Key Points
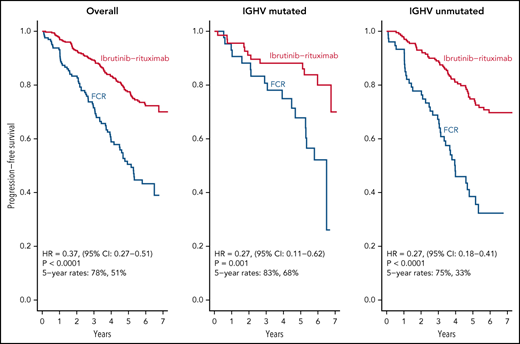
After a median follow-up of 6 years, IR led to superior PFS relative to FCR in patients with both IGHV muted and IGHV unmutated CLL.
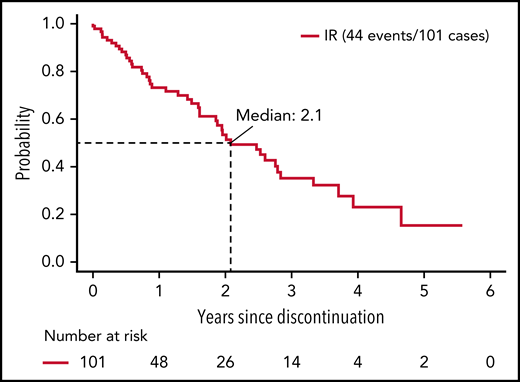
Among ibrutinib-treated patients who discontinued treatment for a reason other than progression, median PFS was 25 months.
Abstract
Herein, we present the long-term follow-up of the randomized E1912 trial comparing the long-term efficacy of ibrutinib–rituximab (IR) therapy to fludarabine, cyclophosphamide, and rituximab (FCR) and describe the tolerability of continuous ibrutinib. The E1912 trial enrolled 529 treatment-naïve patients aged ≤70 years with chronic lymphocytic leukemia (CLL). Patients were randomly assigned (2:1 ratio) to receive IR or 6 cycles of FCR. With a median follow-up of 5.8 years, median progression-free survival (PFS) is superior for IR (hazard ratio [HR], 0.37; P < .001). IR improved PFS relative to FCR in patients with both immunoglobulin heavy chain variable region (IGHV) gene mutated CLL (HR: 0.27; P < .001) and IGHV unmutated CLL (HR: 0.27; P < .001). Among the 354 patients randomized to IR, 214 (60.5%) currently remain on ibrutinib. Among the 138 IR-treated patients who discontinued treatment, 37 (10.5% of patients who started IR) discontinued therapy due to disease progression or death, 77 (21.9% of patients who started IR) discontinued therapy for adverse events (AEs)/complications, and 24 (6.8% of patients who started IR) withdrew for other reasons. Progression was uncommon among patients able to remain on ibrutinib. The median time from ibrutinib discontinuation to disease progression or death among those who discontinued treatment for a reason other than progression was 25 months. Sustained improvement in overall survival (OS) was observed for patients in the IR arm (HR, 0.47; P = .018). In conclusion, IR therapy offers superior PFS relative to FCR in patients with IGHV mutated or unmutated CLL, as well as superior OS. Continuous ibrutinib therapy is tolerated beyond 5 years in the majority of CLL patients. This trial was registered at www.clinicaltrials.gov as #NCT02048813.
Introduction
The last decade has been a time of incredible progress in the treatment of patients with chronic lymphocytic leukemia (CLL). During this interval, oral inhibitors of Bruton's tyrosine kinase (BTKi) and the antiapoptotic protein BCL-2 were developed and found to have robust efficacy in heavily pretreated relapsed or refractory patients.1,2 Ibrutinib was the first in class BTKi while venetoclax was the first in class BCL2 inhibitor used in these studies. After subsequent pilot testing as first-line therapies,3-5 phase 3 trials comparing these agents alone or in combination with anti-CD20 monoclonal antibodies were conducted. Between 2018 and 2020, 5 phase 3 trials comparing these approaches to standard chemoimmunotherapy (CIT) approaches were reported and led to a paradigm shift in first-line treatment.6-9 Venetoclax in combination with obinutuzumab therapy proved superior to chlorambucil in combination with obinutuzumab9 while BTKi-based therapy proved superior to chlorambucil,4 chlorambucil–obinutuzumab,8,10 bendamustine–rituximab,6 and the prior “gold standard” CIT approach, fludarabine, cyclophosphamide, and rituximab (FCR).7
The E1912 trial was the first study to compare ibrutinib-based therapy to FCR, the previous gold standard first-line therapy for patients fit enough to tolerate this approach.11-13 At the time of the initial E1912 report, with a median follow-up of 34 months, ibrutinib with rituximab (IR) demonstrated superior progression-free survival (PFS) and overall survival (OS) relative to that seen with FCR. Although statistically significant, the OS advantage was small and appeared to primarily be due to early deaths in the FCR arm. On subgroup analysis of PFS, ibrutinib-based therapy appeared superior to FCR in all prognostic molecular and biologic subgroups; however, its superiority over FCR in patients with mutated immunoglobulin variable heavy chain (IGVH) did not reach the threshold of statistical significance at the time of the initial report.
The E1912 trial also provided information on the short-term tolerability profile of FCR and ibrutinib-based therapies. Although the overall frequency of grade ≥3 adverse events (AEs) was similar between treatment arms, the toxicity profiles were distinct. More frequent cytopenias (anemia, neutropenia, thrombocytopenia) and infection events were observed in the FCR arm, while hypertensive events and cardiac arrhythmia/atrial fibrillation were more common in the IR arm.
Here, we present an updated report from the E1912 trial with information on AEs, PFS, and OS after 3 additional years of follow-up. We also present data on the long-term tolerability of single-agent ibrutinib, the proportion of patients discontinuing treatment for reasons other than progression, and analysis of pretreatment factors that predict ibrutinib discontinuation. Finally, we provide an updated analysis of clinical outcomes by prognostic characteristics and CLL International Prognostic Index (CLL-IPI) subgroup.
Methods
The E1912 study was designed, led, and coordinated by the Eastern Cooperative Oncology Group (ECOG) – American College of Radiology Imaging Network (ACRIN) Cancer Research Group in partnership with the other NCTN (National Clinical Trials Network) cooperative groups. The trial eligibility has been previously reported.7 Briefly, patients aged ≤70 years with previously untreated CLL or small lymphocytic lymphoma and in need of therapy according to the International Workshop on CLL (iwCLL) criteria14 were eligible for participation. Patients with deletion 17p13 by fluorescent in situ hybridization (FISH) analysis were excluded from participation due to the poor response of such patients to FCR therapy.15 The National Cancer Institute (NCI) Central Institutional Review Board, as well as a local institutional review board, as required by treating institutions, approved the study in accord with the principles of the Declaration of Helsinki. Ibrutinib was provided by Pharmacyclics under a cooperative research and development agreement with the NCI. Accrual to the E1912 trial began in March 2014 and was completed in June 2016.
Study participants provided written informed consent followed by randomization in a 2:1 ratio to receive either IR or FCR CIT. Age (<60 vs ≥60), disease stage (Rai 0-II vs III-IV), ECOG performance status (0-1 vs ≥2), and the presence of chromosome 11q22.3 deletion by FISH were used as stratification factors in the randomization. The number and severity of coexistent health conditions were assessed at enrollment using the Cumulative Illness Rating Scale,16 a well-established assessment of comorbidity that has been used in previous trials of patients with CLL.17 Functional status at enrollment was also assessed by the “Timed Up and Go” test.18,19 CLL-IPI risk category was determined for each patient based on age, stage, serum β-2-microglobulin, TP53 mutation status by sequencing (research assay) and/or FISH, and IGHV status (research assay).20
Patients assigned to FCR therapy received 6 cycles of treatment using the standard schedule.11,12 Those assigned to IR received ibrutinib 420 mg/d until disease progression or unacceptable toxicity. Six cycles of concomitant rituximab were administered during cycles 2 through 7, as detailed in the original publication.7 Standard supportive care measures were allowed for all patients independent of the treatment arm.
Toxicity was graded according to NCI Common Toxicity Criteria Version 4.0 with dose modifications as described in the previous publication.7 All AEs of grade ≥3 throughout the entirety of follow-up on both treatment arms (ie, even after patients assigned to the FCR arm had completed therapy) were recorded so that toxicity monitoring was equivalent between arms. Response to therapy was assessed using the 2008 iwCLL Working Group criteria14 in effect at the trial initiation along with the modification to the criteria published in 2018,21 which added the category of complete response with incomplete bone marrow recovery. The primary response evaluation occurred 12 months from the beginning of treatment and included assessment of lymphadenopathy and organomegaly by physical exam as well as bone marrow aspirate and biopsy. Computed tomography imaging was also performed at 12 months.
Statistical analysis
The primary trial endpoint was PFS, defined as the time from randomization to progression or death without documented progression. Patients alive without documented progression were censored at the time of last disease evaluation. OS was a secondary endpoint, defined as the time from randomization to death from any cause. Patients alive were censored at the date of the last contact. As previously reported,7 the primary study results were released by the Data and Safety Monitoring Board at the time of the first interim analysis (September 2018) due to PFS and OS advantages favoring the IR treatment arm, which met the protocol-specified criteria for immediate reporting.
The primary efficacy analysis was based on the intent-to-treat population, which included all randomized patients regardless of eligibility or treatment status. The toxicity analysis included all treated patients. The Kaplan-Meier method was used to estimate time-to-event distributions. Stratified log-rank tests were used to compare PFS and OS between the 2 arms overall and PFS within subgroups defined by IGHV mutation status. Corresponding hazard ratios (HRs) were estimated using the stratified Cox proportional hazard models. Unstratified versions were used for the comparison of PFS and estimation of HRs within CLL-IPI categories. Fisher’s exact tests were used to compare AE frequencies. P values are 2-sided and were not corrected for multiple testing.
Results
Between 31 January 2014 and 9 June 2016, 529 patients (FCR: 175; IR: 354) were accrued to the E1912 trial, of which 498 were eligible. The clinical and disease characteristics of patients have been previously reported (supplemental Table 1). At the time of the present analyses (9 August 2021), the median follow-up among the 490 living patients is 70 months. Among the 354 patients randomized to IR, 214 (60.5%) continue ibrutinib, and 115 of 175 (65.7%) patients randomized to FCR remain on surveillance at the time of this report.
AEs and treatment discontinuation
With extended follow-up, grade ≥3 treatment-related AEs were observed in 73.0% of IR- and 83.5% of FCR-treated patients (odds ratio [OR], 0.53; 95% CI, 0.32-0.88; P = .0097). A summary of grade 3 to 5 treatment-related AEs throughout the entire study period is shown in Table 1. With respect to bleeding sequelae, 4 patients (1.1%) on the IR arm have experienced grade 3+ hemorrhage (2 upper gastrointestinal hemorrhage, 1 intraoperative hemorrhage, 1 renal hemorrhage). A summary of all (ie, grade 1-5) AEs regardless of treatment attribution throughout the entire study period is shown in supplemental Table 4.
Grade 3 to 5 treatment-related AEs throughout the entire study period
| . | IR, % (n = 352) . | FCR, % (n = 158) . | P value . |
|---|---|---|---|
| Anemia | 4.3 | 15.8 | <.001 |
| Arthralgia | 5.4 | 0.6 | .011 |
| Diarrhea | 2.6 | 0.6 | .185 |
| Fatigue | 2.0 | 2.5 | .745 |
| Hemolysis | 0 | 2.5 | .009 |
| Hypertension | 11.4 | 1.9 | <.001 |
| Leukocytosis | 9.7 | 0.6 | <.001 |
| Lymphocyte count decreased | 6.8 | 65.2 | <.001 |
| Lymphocyte count increased | 28.1 | 13.9 | <.001 |
| Neutrophil count decreased | 28.4 | 45.6 | <.001 |
| Platelet count decreased | 3.4 | 16.5 | <.001 |
| Rash maculo-papular | 2.6 | 1.9 | .762 |
| White blood cell decreased | 6.5 | 40.5 | <.001 |
| Infection | 11.4 | 19.6 | .018 |
| Lung infection | 4.5 | 2.5 | .333 |
| Febrile neutropenia | 2.3 | 15.8 | <.001 |
| Sepsis | 1.1 | 3.2 | .144 |
| Other infection | 9.7 | 6.3 | .237 |
| Cardiac | 7.7 | 0 | <.001 |
| Atrial fibrillation | 4.5 | 0 | .004 |
| Other Cardiac* | 4.3 | 0 | .008 |
| Any grade 3-5 treatment related AEs | 73.0 | 83.5 | .010 |
| . | IR, % (n = 352) . | FCR, % (n = 158) . | P value . |
|---|---|---|---|
| Anemia | 4.3 | 15.8 | <.001 |
| Arthralgia | 5.4 | 0.6 | .011 |
| Diarrhea | 2.6 | 0.6 | .185 |
| Fatigue | 2.0 | 2.5 | .745 |
| Hemolysis | 0 | 2.5 | .009 |
| Hypertension | 11.4 | 1.9 | <.001 |
| Leukocytosis | 9.7 | 0.6 | <.001 |
| Lymphocyte count decreased | 6.8 | 65.2 | <.001 |
| Lymphocyte count increased | 28.1 | 13.9 | <.001 |
| Neutrophil count decreased | 28.4 | 45.6 | <.001 |
| Platelet count decreased | 3.4 | 16.5 | <.001 |
| Rash maculo-papular | 2.6 | 1.9 | .762 |
| White blood cell decreased | 6.5 | 40.5 | <.001 |
| Infection | 11.4 | 19.6 | .018 |
| Lung infection | 4.5 | 2.5 | .333 |
| Febrile neutropenia | 2.3 | 15.8 | <.001 |
| Sepsis | 1.1 | 3.2 | .144 |
| Other infection | 9.7 | 6.3 | .237 |
| Cardiac | 7.7 | 0 | <.001 |
| Atrial fibrillation | 4.5 | 0 | .004 |
| Other Cardiac* | 4.3 | 0 | .008 |
| Any grade 3-5 treatment related AEs | 73.0 | 83.5 | .010 |
Includes grade 3 to 5 treatment-related AEs that occurred in ≥2% of the treated patients in either arm. The worst grade is based on all grade 3 to 5 treatment-related AEs.
Pericardial effusion, heart failure, chest pain-cardiac, sinus tachycardia, supraventricular tachycardia, atrial flutter, ventricular tachycardia, cardiac arrest.
To date, 3 patients on the FCR arm (1.7%) have developed a myelodysplastic syndrome or acute myeloid leukemia (8 months, 31 months, and 25 months after randomization; no cases on the IR arm). Three patients developed Richter's transformation (1 [0.6%] on the FCR arm at 29 months after randomization and 2 on the IR arm 27 and 43 months after randomization.
The proportion of patients developing a second primary cancer was higher in patients on the IR arm relative to the FCR arm (IR = 55 [15.6%], FCR = 17 [10.8%]; P = .17; OR = 0.65; 95% CI 0.34-1.19). The total number of second primary cancers (some patients developed >1 second primary) other than nonmelanoma skin cancer was reported in 15 of 158 (9.5%) patients on the FCR arm and 47 of 352 (13.4%; P = .150) patients on the IR arm (see details in supplemental Table 2). Melanoma was observed in 1 of 158 (0.6%) patients on FCR and 9 of 352 (2.6%; P = .185) patients on the IR arm. Nonmelanoma skin cancer was reported in 6 of 158 (3.8%) patients on FCR and 26 of 352 (7.4%; P = .083) patients in the IR arm.
Among IR-treated patients, the median time of treatment was 58.9 months (range, 0.2-87.3). Among the 138 IR-treated patients who discontinued treatment, 37 (10.5% of patients randomized to and started IR) discontinued therapy due to disease progression or death, and 77 (21.9% of patients randomized to and started IR) discontinued therapy for AEs or complication. The remaining 24 patients (6.8% of patients randomized to and started IR) withdrew for other reasons (Table 2). Thus, among the patients who discontinued ibrutinib treatment, 26.8% did so due to disease progression/death, 55.8% did so due to an AE or complication, and 17.4% withdrew for other reasons. Among the 101 patients who discontinued ibrutinib for a reason other than progression or death, the median time on ibrutinib was 25.9 months (range, 0.2-82.0 months). The median time from ibrutinib discontinuation to disease progression or death in these patients was 25 months (Figure 1). We next evaluated PFS after ibrutinib discontinuation based on the duration of ibrutinib exposure. Among patients receiving <12 months, 12 to 24 months, and >24 months of ibrutinib before discontinuation, the median PFS after discontinuation of ibrutinib was 19.3, 30.3, and 25.0 months, respectively; however, these differences were not statistically significant (P = .47) (supplemental Figure 2).
Reason for ibrutinib discontinuation
| Reason for discontinuation . | Number of patients who discontinued . | Percentage among all IR patients who started IR, n = 352 . | Percentage among IR patients discontinuing treatment, n = 138 . |
|---|---|---|---|
| Progression or death | 37 | 10.5 | 26.8 |
| AE or complications | 77 | 21.9 | 55.8 |
| Withdrawal or other reason* | 24 | 6.8 | 17.4 |
| Reason for discontinuation . | Number of patients who discontinued . | Percentage among all IR patients who started IR, n = 352 . | Percentage among IR patients discontinuing treatment, n = 138 . |
|---|---|---|---|
| Progression or death | 37 | 10.5 | 26.8 |
| AE or complications | 77 | 21.9 | 55.8 |
| Withdrawal or other reason* | 24 | 6.8 | 17.4 |
Other reasons: lost insurance (n = 2), missed visits or noncompliance (n = 5), moved to a new location or site (n = 3).
PFS from discontinuation of ibrutinib. Includes patients who discontinued ibrutinib for reasons other than progression or death and known to be progression-free at the time of discontinuation.
PFS from discontinuation of ibrutinib. Includes patients who discontinued ibrutinib for reasons other than progression or death and known to be progression-free at the time of discontinuation.
We next conducted a multivariable analysis evaluating the relationship between baseline characteristics (age, gender, Timed Up and Go test, Cumulative Illness Rating Scale [CIRS] score, ECOG performance status, creatinine clearance, and baseline anemia/thrombocytopenia) and discontinuation of ibrutinib for a reason other than progression or death during follow-up. As compared with those with a baseline ECOG performance status 0, ECOG performance status 1 (HR, 1.65; 95% CI, 1.09-2.49; P = .017) and ECOG performance status 2 (HR, 2.30; 95% CI, 0.83-6.37; P = .109) were associated with future discontinuation of ibrutinib for a reason other than progression or death.
Clinical outcomes
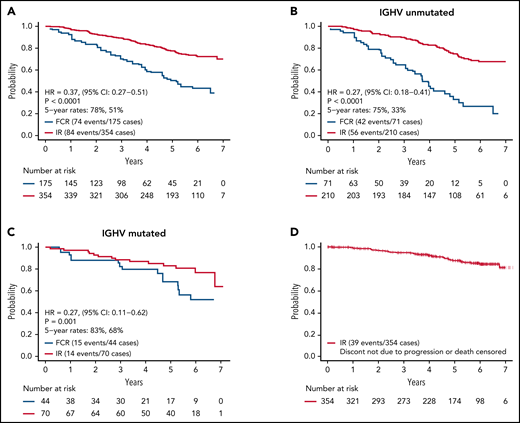
As of August 9, 2021, we have observed 158 PFS events and 39 deaths for both arms. Updated analysis of PFS between arms is shown in Figure 2A. Patients on the IR arm had superior PFS among both patients with unmutated IGHV (HR, 0.27; P < .001) (Figure 2B) and mutated IGHV CLL (HR = 0.27, P = .001) (Figure 2C). PFS for patients remaining on ibrutinib (ie, censoring patients who went off ibrutinib for AEs or other reasons) is shown in Figure 2D.
PFS. (A) PFS among all patients; (B) PFS among patients with unmutated IGHV; (C) PFS among patients with mutated IGHV; (D) PFS for patients remaining on ibrutinib. Patients who went off ibrutinib for AEs or reasons other than progression are censored at the time of ibrutinib discontinuation.
PFS. (A) PFS among all patients; (B) PFS among patients with unmutated IGHV; (C) PFS among patients with mutated IGHV; (D) PFS for patients remaining on ibrutinib. Patients who went off ibrutinib for AEs or reasons other than progression are censored at the time of ibrutinib discontinuation.
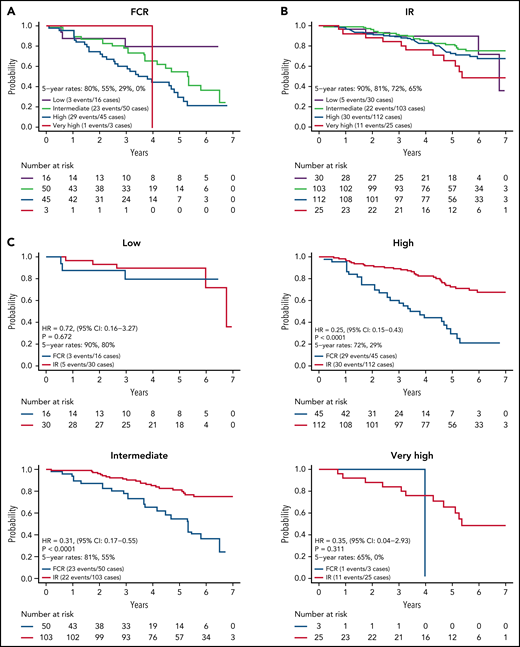
We next evaluated PFS by CLL IPI risk category for the 384 patients with results for the variables needed to calculate the CLL IPI score. CLL IPI risk category at baseline stratified PFS for patients on the FCR arm (Figure 3A) with 5-year PFS of 80%, 56%, and 30% for those in the low-, intermediate-, and high-risk categories (P = .018; too few patients in very high-risk category to analyze). However, the CLL IPI risk category at baseline stratified PFS more modestly for patients on the IR arm (Figure 3B) with a 5-year PFS of 90%, 81%, 70%, and 65% for those in the low-, intermediate-, high-, and very high-risk categories.
PFS by CLL IPI risk group. (A) PFS by CLL IPI risk group for FCR-treated patients; (B) PFS by CLL IPI risk group for IR-treated patients; (C) PFS by treatment arm within each CLL IPI risk group.
PFS by CLL IPI risk group. (A) PFS by CLL IPI risk group for FCR-treated patients; (B) PFS by CLL IPI risk group for IR-treated patients; (C) PFS by treatment arm within each CLL IPI risk group.
Among IR treated patients, PFS for patients in the very high-risk category was lower than those in the low (P = .055) and intermediate (P = .022) categories. A comparison of PFS between arms within each CLL IPI risk category is shown in Figure 3C. Although sample size limits power for comparison between arms in some CLL IPI risk groups, IR demonstrated a statistically significant advantage in PFS relative to FCR for patients in the intermediate (HR, 0.31; P < .001) and high-risk IPI categories (HR, 0.25; P < .001).
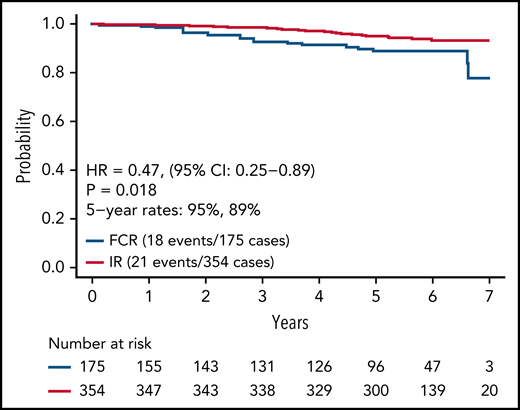
Updated analysis of OS for patients on both arms is shown in Figure 4. A small but statistically significant improvement in OS was observed for patients on the IR arm. Information on known causes of death and salvage therapy is provided in supplemental Table 3A-B. A separate analysis of OS for patients with IGHV mutated and unmutated CLL is shown in supplemental Figure 1A-B. Although power for this secondary analysis is limited, a statistically significant advantage in OS was observed for patients with unmutated IGHV (HR, 0.35; 95% CI, 0.15-0.80; P = .01) but not for patients with mutated IGHV (HR, 0.72; 95% CI, 0.15-3.47; P = .68).
Discussion
We report here longer-term results of the E1912 trial after a median of 70 months of follow-up. The initial results from this phase 3 trial established that ibrutinib-based therapy offered superior PFS and OS to FCR, the most effective CIT regimen for CLL patients.11,12 With longer follow-up, IR therapy offers statistically superior PFS to FCR overall and in patients with IGHV mutated or unmutated CLL. Some,13,22-24 although not all,25 studies of FCR-treated patients suggest there may be a plateau in the PFS curve in patients with IGHV mutated CLL that occurs 8 to 10 years after therapy, and longer follow-up from E1912 will continue to be illustrative for that subset of patients. Nonetheless, IR therapy exhibits a markedly superior PFS to FCR in patients with mutated IGHV (HR, 0.27; 95% CI, 0.11-0.62) with current follow-up. The advantage in OS observed with IR therapy in the initial report also persists with longer follow-up.
With approximately 6 years of median follow-up, 60% of patients randomized to ibrutinib-based therapy have remained on treatment. The most common reason for discontinuing ibrutinib was AEs. Approximately 1 in 5 patients randomized to the ibrutinib arm have discontinued treatment due to an AE with current follow-up. It should be noted that the median age of patients enrolled in the E1912 trial was 58 years, where age may influence tolerability and the ability to remain on long-term treatment with ibrutinib-based therapy. Higher baseline ECOG performance status were independent predictors of discontinuation of ibrutinib for a reason other than progression or death. The relationship between performance status and discontinuation is consistent with the findings of reports in older patients outside of clinical trials, which identified ECOG performance status as a predictor of ibrutinib treatment feasibility and outcome.26,27 In contrast, the lack of an association between baseline CIRS score and early discontinuation of ibrutinib is distinct from prior reports in older patients which reported an increased likelihood of ibrutinib discontinuation among patients with CIRS scores of ≥6.26,28 This difference is likely due to the younger patient age and lower baseline CIRS scores of the patients in E1912. These observations from E1912 may allow expanded use of performance status to identify patients who will struggle with long-term ibrutinib treatment and define a population for participation in trials of alternative treatment approaches designed for less fit individuals.
In late 2021, the initial results of a second randomized trial comparing FCR to IR (ie, the FLAIR [Front-Line therapy in CLL: Assessment of Ibrutinib-containing Regimens] trial) were reported in abstract form.29 After a median follow-up of 52.7 months, this trial confirmed a PFS advantage for IR relative to FCR (HR, 0.44; 95% CI, 0.32-0.6; P < .001)29 that was similar in magnitude to that reported in the initial results of the E1912 trial (HR, 0.35; 95% CI, 0.22-0.56; P < .001).7 Unlike E1912, no difference in OS has been observed in the FLAIR trial to date. Differences in salvage therapy may be one explanation for this difference. It is important to note that the E1912 trial began earlier than FLAIR and before ibrutinib therapy for relapsed patients had fully penetrated routine clinical practice in the United States. Not all of the 6 patients on the FCR arm of E1912 who died due to CLL received BTKi therapy before death. Patient age and its relationship to toxicity and ibrutinib-related cardiac events may also be one factor that explains the difference in the OS results between E1912 and FLAIR. The median age of the patients on E1912 was 58 years (7.9% >65 years) as compared with the median age of 63 years (24.2% >65 years) in FLAIR. Among the 386 ibrutinib-treated patients in the FLAIR trial, 9 died of cardiac events as compared with 1 cardiac death to date in E1912. Accordingly, one hypothesis for the difference in the OS results between trials is that the increased number of cardiac deaths in FLAIR, which may relate to the older age of the patients treated, eliminates the survival benefit for first-line ibrutinib in younger patients, as observed in E1912.
The 7-year PFS is ∼80% for patients who were able to remain on ibrutinib therapy, suggesting that disease progression for patients who remain on therapy is relatively uncommon. Among those who discontinued ibrutinib for a reason other than progression (median time on ibrutinib before discontinuation, 25 months), the median time to progression exceeded 2 years. These results suggest that many patients who have had a clinical response and discontinued ibrutinib for unacceptable toxicity have a protracted interval before requiring additional therapy. Although the baseline CLL-IPI risk category did not predict PFS as effectively in IR-treated patients as it did for FCR, a statistically shorter PFS was observed for patients in the very high CLL-IPI risk category relative to patients in the IPI low- and intermediate-risk categories. Thus, the CLL-IPI still has predictive value in patients initiating ibrutinib-based therapy; however, new predictive models that stratify PFS with this treatment approach more precisely would be beneficial for patient counseling and management.
The present update also provides more mature information regarding longer-term toxicity in both treatment arms. Ibrutinib-based therapy was associated with fewer grade 3+ AEs relative to FCR across the full length of treatment and follow-up on both arms. The difference in the prevalence of specific AEs remains strongly consistent with the initial report. Ibrutinib-based therapy was associated with higher rates of hypertension, atrial fibrillation, and other cardiac complications, while FCR-based therapy was associated with higher rates of cytopenias and infectious complications. To date, few patients on either arm have developed myelodysplastic syndrome or AML or Richter's transformation. The latter may be due in part to the exclusion of patients with deletion 17p from participating in the E1912 trial.
With current follow-up, the proportion of patients developing a second primary malignancy is higher on the ibrutinib arm, although this difference is not statistically significant. An increased risk of second primary cancers has not been reported in other trials of BTKi’s. A recent single-center report from The Ohio State University suggested a 2.2-fold increase in observed vs expected second primary cancers among patients treated with a BTKi relative to the general population,30 a rate similar to that reported in previous trials of CLL patients treated with FCR.31,32 An increase in second primary cancers was not observed on the ibrutinib arm of the FLAIR trial after a median follow-up of 52.7 months.29 It is uncertain why BTKi therapy would increase the risk of other cancers from a mechanistic standpoint. It has been hoped that first-line BTKi-based therapy may reduce second cancer risk in CLL patients due to restoration of certain components of immune function.30 Additional follow-up will be necessary to determine if a difference in second primary malignancies exists between the arms of E1912, and information on increased second cancer incidence from other ibrutinib trials will also be of interest.
The updated results from E1912 continue to cement a treatment paradigm favoring targeted therapy, including BTKi-based therapy, rather than CIT as the preferred approach for previously untreated CLL patients.6-10 In the E1912 trial, this approach appears to offer more favorable. The PFS, superior OS, as well as better tolerability. PFS of 72.6% at 6 years for ibrutinib-treated patients with current follow-up is superior not only to the FCR arm of the E1912 trial but also that reported with nearly all previous phase 3 trials of CIT.6-12,17 The 6-year PFS of 43.3% for the FCR-treated patients in E1912 is nearly identical to that observed in both the CLL-8 and CLL-10 trials.11,12
It should be noted that although patients on the ibrutinib arm of the E1912 trial also received rituximab, the benefit of adding anti-CD20 therapy with BTKi is unclear and remains an active area of investigation. Two previous randomized trials have demonstrated that while adding rituximab to ibrutinib may improve the depth of remission, it does not improve PFS.6,33 Based on this data, most experienced clinicians favor ibrutinib monotherapy when using ibrutinib as first-line therapy in routine practice. A more recent randomized trial comparing the BTKi acalabrutinib alone or in combination with the more efficient anti-CD20 monoclonal antibody obinutuzumab found a PFS advantage for the obinutuzumab combination arm.10 Additional studies are needed to determine the benefits of adding obinutuzumab or other more effective anti-CD20 monoclonal antibodies to BTKi therapy in previously untreated CLL patients.
Where do efforts to improve therapy for previously untreated CLL patients go from here? It is important to emphasize that the E1912 trial excluded patients with deletion 17p. These individuals typically represent 5% to 10% of previously untreated patients in need of therapy. Although these individuals respond to BTKi and BCL-2 inhibitor approaches, they typically have a shorter duration of response. More efficacious approaches are needed for that subset and are in development.34-37 For the remaining 90% to 95% of patients starting first-line therapy, appropriate goals may include durable remission, favorable toxicity profile, eliminating the need for continuous therapy, and an economically viable approach that makes the most effective therapies available to patients worldwide. Some progress has already been made toward these goals. Second generation BTKi’s have been developed, and early results from phase 3 trials of relapsed/refractory patients suggest these novel targeted agents may have similar efficacy but lower rates of side effects, including less hypertension, arthralgia, muscle spasms, diarrhea, and atrial fibrillation (although they may have higher rates of headache and cough).38 Multiple phase 3 trials are currently comparing BTKi’s to BCL2 inhibitors, comparing newer generation BTKi’s to ibrutinib-based approaches, and evaluating combinations of BTK and BCL2 inhibitors in previously untreated patients with the goal of improving tolerability, achieving undetectable measurable residual disease, longer-lasting remissions, and eliminating the need for continuous therapy.
In conclusion, with a median follow-up of approximately 6 years, the long-term results of the E1912 trial continue to demonstrate superior PFS and OS for IR therapy relative to FCR in patients with mutated or unmutated IGHV. First-line IR was generally well tolerated, with a majority of patients randomized to the ibrutinib arm continuing treatment as of the time of this report. CLL disease progression while on ibrutinib is an uncommon event. Among patients on E1912 who discontinue ibrutinib for a reason other than progression, the median PFS is 2 years. Additional longer-term follow-up is necessary to provide further insights on late toxicities and complications. These results reaffirm ibrutinib-based treatment as an appropriate first-line therapy option for the majority of CLL patients aged ≤70.
Acknowledgments
This study was conducted by the ECOG-ACRIN Cancer Research Group (Peter J. O'Dwyer and Mitchell D. Schnall, Group Co-Chairs) and supported by the National Cancer Institute of the National Institutes of Health under the following award numbers: U10CA180794, U10CA180821, U10CA180820, U10CA180888, UG1CA189859, UG1CA189863, UG1CA190140, UG1CA232760, UG1CA233180, UG1CA233230, UG1CA233253, UG1CA233290, and UG1CA233339. Correlative studies were supported by CA193541. The study was also supported in part by Pharmacyclics, Inc. (a subsidiary of AbbVie).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: T.D.S., X.V.W., and N.E.K. designed research; T.D.S., X.V.W., C.A.H., E.M.P., S.O., J.B., D.F.J., E.B., J.F.L., C.C.Z., S.E.C., P.M.B., A.F.C., A.R.M., A.K.S., M.P.M., R.F.L., H.E., R.M.S., M.L., M.T., and N.E.K. performed research; T.D.S., X.V.W., C.A.H., E.M.P., S.O., J.B., D.F.J., E.B., J.F.L., C.C.Z., S.E.C., P.M.B., A.F.C., A.R.M., A.K.S., M.P.M., R.F.L., H.E., R.M.S., M.L., M.T., and N.E.K. contributed patients or analytical tools; X.V.W. analyzed data; T.D.S. and X.V.W. wrote the paper; and N.E.K., C.A.H., E.M.P., S.O., J.B., D.F.J., E.B., J.F.L., C.C.Z., S.E.C., P.M.B., A.F.C., A.R.M., A.K.S., M.P.M., R.F.L., H.E., R.M.S., M.L., M.T., and N.E.K. critically revised the paper.
Conflict-of-interest disclosure: T.D.S. reports receiving grant support from Pharmacyclics, AbbVie, and Genentech; and holds a patent (US14/292 075) on green tea extract epigallocatechin gallate in combination with chemotherapy for chronic lymphocytic leukemia. N.E.K. reports serving on the advisory board for AbbVie, AstraZeneca, Beigene, Behring, Cytomx Therapy, Dava Oncology, Janssen, Juno Therapeutics, Oncotracker, Pharmacyclics, and Targeted Oncology; serves on the DSMC (Data Safety Monitoring Committee) for Agios Pharm, AstraZeneca, BMS–Celgene, Cytomx Therapeutics, Janssen, Morpho-sys, and Rigel; and has received research funding from AbbVie, Acerta Pharma, Bristol Myers Squibb, Celgene, Genentech, MEI Pharma, Pharmacyclics, Sunesis, TG Therapeutics, and Tolero Pharmaceuticals. A.R.M. receives research support from TG Therapeutics, Pharmacyclics, AbbVie, Johnson and Johnson, Acerta, AZ, Regeneron, DTRM BioPharma, Sunesis, Loxo Oncology Adaptive; and advisory/consultancy/DSMB for TG Therapeutics, Pharmacyclics, AbbVie, Johnson and Johnson, Acerta, AZ, DTRM BioPharma, Sunesis, and Adaptive. S.O. receives research support from Kite, Regeneron, and Gilead; consultant and research support from Gilead, Pharmacyclics, TG Therapeutics, Pfizer, and Sunesis; consultant for Amgen, Astellas, Celgene, GSK, Janssen Oncology, Aptose Biosciences Inc., Vaniam Group LLC, AbbVie, Alexion, Verstem, Eisai, Juno Therapeutics, Vida Ventures, Autolus, Johnson and Johnson, and Merck. J.B. receives research funding from Oncternal and Velosbio; consultant for AbbVie, AstraZeneca, Pharmacyclics/AbbVie, and Kite/Gilead; grant support and advisory board fees from Pharmacyclics-AbbVie; and lecture fees and travel support from Janssen, Gilead Sciences, and Genentech. E.B. reports consulting fees from DASA. S.E.C. receives funds as follows: institutional research funding from AbbVie, Acerta, Gilead, Janssen, Pharmacyclics, Takeda; Data Safety Monitoring Committee for Beigene; Clinical Trial Steering Committee for Acerta; consultancy from AbbVie, Adaptive, Astellas, AstraZeneca, Genentech, Gilead, Janssen, and Pharmacyclics; honoraria from Janssen, Pharmacyclics, (CME accredited) Imedex, and Medscape; travel expenses from AbbVie, Beigene, Genentech, Janssen, and Pharmacyclics; and expert witness for Genentech. P.M.B. consulted for Pharmacyclics, AbbVie, Genentech, Gilead, AstraZeneca, Bayer, Merck, Celgene/BMS, Morphosys, TG Therapeutics, and Seattle Genetics. A.F.C. reports serving on the advisory board for SecuraBio and ADC Therapeutics and receiving research funding from SecuraBio. A.R.M. receives grant support, consulting fees, fees for serving on a data and safety monitoring board, and advisory board fees from TG Therapeutics; grant support, consulting fees, and advisory board fees from Pharmacyclics, Johnson & Johnson, AbbVie, and AstraZeneca; grant support from Regeneron; fees for serving on a data and safety monitoring board and advisory board fees from Celgene; grant support and advisory board fees from Sunesis Pharmaceuticals and Loxo Oncology; and lecture fees, fees for continuing medical education events and other events from prIME Oncology. H.E. receives research funding from AbbVie, Agios, Amgen, Daiichi Sankyo, Forma, Forty Seven/Gilead, Glycomimetics, ImmunoGen, Jazz, Macrogenics, and Novartis; member of advisory boards for AbbVie, Agios, Astellas, Celgene/BMS, Daiichi Sankyo, Genentech, Glycomimetics, Incyte, Jazz, Kura Oncology, Novartis, Takeda, and Trillium; speakers bureau for AbbVie, Agios, Amgen Celgene/BMS, Incyte, Jazz, and Novartis; financial or material support from AbbVie (Chair, Independent Review Committee for VIALE A and VIALE C) and Celgene/BMS (Chair, AML Repository Study). M.T. receives research funding from AbbVie, Cellerant, Orsenix, ADC Therapeutics, Biosight, Glycomimetics, Rafael Pharmaceuticals, and Amgen; member of advisory board for AbbVie, BioLineRx, Daiichi-Sankyo, Orsenix, KAHR, Rigel, Nohla, Δ Fly Pharma, Tetraphase, Oncolyze, Jazz Pharma, Roche, Biosight, and Novartis; and receives royalties from UpToDate. R.M.S. reports grants and personal fees from AbbVie, Agios, and Novartis; grants from Arog; personal fees from Actinium, Argenx, Astellas, AstraZeneca, Biolinerx, Celgene, Daiichi-Sankyo, Elevate, Gemoab, Janssen, Jazz, Macrogenics, Otsuka, Pfizer, Hoffmann La Roche, Stemline, Syndax, Syntrix, Syros, Takeda, and Trovagene outside the submitted work. M.L. receives grant support and consulting fees from Amgen; grant support from Astellas Pharma, AbbVie, Actinium Pharmaceuticals, Novartis, and Pluristem Therapeutics; and consulting fees from Sanofi and NewLink Genetics. The remaining authors declare no competing financial interests.
Correspondence: Tait Shanafelt, Division of Hematology, Department of Medicine, Stanford University School of Medicine, Stanford, CA 94305-5101; e-mail: tshana@stanford.edu.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal