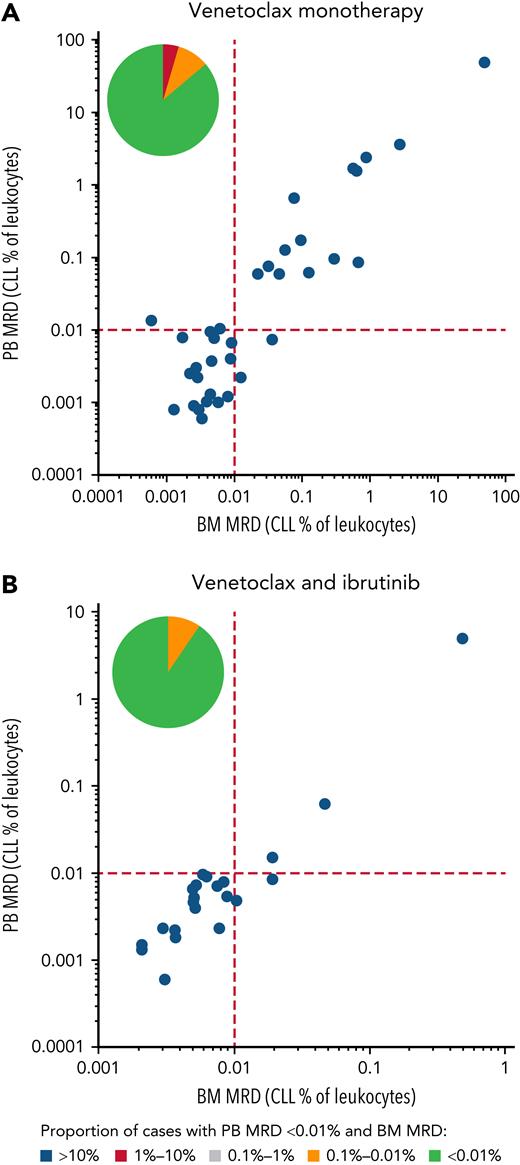
Thirty-six of 38 patients completed 12 months of venetoclax treatment and were assessed for response and MRD at Cycle 12 Day 1. Before Cycle 12 Day 1, one patient (previously treated with 4 earlier lines of chemoimmunotherapy, including autologous stem cell transplantation) discontinued treatment due to an AE (myelodysplasia, grade 4, deemed unrelated to venetoclax) and one patient progressed on venetoclax monotherapy, and thus were not assessed for MRD. Nineteen (53%) of 36 evaluable patients achieved uMRD4 in PB at Cycle 12 Day 1, and 17 of 19 (45% of the whole population) also had uMRD4 in BM (concordance, 89%) (Figure 1A). The ORR at this time point was 95% (36 of 38; 95% confidence interval [CI], 83-99), with 20 (53%) CRs and 16 (42%) PRs. uMRD4 CR in those receiving venetoclax monotherapy were 65% (11 of 17; 95% CI, 41-83). Sixteen of 17 uMRD4 patients discontinued venetoclax as planned (Figure 2A). The median time to uMRD4 in the 17 patients who achieved uMRD4 with venetoclax monotherapy was 5 months (range, 5-10 months) for PB and 10 months (range, 10-11 months) for PB and BM. One patient erroneously received combination treatment with ibrutinib and venetoclax for 6 months although displaying uMRD4 in both PB and BM at Cycle 12 Day 1 assessment, and was excluded from this analysis. All remaining 19 MRD+ patients continued venetoclax and added ibrutinib.
PB and BM MRD assessments. On the x-axis, the MRD assessment in the BM is given, on the y-axis the MRD value in the PB is given; both are expressed as percentage of leukocytes. (A) Patients treated with venetoclax only. (B) Patients treated with venetoclax monotherapy followed by venetoclax combined with ibrutinib until uMRD4, progressive disease, unacceptable toxicity, or Cycle 24 Day 28, whichever occurs first. The pie chart in each graph illustrates the percentage of PB uMRD4 with concordant (in green) or discordant (in yellow and red) BM MRD levels.
PB and BM MRD assessments. On the x-axis, the MRD assessment in the BM is given, on the y-axis the MRD value in the PB is given; both are expressed as percentage of leukocytes. (A) Patients treated with venetoclax only. (B) Patients treated with venetoclax monotherapy followed by venetoclax combined with ibrutinib until uMRD4, progressive disease, unacceptable toxicity, or Cycle 24 Day 28, whichever occurs first. The pie chart in each graph illustrates the percentage of PB uMRD4 with concordant (in green) or discordant (in yellow and red) BM MRD levels.
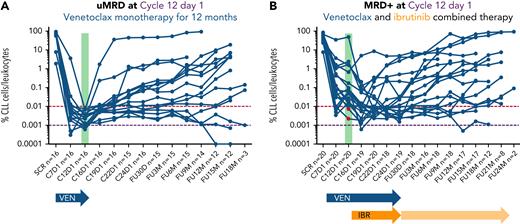
PB MRD assessments over time. On the x-axis, the different time points when MRD was assessed as per protocol are shown; on the y-axis, the MRD value expressed as percentage of leukocytes is shown. (A) Patients treated with venetoclax only. (B) Patients treated with venetoclax monotherapy followed by venetoclax combined with ibrutinib until uMRD4, progressive disease, unacceptable toxicity, or Cycle 24 Day 28, whichever occurs first. Two patients (highlighted in red) had uMRD4 in PB at Cycle 12 Day 1 assessment but were MRD+ in the BM, thus fulfilling per protocol MRD+ criteria. IBR, ibrutinib; VEN, venetoclax.
PB MRD assessments over time. On the x-axis, the different time points when MRD was assessed as per protocol are shown; on the y-axis, the MRD value expressed as percentage of leukocytes is shown. (A) Patients treated with venetoclax only. (B) Patients treated with venetoclax monotherapy followed by venetoclax combined with ibrutinib until uMRD4, progressive disease, unacceptable toxicity, or Cycle 24 Day 28, whichever occurs first. Two patients (highlighted in red) had uMRD4 in PB at Cycle 12 Day 1 assessment but were MRD+ in the BM, thus fulfilling per protocol MRD+ criteria. IBR, ibrutinib; VEN, venetoclax.
The 19 MRD+ patients continued on to the combined treatment with venetoclax and ibrutinib. With a median duration of combined treatment of 7 months (range, 3-10 months), the uMRD4 rate improved over time (Figure 2B). Sixteen of 19 patients obtained uMRD4 in both PB and BM (84%; 95% CI, 62-94; concordance, 90%) as best response over combined treatment (from Cycle 16 Day 1 to Cycle 24 Day 1), including 2 patients with uMRD4 only in PB at the time of ibrutinib initiation (Figure 1B). The ORR was 100% (14 CR and 5 PR); median time to best response was 10 months since Cycle 1 Day 1 (range, 10-21 months). uMRD4 CR with the combination of venetoclax and ibrutinib was obtained in 13 (68%) of 19 patients. At Cycle 24 Day 28, three subjects had never achieved uMRD4 in PB; 2 of them, per protocol, continued ibrutinib monotherapy after discontinuing venetoclax while 1 subject still MRD+ had previously discontinued both ibrutinib and venetoclax after 6 months of combined treatment due to an unrelated AE and progressed 12 months after treatment interruption (Figure 3).
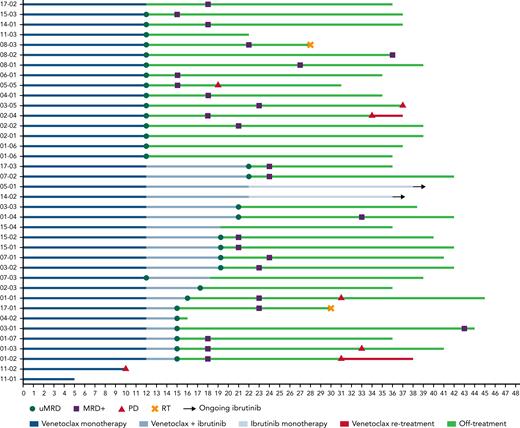
Response to treatment and patient outcome. The swimmer’s plot is showing MRD assessment and treatment outcome of all 38 eligible patients enrolled into the study. PD, progressive disease; RT, Richter transformation.
Response to treatment and patient outcome. The swimmer’s plot is showing MRD assessment and treatment outcome of all 38 eligible patients enrolled into the study. PD, progressive disease; RT, Richter transformation.
After a median follow-up of 36.5 months (range, 5-44 months), the median PFS was not reached, and the estimated 36-month PFS was 74.5% (95% CI, 53-86) (Figure 4). Ten patients progressed: 2 developed Richter transformation (RT), both 16 months after stopping venetoclax (1) or venetoclax + ibrutinib (1) treatment, 1 progressed during venetoclax monotherapy, and all 3 went off-study. Of the remaining 7 patients (6 of 7 uMRD4 at the time of therapy discontinuation), 4 experienced CLL progression requiring treatment after combination treatment discontinuation (2 of them carrying TP53 aberrations), 3 experienced progressive disease without treatment need. The median time to progression was 30.5 months (range, 10-37 months). Among the 4 patients who required a subsequent treatment, at the time of data-cut, 1 patient dropped out, 1 patient had not started treatment yet, and 2 patients started retreatment with venetoclax (1 patient [time to next treatment, 35 months] for 3 months, achieving a MRD+ PR, and 1 patient [time to next treatment, 32 months] achieved a MRD+ PR, then progressed after 7 months).
Thirty-two of 33 uMRD4 patients who discontinued treatment had MRD status assessed every 3 months up to 3 years and then every 6 months. Twenty-five (78%) of 32 experienced MRD relapse but only 8 of 25 patients later developed clinical progression (2 with RT, 2 patients without need for treatment, 3 patients who underwent venetoclax re-treatment, and 1 dropout). The median time to MRD relapse was 7 months (95% CI, 3.6-10.4), and the median time from MRD relapse to clinical progression was not reached (ranging from 5 months to not reached). No statistically significant difference in time to MRD relapse was identified in patients who achieved uMRD4 with venetoclax monotherapy compared with those who reached uMRD4 by combining ibrutinib with venetoclax, although with the caveat of small numbers.
Safety
Venetoclax monotherapy and the combination of venetoclax and ibrutinib were well tolerated. At the time of data lock (September 19, 2021), 7 serious AEs, 25 grade 3 to 4 AEs, and 160 AEs were reported in 32 patients; 6 patients did not experience any AEs. The most frequent AEs of any grade included: neutropenia (any grade, 19; grade 3-4, 17), cough (any grade, 14; grade 3-4, none), fever (any grade, 13; grade 3-4, none), diarrhea (any grade, 10; grade 3-4, none), upper respiratory tract infection (any grade, 9; grade 3-4, none), and bronchitis (any grade, 7; grade 3-4, 2) (Table 2), with no grade 5 events. AEs higher than grade 3 to 4 were rare, with only 25 events (mostly neutropenia), and only neutropenia and bronchitis occurring in ≥2 patients. No laboratory or clinical TLS was observed. No patients discontinued treatment with ibrutinib and/or venetoclax due to treatment-related toxicities (2 patients discontinued due to AEs not related to study drugs). Eight patients had temporary interruption of any of the 2 drugs due to 11 AEs. The mean treatment interruption for venetoclax was 9 days (range, 2-26 days), while only 1 patient interrupted ibrutinib for 4 days.
AEs in 32 of 39 patients enrolled into the study
| AE . | Any grade . | Grade 3 to 4 . | Missing . | ||
|---|---|---|---|---|---|
| No. . | % . | No. . | % . | ||
| Neutropenia | 19 | 48.7 | 17 | 43.6 | 0 |
| Cough | 14 | 35.9 | 0 | 0.0 | 3 |
| Fever | 13 | 33.3 | 0 | 0.0 | 1 |
| Diarrhea | 10 | 25.6 | 0 | 0.0 | 0 |
| Upper respiratory tract infection | 9 | 23.1 | 0 | 0.0 | 1 |
| Bronchitis | 7 | 17.9 | 2 | 5.1 | 1 |
| Herpes zoster | 5 | 12.8 | 1 | 2.6 | 0 |
| Rash | 5 | 12.8 | 0 | 0.0 | 1 |
| Bleeding | 5 | 12.8 | 0 | 0.0 | 0 |
| Asthenia | 4 | 10.3 | 0 | 0.0 | 0 |
| Hypertension | 4 | 10.3 | 0 | 0.0 | 0 |
| Nausea | 4 | 10.3 | 0 | 0.0 | 0 |
| Non-melanoma skin cancer | 4 | 10.3 | 0 | 0.0 | 1 |
| Pneumonia | 3 | 7.7 | 1 | 2.6 | 1 |
| Epigastric pain | 3 | 7.7 | 0 | 0.0 | 0 |
| Fatigue | 3 | 7.7 | 0 | 0.0 | 0 |
| Hypocalcemia | 3 | 7.7 | 0 | 0.0 | 0 |
| Urinary disorders | 3 | 7.7 | 0 | 0.0 | 1 |
| Thrombocytopenia | 2 | 5.1 | 1 | 2.6 | 0 |
| AST/ALT elevation | 2 | 5.1 | 0 | 0.0 | 0 |
| Dizziness | 2 | 5.1 | 0 | 0.0 | 0 |
| Genital tract infection | 2 | 5.1 | 0 | 0.0 | 0 |
| Hyperkeratosis | 2 | 5.1 | 0 | 0.0 | 0 |
| Presyncope | 2 | 5.1 | 0 | 0.0 | 0 |
| Urinary tract infection | 2 | 5.1 | 0 | 0.0 | 0 |
| Vomiting | 2 | 5.1 | 0 | 0.0 | 1 |
| Allergic reaction | 2 | 5.1 | 0 | 0.0 | 1 |
| COVID-19 pneumonia | 1 | 2.6 | 1 | 2.6 | 0 |
| Febrile neutropenia | 1 | 2.6 | 1 | 2.6 | 0 |
| Myelodysplastic syndrome | 1 | 2.6 | 1 | 2.6 | 0 |
| Arthralgia | 1 | 2.6 | 0 | 0.0 | 0 |
| Atrial fibrillation | 1 | 2.6 | 0 | 0.0 | 0 |
| Biliary colic | 1 | 2.6 | 0 | 0.0 | 0 |
| Bone pain | 1 | 2.6 | 0 | 0.0 | 0 |
| Cholecystitis | 1 | 2.6 | 0 | 0.0 | 0 |
| Clotting | 1 | 2.6 | 0 | 0.0 | 0 |
| Ear pain | 1 | 2.6 | 0 | 0.0 | 1 |
| Eye infection | 1 | 2.6 | 0 | 0.0 | 0 |
| Gastrointestinal infection | 1 | 2.6 | 0 | 0.0 | 0 |
| Hyperphosphatemia | 1 | 2.6 | 0 | 0.0 | 0 |
| Inguinal hernia | 1 | 2.6 | 0 | 0.0 | 0 |
| Melanoma | 1 | 2.6 | 0 | 0.0 | 1 |
| Noncardiac chest pain | 1 | 2.6 | 0 | 0.0 | 0 |
| Oral mucositis | 1 | 2.6 | 0 | 0.0 | 0 |
| Other infections | 1 | 2.6 | 0 | 0.0 | 0 |
| Palpitations | 1 | 2.6 | 0 | 0.0 | 0 |
| Paresthesia | 1 | 2.6 | 0 | 0.0 | 1 |
| Pleural effusion | 1 | 2.6 | 0 | 0.0 | 0 |
| Shoulder dislocation | 1 | 2.6 | 0 | 0.0 | 0 |
| Sore throat | 1 | 2.6 | 0 | 0.0 | 0 |
| Testicular disorders | 1 | 2.6 | 0 | 0.0 | 1 |
| Total | 160 | 25 | 16 | ||
| AE . | Any grade . | Grade 3 to 4 . | Missing . | ||
|---|---|---|---|---|---|
| No. . | % . | No. . | % . | ||
| Neutropenia | 19 | 48.7 | 17 | 43.6 | 0 |
| Cough | 14 | 35.9 | 0 | 0.0 | 3 |
| Fever | 13 | 33.3 | 0 | 0.0 | 1 |
| Diarrhea | 10 | 25.6 | 0 | 0.0 | 0 |
| Upper respiratory tract infection | 9 | 23.1 | 0 | 0.0 | 1 |
| Bronchitis | 7 | 17.9 | 2 | 5.1 | 1 |
| Herpes zoster | 5 | 12.8 | 1 | 2.6 | 0 |
| Rash | 5 | 12.8 | 0 | 0.0 | 1 |
| Bleeding | 5 | 12.8 | 0 | 0.0 | 0 |
| Asthenia | 4 | 10.3 | 0 | 0.0 | 0 |
| Hypertension | 4 | 10.3 | 0 | 0.0 | 0 |
| Nausea | 4 | 10.3 | 0 | 0.0 | 0 |
| Non-melanoma skin cancer | 4 | 10.3 | 0 | 0.0 | 1 |
| Pneumonia | 3 | 7.7 | 1 | 2.6 | 1 |
| Epigastric pain | 3 | 7.7 | 0 | 0.0 | 0 |
| Fatigue | 3 | 7.7 | 0 | 0.0 | 0 |
| Hypocalcemia | 3 | 7.7 | 0 | 0.0 | 0 |
| Urinary disorders | 3 | 7.7 | 0 | 0.0 | 1 |
| Thrombocytopenia | 2 | 5.1 | 1 | 2.6 | 0 |
| AST/ALT elevation | 2 | 5.1 | 0 | 0.0 | 0 |
| Dizziness | 2 | 5.1 | 0 | 0.0 | 0 |
| Genital tract infection | 2 | 5.1 | 0 | 0.0 | 0 |
| Hyperkeratosis | 2 | 5.1 | 0 | 0.0 | 0 |
| Presyncope | 2 | 5.1 | 0 | 0.0 | 0 |
| Urinary tract infection | 2 | 5.1 | 0 | 0.0 | 0 |
| Vomiting | 2 | 5.1 | 0 | 0.0 | 1 |
| Allergic reaction | 2 | 5.1 | 0 | 0.0 | 1 |
| COVID-19 pneumonia | 1 | 2.6 | 1 | 2.6 | 0 |
| Febrile neutropenia | 1 | 2.6 | 1 | 2.6 | 0 |
| Myelodysplastic syndrome | 1 | 2.6 | 1 | 2.6 | 0 |
| Arthralgia | 1 | 2.6 | 0 | 0.0 | 0 |
| Atrial fibrillation | 1 | 2.6 | 0 | 0.0 | 0 |
| Biliary colic | 1 | 2.6 | 0 | 0.0 | 0 |
| Bone pain | 1 | 2.6 | 0 | 0.0 | 0 |
| Cholecystitis | 1 | 2.6 | 0 | 0.0 | 0 |
| Clotting | 1 | 2.6 | 0 | 0.0 | 0 |
| Ear pain | 1 | 2.6 | 0 | 0.0 | 1 |
| Eye infection | 1 | 2.6 | 0 | 0.0 | 0 |
| Gastrointestinal infection | 1 | 2.6 | 0 | 0.0 | 0 |
| Hyperphosphatemia | 1 | 2.6 | 0 | 0.0 | 0 |
| Inguinal hernia | 1 | 2.6 | 0 | 0.0 | 0 |
| Melanoma | 1 | 2.6 | 0 | 0.0 | 1 |
| Noncardiac chest pain | 1 | 2.6 | 0 | 0.0 | 0 |
| Oral mucositis | 1 | 2.6 | 0 | 0.0 | 0 |
| Other infections | 1 | 2.6 | 0 | 0.0 | 0 |
| Palpitations | 1 | 2.6 | 0 | 0.0 | 0 |
| Paresthesia | 1 | 2.6 | 0 | 0.0 | 1 |
| Pleural effusion | 1 | 2.6 | 0 | 0.0 | 0 |
| Shoulder dislocation | 1 | 2.6 | 0 | 0.0 | 0 |
| Sore throat | 1 | 2.6 | 0 | 0.0 | 0 |
| Testicular disorders | 1 | 2.6 | 0 | 0.0 | 1 |
| Total | 160 | 25 | 16 | ||
ALT/AST, alanine aminotransferase/aspartate aminotransferase.
Discussion
To the best of our knowledge, this is one of the few studies, together with the recently published HOVON141/VISION (A Prospective, Multicenter, Phase-II Trial of Ibrutinib Plus Venetoclax in Patients With Creatinine Clearance ≥30 mL/min Who Have R/R CLL With or Without TP53 Aberrations),20 designed with a MRD-driven strategy to intensify treatment and define treatment duration in patients with R/R CLL. Based on the results presented here, this innovative strategy proved to be feasible and effective, and has the potential of avoiding unnecessary drug exposure in previously treated patients with CLL.
Recent studies explored the combination of BTK inhibitors and venetoclax ± anti-CD20 monoclonal antibodies both in first-line and in R/R CLL.13,14 All these studies are based on a similar fixed duration approach; that is, all patients receive the same combination and are then elected to stop therapy after a fixed time. Here, we adopted a tailored approach, in which venetoclax monotherapy was initiated and, based on MRD response, discontinued or intensified by adding ibrutinib. Following this design, we were able to obtain a 95% ORR, with 87% uMRD4, of which 45% achieved uMRD4 already after venetoclax monotherapy. This impressive efficacy has been obtained in a population highly enriched of unfavorable disease features, including 79% of patients with unmutated IGHV and 29% with TP53 aberrations. The results of our study look even better if we consider recent data on responses achieved with a triplet regimen (obinutuzumab, venetoclax, and ibrutinib) in treatment-naive patients with high-risk features. In a selected population of treatment-naive patients with CLL carrying TP53 abnormalities, Huber et al21 showed that the uMRD rate after 14 months of combined treatment with obinutuzumab, venetoclax, and ibrutinib was 78% and 65.9% in PB and BM, respectively, with a median PFS of 33.5 months. This outcome thus questions the real added value of anti-CD20 monotherapy, particularly in TP53-aberrant patients. Interestingly, 7 patients (3 treated only with venetoclax monotherapy) in our study remain in uMRD4 after a median follow-up of 36.5 months, suggesting that even among R/R patients, some may still benefit from limited-intensity treatment. Conversely, only 2 of 38 patients had to continue ibrutinib single agent because of MRD persistence, thus identifying those patients who need continuous treatment for a long-term disease control.
The current study also has the merit of reporting prospective data on the efficacy of venetoclax alone in R/R CLL, as very limited data exist on MRD efficacy with venetoclax 400 mg alone in R/R CLL, with a pooled analysis of phase 1 to 2 studies documenting an ORR of 73.5% and uMRD (not routinely tested) of 27.1% in PB and 10.7% in BM.22 At variance, in our study, we report prospective and systematic MRD analyses on a homogeneous venetoclax-treated cohort, less heavily pretreated than the one in the early studies and thus more in line with the patient population currently encountered in clinical practice.
The IMPROVE results also compare very favorably with those of the MURANO study (A Study to Evaluate the Benefit of Venetoclax Plus Rituximab Compared With Bendamustine Plus Rituximab in Participants With R/R CLL),23 in which patients with R/R CLL were treated with venetoclax + rituximab for 24 months, showing 62% uMRD in PB after the first year of treatment and remaining unchanged in the second year of fixed-duration therapy. This evidence suggests a limited value of an additional second year of treatment with venetoclax, at least in the R/R setting. This seems apparent also in our study in which >50% of patients not achieving uMRD4 after 12 months of venetoclax monotherapy (Figure 2B) had stable if not increasing MRD levels right before the addition of ibrutinib that associated with a sharp change in the direction of the MRD curve. Thus, these data offer reassurance regarding the appropriateness of the timing of treatment intensification in our study: the addition of ibrutinib to venetoclax in the second year leads to uMRD4 in the BM in almost all patients (87%) with a median duration of combined treatment of only 7 months, again comparing well with 53% uMRD4 in the CLARITY study (Assessment of VenetoCLAx (ABT-199) in combination with IbRutInib in relapsed/refracTory Chronic LymphocYtic Leukaemia)14 after a full year of the 2-drug regimen in R/R CLL. The strategy of discontinuing treatment upon uMRD4 adopted in our study was also supported by the recent results of the HOVON141/VISION in which no benefit in terms of PFS was documented for patients continuing ibrutinib therapy after achieving uMRD4 with the combination of ibrutinib and venetoclax if compared with treatment cessation and MRD monitoring.20 It should be noted that in the HOVON study, retreatment with ibrutinib was guided by MRD relapse, whereas in our study retreatment with venetoclax relies on clinical progression. Although it is still too early to provide valid data on venetoclax retreatment in our patient cohort, considering the small number of patients requiring treatment and the short follow-up after progression, the recent update of the MURANO study is lending support to retreatment with venetoclax-based regimens after initial response to fixed-duration combinations showing a best ORR of 72.2%.24
In our study, the limited exposure to study drugs translated into a very low rate of AEs, with few patients experiencing grade 3/4 AEs, which were mainly neutropenia, as expected given the backbone treatment with venetoclax. Interestingly, no patients experienced clinical or laboratory TLS. This is particularly notable for 2 reasons; first, 37% of patients were considered at high risk before treatment initiation; and second, the IMPROVE study differs from all other published works on venetoclax in combination with BTK inhibitors, as it explores the addition of ibrutinib after venetoclax (while the former drug is typically administered before the BCL2 inhibitor as a debulking strategy in other published works to decrease the TLS risk).13,16 It is not surprising, although reassuring, that by complying with the well-established ramp-up and electrolyte testing procedures, it has been possible to safely administer the drug in a multicenter setting.
In our study, median PFS is not yet reached and 36-month PFS was 74.5%, comparing favorably to the 36-month PFS of 71.4% reported for the combination of venetoclax + rituximab23 in the MURANO study. This is even more relevant when we consider that the 28 patients who obtained uMRD4 at any time point and stopped treatment remained treatment-free for a median of 23 months (range, 1-29 months) with a median treatment duration of only 14 months (range, 11-22 months) instead of the 2 years of the venetoclax + rituximab combination. In our study, MRD became detectable again after a median of 7 months in 25 patients, but only 4 experienced CLL progression requiring treatment and 2 additional patients actually developed RT, unfortunately an expected outcome in R/R CLL. The PFS benefit (15-month PFS of 97.3%) in our patient cohort compares favorably also with the phase 1 study of venetoclax monotherapy with a 15-month PFS of 69% for patients on the 400-mg dose, where more heavily pretreated patients were enrolled, thus supporting the use of venetoclax-based combination in earlier treatment lines.4
Focusing on depth of response with the combination of ibrutinib and venetoclax in the R/R patients, our results show a 84% BM uMRD rate compared with 36% to 67% in other studies exploring the 2-drug combination.14,17 Only 2 of 38 patients progressed with treatment need by month 21 (21-month PFS, 94.5%), in line with the experience in the CLARITY study (only 1 progressive case after a median follow-up of 21.1 months).14
Characteristics of patients experiencing disease progression with treatment need after discontinuing study treatment (venetoclax ± ibrutinib) included the presence of TP53 aberrations at the time of enrollment (in 50% of them). A more thorough characterization at the time of progression is needed.
Our approach also has the great advantage of the full oral administration and a duration tailored according to the MRD status. In the end, this approach led to a median treatment duration of 14 months with the obvious advantages in terms of clinical and financial toxicity.
The IMPROVE study can be considered a proof-of-principle that an intra-patient strategy of treatment intensification is feasible when using MRD assessment as a biomarker of efficacy. We are aware of the limitations of our study, including the phase 2 design without a standard arm comparator, and the limited sample size. It is also reasonable to speculate that our results may benefit from the addition of anti-CD20 monoclonal antibodies when initiating venetoclax, although again this could be incorporated in the progressive escalation of therapy (eg, in patients not achieving uMRD4 after 5 months of monotherapy, which is the median time needed in our cohort to achieve eradication of the disease). The role of obinutuzumab in the R/R setting is also worth further exploration.
In conclusion, this MRD-driven strategy allowed identical depth of response to be reached in each patient with an individualized time-limited approach, preventing overtreatment in patients who achieve uMRD with monotherapy, and ultimately identifying the few patients who may benefit from continuous treatment. Time to clinical progression, response to venetoclax retreatment, and disease characteristics at the time of clinical relapse as well as in those with persistent MRD remain to be established with longer follow-up. This MRD-driven intrapatient treatment intensification could lead to substantial improvements for patients avoiding unnecessary drug exposure and for the health systems promoting a real evidence-based allocation of resources and with the potential of being quickly transferred in the first line setting.
Acknowledgments
This work was supported by AbbVie (donation of Venetoclax as well as financial support).
Authorship
Contribution: P.G. was responsible for conception and design; P.G., L. Scarfò, E.A., L. Schiattone, A.C., L.F., R.M., M.D., A.F., M.M., G.R., R.S., M.C., P.R., L.L., and M.V. provided study materials or patients; S.H., E.A., L. Scarfò, E.S., L. Schiattone, E.P., A.C., P.R., and M.C collected and assembled the data; all authors contributed to data analysis and interpretation, as well as writing of the manuscript; and all authors gave final approval of the manuscript and agree to be accountable for all aspects of the work.
Conflict-of-interest disclosure: P.G. received honoraria from AbbVie, ArQule/MSD, AstraZeneca, Celgene/Juno/BMS, Janssen, Loxo/Lilly, Roche; and research support from AbbVie, AstraZeneca, Janssen, Gilead, and Sunesis. L. Scarfò received honoraria from AbbVie, AstraZeneca, and Janssen outside the submitted work. L.F. received honoraria from AbbVie, AstraZeneca, and Janssen outside the submitted work. The remaining authors declare no competing financial interests.
The current affiliation for L. Schiattone is UOSD Centro Diagnosi e Terapia dei Linfomi, Presidio Ospedaliero Santo Spirito, Pescara, Italy.
Correspondence: Paolo Ghia, Strategic Research Program on Chronic Lymphocytic Leukemia (CLL), Università Vita Salute and Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ospedale San Raffaele, Via Olgettina 60, 20132 Milano, Italy; e-mail: ghia.paolo@hsr.it.
References
Author notes
∗L. Scarfò and S.H. are joint first authors.
Individual participant data that underlie the results reported in this article after de-identification, the study protocol, and related documents are available upon request to the corresponding author (ghia.paolo@hsr.it).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal