In this issue of Blood, complementary manuscripts by Thomalla et al1 and Thijssen et al2 expand our understanding of the adaptive mechanisms associated with resistance to venetoclax (see figure). Venetoclax blocks the ability of BCL2 to inhibit proapoptotic proteins such as BAX, leading in turn to permeabilization of the mitochondrial outer membrane and committing the cell to apoptosis.3,4 Like BCL2, the antiapoptotic protein MCL1 also interacts with proapoptotic BAX proteins to block their function, but MCL1 is not affected by venetoclax. PUMA and NOXA interact with BCL2, thus freeing BAX, which is then able to signal apoptosis to the mitochondria.5
The mechanisms that block or blunt the activity of venetoclax are unexpectedly heterogeneous.6-11 Recurrent mutations in BCL2 lead to resistance due to decreased affinity of BCL2 for venetoclax.6-8 Upon treatment with venetoclax, BAX mutation can occur in the myeloid compartment.9BAX mutations abrogate the outer mitochondrial membrane localization of BAX, thus keeping it in its inactive form, and are associated with the development of lineage-specific clonal hematopoiesis. Beside mutations, upregulation of MCL1 due to amplification of chromosome 1 is associated with resistance to venetoclax.10,11
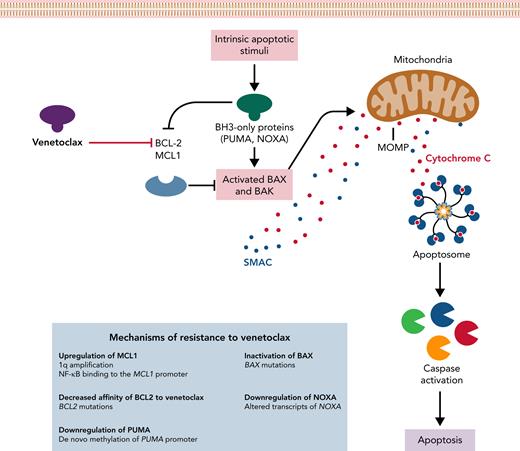
Mechanisms of resistance to venetoclax. The intrinsic apoptotic pathway can be engaged by stimuli that regulate BCL-2 and MCL1 interactions with BH3-only proteins (ie, PUMA and NOXA), modulating the activation of the effector proteins BAX and BAK. Once activated, BAX and BAK cause MOMP, leading to the release of proapoptotic proteins. Cytochrome C leads to apoptosome formation that recruits and activates caspase. Mechanisms of resistance to venetoclax described in the papers by Thijssen et al and Thomalla et al are shown in the blue box. MOMP, mitochondrial outer membrane permeabilization; SMAC, second mitochondria-derived activator of caspase.
Mechanisms of resistance to venetoclax. The intrinsic apoptotic pathway can be engaged by stimuli that regulate BCL-2 and MCL1 interactions with BH3-only proteins (ie, PUMA and NOXA), modulating the activation of the effector proteins BAX and BAK. Once activated, BAX and BAK cause MOMP, leading to the release of proapoptotic proteins. Cytochrome C leads to apoptosome formation that recruits and activates caspase. Mechanisms of resistance to venetoclax described in the papers by Thijssen et al and Thomalla et al are shown in the blue box. MOMP, mitochondrial outer membrane permeabilization; SMAC, second mitochondria-derived activator of caspase.
Using functional genomic screens followed by validation in primary tumor samples, Thomalla et al identify an epigenetic mechanism of adaptation of tumor cells to venetoclax. The authors show by multiple convincing experiments in cell lines and primary samples that resistance toward venetoclax is mediated by de novo methylation of the PUMA promoter and subsequent downregulation of PUMA expression.
By using single-cell approaches, Thijssen et al clearly show that MCL1 overexpression dominates in venetoclax-resistant clones. However, MCL1 gene amplification accounted for increased MCL1 expression in only a limited number of tumors that became resistant to venetoclax. Conversely, Thijssen et al show that it was more common that marked NF-κB activation and NF-κB binding to the MCL1 promoter resulting in increased MCL1 expression resulted in relapses occurring on venetoclax therapy.
The high granularity of the approach used by Thijssen et al also demonstrated large intra- and inter-patient heterogeneity. Multiple mechanisms of escape (eg, 1q24 amplification, BCL2 mutations, BAX mutations, and altered transcript of proapoptotic NOXA) may coexist in the same tumor, usually restricted to different subclones. While some of these escape mechanisms are unique, others are found in multiple patients, indicating that tumor cells intrinsically adapt in multiple ways to selection pressure on cell survival exerted by venetoclax treatment.
The articles by Thijssen et al and Thomalla et al provide two translational implications. Epigenetic changes of resistance (ie, NF-κB–promoted upregulation of MCL1) are driven and sustained by ongoing venetoclax therapy. Indeed, they disappear once venetoclax therapy is stopped. Hence, limited duration venetoclax therapy is an appealing strategy to prevent the emergence of resistance. Mechanisms of resistance to venetoclax use the regulators of the intrinsic apoptotic pathway. Hence, therapeutic approaches that induce apoptosis through the extrinsic pathway (ie, tumor necrosis factor–related apoptosis-inducing ligand TRAIL-mediated apoptosis) can be effective in resistant cells.
Conflict-of-interest disclosure: A.C. received honoraria from AbbVie and AstraZeneca and research grants from Gilead and Pfizer. D.R. received honoraria from AbbVie, AstraZeneca, BeiGene, and Janssen and research grants from AbbVie, AstraZeneca, and Janssen.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal