Key Points
EBIs contain differentiating granulocyte precursors and serve as dual niches for terminal erythropoiesis and granulopoiesis.
Single-cell RNA sequencing and CITE sequencing suggest significant heterogeneity and plasticity within EBI macrophages.
Abstract
The erythroblastic island (EBI), composed of a central macrophage surrounded by maturing erythroblasts, is the erythroid precursor niche. Despite numerous studies, its precise composition is still unclear. Using multispectral imaging flow cytometry, in vitro island reconstitution, and single-cell RNA sequencing of adult mouse bone marrow (BM) EBI-component cells enriched by gradient sedimentation, we present evidence that the CD11b+ cells present in the EBIs are neutrophil precursors specifically associated with BM EBI macrophages, indicating that erythro-(myelo)-blastic islands are a site for terminal granulopoiesis and erythropoiesis. We further demonstrate that the balance between these dominant and terminal differentiation programs is dynamically regulated within this BM niche by pathophysiological states that favor granulopoiesis during anemia of inflammation and favor erythropoiesis after erythropoietin stimulation. Finally, by molecular profiling, we reveal the heterogeneity of EBI macrophages by cellular indexing of transcriptome and epitope sequencing of mouse BM EBIs at baseline and after erythropoietin stimulation in vivo and provide a searchable online viewer of these data characterizing the macrophage subsets serving as hematopoietic niches. Taken together, our findings demonstrate that EBIs serve a dual role as niches for terminal erythropoiesis and granulopoiesis and the central macrophages adapt to optimize production of red blood cells or neutrophils.
Introduction
The superb efficiency of the mammalian bone marrow (BM) to produce red blood cells at a rate of 2.5 million/second for an adult human at homeostasis1 is provided by the erythropoietic niche, where erythroblasts differentiate in close association with a central macrophage (Mφ), forming the erythroblastic island (EBI), described by Marcel Bessis in 1958 as the first hematopoietic niche.2 The EBI macrophages secrete growth factors that promote survival and proliferation of erythroblasts, provide iron for hemoglobin synthesis, and phagocytose the extruded nuclei, preventing toxicity from free DNA.3-6 Terminal erythroid differentiation, from the late erythroid colony-forming unit (CFU-E) to the reticulocyte stage, occurs within the EBIs.2,4,7 EBIs anatomically and functionally are known to comprise the erythropoietic niches, where positive and negative regulation of terminal erythroid maturation is organized and coordinated.3,4
Many disorders affecting red cell production, such as thalassemias, congenital dyserythropoietic anemias, and acquired myelodysplasia, as well as the widespread anemia of inflammation (AoI), arise from defects in the final stages of erythropoiesis, when erythroblasts mature around the Mφ within EBIs.3 AoI occurs in patients with chronic or acute immune activation as a result of infectious, malignant, or autoimmune diseases and affects the quality of life of millions of people worldwide.8 Erythropoiesis in AoI is characterized by underutilization of iron despite adequate iron stores and blunted response to erythropoietin (Epo) caused by proinflammatory cytokines, which promote myelopoiesis.9-11 Treatment for AoI is currently limited to transfusions and resolution of the underlying disease, which is frequently not feasible.
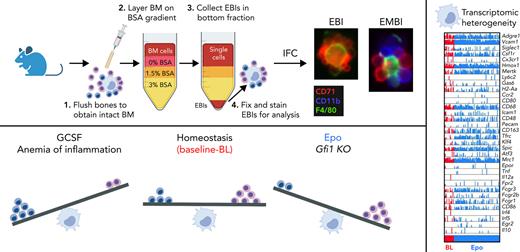
Therefore, functional characterization of the erythropoietic niche and the EBI Mφ and better understanding of the identity and interactions within the EBI are needed. F4/80, VCAM1, CD169, and CD11b have been reported as markers for EBI Mφs based on non–imaging flow cytometry (IFC) studies of events double positive for CD71 and F4/80.12,13 We used gravity sedimentation to isolate BM clusters enriched for EBIs and performed unbiased analysis by IFC, focused on the morphological identity of a macrophage tightly associated with surrounding erythroblasts. Our previous studies with IFC revealed that F4/80, VCAM1, and CD169 are indeed detected on EBI Mφs, however heterogeneously, pointing to the heterogeneity and plasticity of the Mφ population serving as erythropoietic niches. In contrast, anti-CD11b does not label the EBI Mφs; it labels a third cell population consistently present within the islands.14
Here, we demonstrate that the CD11b+ cells in intimate contact with the central EBI Mφ are granulocytic precursors, providing evidence that terminal erythropoiesis proceeds in parallel with terminal granulopoiesis within EBIs. In mice stimulated by granulocyte colony-stimulating factor (GCSF) and models of AoI, we demonstrate increased numbers of CD11b+ cells within the islands along with suppression of erythropoiesis, whereas a reverse balance of increased proportion of erythroblasts to granulocyte precursors within the EBIs is observed in mouse models with inherently decreased granulopoiesis (Gfi1−/−) or after stimulation by Epo. Further evaluation of the EBI Mφs by cellular indexing of transcriptomes and epitopes by sequencing (CITE-seq) revealed several distinct macrophage populations. Our data display the heterogeneous composition of macrophages within the BM erythropoietic niche, enabling their plasticity in supporting erythropoiesis and granulopoiesis.
Methods
Mice
All mouse protocols were approved by the institutional animal care and use committee of Cincinnati Children’s Hospital Medical Center and Feinstein Institutes. Wild-type (WT) C57BL/6J mice were used unless otherwise specified. GCSF was administered at 125 or 250 μg/kg body weight once daily for 3 or 4 days, respectively.15 Epo was administered at 1200 U/kg once daily for 10 days.16
EBI isolation and gradient sedimentation
Hindlimb bones were flushed with EBI buffer (Iscove’s modified Dulbecco’s medium containing 5% fetal bovine serum, 1 mM CaCl2, and 1 mM MgCl2). The suspension was gently pipetted, filtered, and layered onto a bovine serum albumin (BSA) density gradient in EBI buffer (5 mL each containing BSA 3%, 1.5%, and 0%) as previously described.17 After 30 minutes of gravity sedimentation, the top layers were removed and the 3% BSA fraction was collected.
IFC
Single-cell genomics
scRNA-seq
Dispersed EBIs from WT BM were divided and subjected to fluorescence-activated cell sorting to sort CD71+, CD11b+, and F4/80+ populations or left unsorted before single-cell RNA sequencing (scRNA-seq). All samples in both sites (Cincinnati Children’s Hospital Medical Center and Feinstein) were diluted to 1,000 cells/mL and loaded in each lane of a Chromium Controller Instrument (10x Genomics) following the 3′ v2 assay protocol. Sequencing was performed on the Illumina NovaSeq (Novogene).
CITE-seq
Single cells after dispersion of EBI clusters were subjected to red blood cell lysis and negative selection via magnetic-activated cell sorting to enrich for EBI macrophages and were labeled with Total-Seq A antibody-derived tags (ADTs) according to the manufacturer’s protocol. CITE-seq was carried out at the Gene Expression Core at Cincinnati Children’s Hospital Medical Center following the 3′v3 10x Genomics Chromium Protocol. Detailed methods are provided in supplemental Materials and Methods.
Results
CD11b+ cells participating in BM EBIs are maturing granulocytes
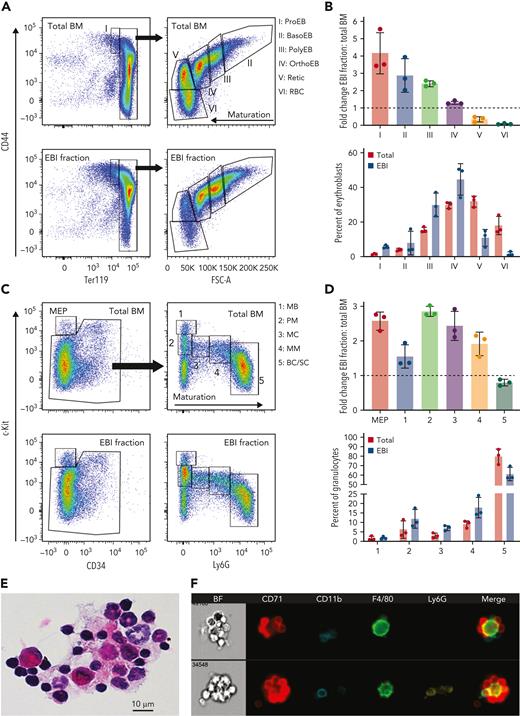
We previously reported that, although EBI macrophages are negative for CD11b, many EBIs contained CD11b+ cells in contact with the Mφ.14 To further investigate the CD11b-expressing cells associated with EBIs in the BM, we first optimized the collection of native EBIs from murine BM using density gradient sedimentation (Figure 1A). Compared with unfractionated total BM, the 3% BSA fraction had a 5-fold higher concentration of large area events (>300 Area_BF) and a 10-fold increase in the number of EBIs relative to total events permitting the analysis of more than 200 EBIs by IFC per mouse/experiment (average of 280 EBIs in n = 10 WT/control experiments; Figure 1B). Surprisingly, only 25% of these clusters were exclusively CD71+ cells surrounding an F4/80+ macrophage in tight, flower-like clusters. The remaining 75% of EBIs contained CD71+ erythroid cells and CD11b+ cells intimately attached to the Mφ (Figure 1C; supplemental Figure 1A). To quantify the relative abundance of CD11b+ vs CD71+ cells within the EBI clusters, we compared the CD11b+ vs CD71+ area within the EBI population by IFC (Figure 1D), which, interestingly, produced a consistent slope (0.690 ± 0.035) among C57BL/6J mice at homeostasis, suggesting a physiological significance for this relationship. Confocal imaging of BM in situ confirmed CD11b+ cells in intimate contact with F4/80+ Mφs, demonstrating that these cells are normally associated in vivo (Figure 1E; supplemental Figure 1B). To further exclude the possibility that nonspecific interaction during the preparation of EBIs caused detection of CD11b+ cells in close association with the Mφs, mixing experiments of WT BM EBIs with BM cells from green fluorescent protein–expressing mice were performed (supplemental Figure 1C-E). There was no tendency of single CD11b+ cells to randomly attach to unfixed WT EBIs during processing, demonstrating that CD11b+ cells observed in the EBIs by IFC are indeed native EBI components.
CD11b+ cells participate in BM EBIs in a constant ratio with erythroblasts. (A) BM clusters, enriched in EBIs, are collected by gravity sedimentation through a BSA gradient. (B) A population of large cell clusters is gated based on their large area in the bright field, which are then gated for double positivity for F4/80 and CD71; EBIs are then selected manually out of this gate after direct visualization.13 Upon visual inspection and identification of a cluster as an EBI (central F4/80+ macrophages surrounded with at least 3 CD71+ erythroblasts), the event is marked in yellow and designated to the manually tagged EBI population. (C) Representative images of EBIs from ImageStream analysis demonstrating varying ratios of CD11b to CD71. (D) Flow cytogram of CD11b+ area vs CD71+ area in the EBIs for one representative experiment. Each point represents an EBI observed by IFC. Upon visual inspection and identification of a cluster as an EBI, the event is marked in yellow and designated as a tagged EBI. All EBIs observed in one biological replicate are shown. Least-square linear regression was used to produce the dotted line, with the constraint to cross the point (X0, Y0), and therefore, because the intercept is 0, the slope represents the ratio of CD11b+ area to CD71+ area within EBIs. The average slope in 5 experiments was in a fairly narrow range of 0.690 ± 0.035, indicating a constant ratio of CD11b+ cells to erythroblasts in the EBIs and implying a physiologic significance. Spearman correlation coefficient r values for all replicates ranged from 0.4 to 0.6 (P < .0001). (E) Whole-mount immunofluorescence staining of intact BM shows cells stained for CD11b (blue) and CD71 (red) intimately associated with an F4/80+ macrophage (green) in situ. Image shown is a maximum-intensity projection of a confocal Z-stack. Scale bar represents 10 μm.
CD11b+ cells participate in BM EBIs in a constant ratio with erythroblasts. (A) BM clusters, enriched in EBIs, are collected by gravity sedimentation through a BSA gradient. (B) A population of large cell clusters is gated based on their large area in the bright field, which are then gated for double positivity for F4/80 and CD71; EBIs are then selected manually out of this gate after direct visualization.13 Upon visual inspection and identification of a cluster as an EBI (central F4/80+ macrophages surrounded with at least 3 CD71+ erythroblasts), the event is marked in yellow and designated to the manually tagged EBI population. (C) Representative images of EBIs from ImageStream analysis demonstrating varying ratios of CD11b to CD71. (D) Flow cytogram of CD11b+ area vs CD71+ area in the EBIs for one representative experiment. Each point represents an EBI observed by IFC. Upon visual inspection and identification of a cluster as an EBI, the event is marked in yellow and designated as a tagged EBI. All EBIs observed in one biological replicate are shown. Least-square linear regression was used to produce the dotted line, with the constraint to cross the point (X0, Y0), and therefore, because the intercept is 0, the slope represents the ratio of CD11b+ area to CD71+ area within EBIs. The average slope in 5 experiments was in a fairly narrow range of 0.690 ± 0.035, indicating a constant ratio of CD11b+ cells to erythroblasts in the EBIs and implying a physiologic significance. Spearman correlation coefficient r values for all replicates ranged from 0.4 to 0.6 (P < .0001). (E) Whole-mount immunofluorescence staining of intact BM shows cells stained for CD11b (blue) and CD71 (red) intimately associated with an F4/80+ macrophage (green) in situ. Image shown is a maximum-intensity projection of a confocal Z-stack. Scale bar represents 10 μm.
To assess the effectiveness of the density gradient sedimentation method to enrich for EBIs, erythroid precursors in total BM and in dissociated EBIs from the 3% BSA fraction were evaluated by flow cytometry (Figure 2A). The 3% BSA fraction was enriched for erythroblasts and depleted of reticulocytes and mature red blood cells (Figure 2B), as expected for erythroid precursors organized in EBIs. We suspected that the CD11b+ cells present within the EBIs might be granulocytes, as mixed hematopoietic clusters containing erythroid and granulocytic precursors have been previously observed by detailed immunohistology20 or electron microscopy.21 Therefore, we evaluated the cells collected in the 3% BSA fraction for granulocytic lineage differentiation (Figure 2C) with flow cytometry as previously described.22 Relative to total BM, the 3% BSA fraction enriched for EBIs was also significantly enriched in promyelocytes (population 2, ckitint,Ly6Gneg) and myelocytes (population 3, ckitint,Ly6Glow), with the most mature neutrophil population (bands and segmented neutrophils, ckitint/low,Ly6Ghigh) depleted (Figure 2D). An increase in the megakaryocyte-erythroid progenitor population was also notable, likely reflecting the presence of erythroid colony-forming unit in the EBIs3,9,23 (Figure 2C-D). In contrast, B-cell and T-cell populations are depleted in the EBI fraction compared with total BM (supplemental Figure 2A-C). Granulocytic precursors were evident within EBIs stained in cytospins with hematoxylin and eosin and by IFC (Figure 2E-F). The specific enrichment of myeloid precursors within the 3% BSA suggests that EBIs are not only erythroblastic but also erythromyeloblastic islands (EMBIs), serving as niches for terminal erythropoiesis and granulopoiesis.
Immature granulocytes are enriched within the EBI fraction, similarly to erythroblasts, and are present in isolated EBIs. Total unfractionated BM and the EBI fraction, after cell dissociation, were evaluated by flow cytometry for erythroblasts and granulocytic precursors. (A) Representative flow plots showing terminal erythropoiesis populations in total unfractionated BM and the EBI fraction. Populations I to VI correspond to proerythroblasts (ProEB), basophilic (BasoEB), polychromatophilic (PolyEB), and orthochromatic (OrthoEB) erythroblasts, reticulocytes (Retic), and mature red blood cells (RBC), respectively. (B) Top graph: the fold change of each population in the EBI fraction vs the whole BM was calculated in 3 biologic repeats (fold change is shown as mean ± SD). Bottom graph: the percentage of each erythroblast population out of total erythroblasts in the total BM (red bars) and the 3% BSA fraction (blue bars) demonstrates the incremental presence of maturing erythroblast populations in total BM and EBI fraction as expected. (C) Representative flow plots showing terminal granulopoiesis populations in total unfractionated BM and EBI fraction. Populations 1 to 5 contain predominantly myeloblasts (MB), promyelocytes (PM), myelocytes (MC), metamyelocytes (MM), and band and segmented cells (BC/SC), respectively. (D) The fold change of each population in the EBI fraction vs the whole BM was calculated in 3 biologic repeats (fold change is shown as mean ± SD). (E) Hematoxylin and eosin staining of cytospins of the EBI fraction showing macrophages surrounded by erythroblasts and granulocytic precursors. (F) IFC of the EBI fraction showing CD11b+Ly6Gneg immature granulocyte precursors and more mature Ly6G+ granulocyte precursors within erythromyeloblastic islands.
Immature granulocytes are enriched within the EBI fraction, similarly to erythroblasts, and are present in isolated EBIs. Total unfractionated BM and the EBI fraction, after cell dissociation, were evaluated by flow cytometry for erythroblasts and granulocytic precursors. (A) Representative flow plots showing terminal erythropoiesis populations in total unfractionated BM and the EBI fraction. Populations I to VI correspond to proerythroblasts (ProEB), basophilic (BasoEB), polychromatophilic (PolyEB), and orthochromatic (OrthoEB) erythroblasts, reticulocytes (Retic), and mature red blood cells (RBC), respectively. (B) Top graph: the fold change of each population in the EBI fraction vs the whole BM was calculated in 3 biologic repeats (fold change is shown as mean ± SD). Bottom graph: the percentage of each erythroblast population out of total erythroblasts in the total BM (red bars) and the 3% BSA fraction (blue bars) demonstrates the incremental presence of maturing erythroblast populations in total BM and EBI fraction as expected. (C) Representative flow plots showing terminal granulopoiesis populations in total unfractionated BM and EBI fraction. Populations 1 to 5 contain predominantly myeloblasts (MB), promyelocytes (PM), myelocytes (MC), metamyelocytes (MM), and band and segmented cells (BC/SC), respectively. (D) The fold change of each population in the EBI fraction vs the whole BM was calculated in 3 biologic repeats (fold change is shown as mean ± SD). (E) Hematoxylin and eosin staining of cytospins of the EBI fraction showing macrophages surrounded by erythroblasts and granulocytic precursors. (F) IFC of the EBI fraction showing CD11b+Ly6Gneg immature granulocyte precursors and more mature Ly6G+ granulocyte precursors within erythromyeloblastic islands.
CD11b+ cells constitute a predominant cell population in EBIs among erythroblasts and macrophages
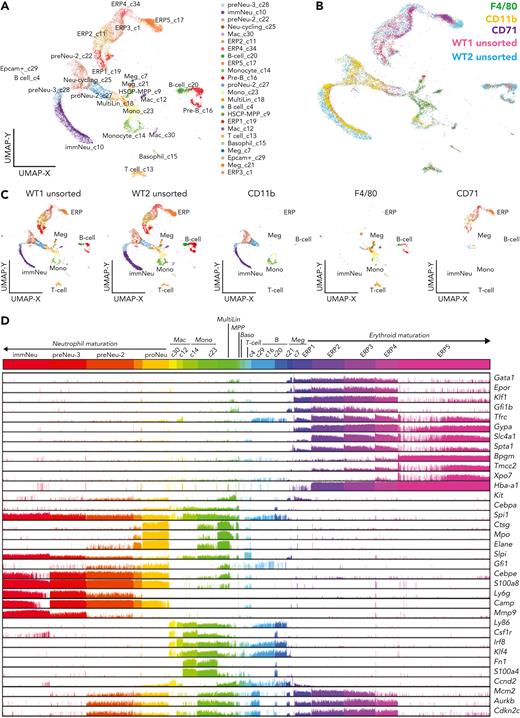
To systematically document the various cell populations participating in the EBIs, we performed scRNA-seq (Chromium; 10x Genomics) on cells collected after dispersing the EBI-enriched BM clusters. These cells were loaded for scRNA-seq unsorted (WT1 unsorted) or after fluorescence-activated cell sorting into 3 populations based on positivity for F4/80, CD71, or CD11b (supplemental Figure 3A-B). A combined unsupervised analysis of the resulting scRNA-seq samples identified a broad range of populations (n = 24) in the unsorted, most enriched in erythroid progenitors (43% of cells), followed by granulocytic progenitors (38% of cells; Figure 3A-B; supplemental Figure 3D). Nearly identical percentages of these major lineages were found in a validation biologic repeat using an unsorted sample from another C57Bl/6J BM specimen (WT2 unsorted) in an independent analysis (41% erythroid, 31% granulocytic). The samples of unsorted EBI-constituent cells represent a superset of the cell populations found in CD71+, CD11b+, and F4/80+ sorted samples (Figure 3B-C). To confirm the population distributions, erythroid and granulocytic lineage-specific genes were selectively queried and visualized on the corresponding Uniform Manifold Approximation and Projection plot (supplemental Figures 4 and 5).
scRNA-seq analysis of EBI constituent cells reveals terminal granulopoiesis alongside terminal erythropoiesis. (A) Uniform Manifold Approximation and Projection (UMAP) plot generated by ICGS2 (Iterative Clustering and Guide-gene Selection version 2) depicting the different populations of cells composing the EBI fraction. The naming of cell populations was marker gene–driven, with an emphasis on prior well-defined hematopoietic lineage notations.20 (B) Visualization in the UMAP plot of the sorted F4/80+ (green), CD11b+ (yellow), and CD71+ (purple) populations, representing the main component populations within the EBIs, showing their alignment to the unsorted cells (pink, blue) of the EBI fraction from C57BL/6 WT BM. (C) Individual contribution of each capture to the UMAP plot pictured in A. Cell numbers for each sample were as follows: WT1 unsorted, n = 4963; WT2 unsorted, n = 7224; CD11b, n = 2668; F4/80 n = 1335; and CD71, n = 1264. (D) Comb plot showing the relative expression level of genes characteristic of each population (erythroblasts, granulocytic precursors, macrophages). ERP1 to ERP4 are marked by high expression of erythroid commitment and differentiation genes such as Gata1, Epor, Klf1, and Gfi1b, whereas transcripts of membrane and cytoskeletal proteins such as transferrin receptor CD71 (Tfrc), glycoprotein A (Gypa), band 3 (Slc4a1), and α-spectrin (Spta1) are most significantly expressed in ERP2 to ERP4. ERP5 was marked by genes known to be expressed in orthochromatic erythroblasts and reticulocytes such as Bpgm and Xpo721-23 and was the most frequent erythroblast population in unsorted and in CD71+ population sorted from the EBIs, as expected for the most mature erythroblasts. CD11b+ sorted cells segregate into 3 transcriptionally distinct clusters that represent granulocytic precursors associated with previously defined neutrophil specification (proNeu) and commitment cell states (preNeu to immNeu).24 Downregulation of cell cycle–related genes Mcm2, Aurkb, and Cdkn2c, especially in ERP5 and immNeu, confirms that these populations consist of terminally differentiated erythroid and granulocytic cells.
scRNA-seq analysis of EBI constituent cells reveals terminal granulopoiesis alongside terminal erythropoiesis. (A) Uniform Manifold Approximation and Projection (UMAP) plot generated by ICGS2 (Iterative Clustering and Guide-gene Selection version 2) depicting the different populations of cells composing the EBI fraction. The naming of cell populations was marker gene–driven, with an emphasis on prior well-defined hematopoietic lineage notations.20 (B) Visualization in the UMAP plot of the sorted F4/80+ (green), CD11b+ (yellow), and CD71+ (purple) populations, representing the main component populations within the EBIs, showing their alignment to the unsorted cells (pink, blue) of the EBI fraction from C57BL/6 WT BM. (C) Individual contribution of each capture to the UMAP plot pictured in A. Cell numbers for each sample were as follows: WT1 unsorted, n = 4963; WT2 unsorted, n = 7224; CD11b, n = 2668; F4/80 n = 1335; and CD71, n = 1264. (D) Comb plot showing the relative expression level of genes characteristic of each population (erythroblasts, granulocytic precursors, macrophages). ERP1 to ERP4 are marked by high expression of erythroid commitment and differentiation genes such as Gata1, Epor, Klf1, and Gfi1b, whereas transcripts of membrane and cytoskeletal proteins such as transferrin receptor CD71 (Tfrc), glycoprotein A (Gypa), band 3 (Slc4a1), and α-spectrin (Spta1) are most significantly expressed in ERP2 to ERP4. ERP5 was marked by genes known to be expressed in orthochromatic erythroblasts and reticulocytes such as Bpgm and Xpo721-23 and was the most frequent erythroblast population in unsorted and in CD71+ population sorted from the EBIs, as expected for the most mature erythroblasts. CD11b+ sorted cells segregate into 3 transcriptionally distinct clusters that represent granulocytic precursors associated with previously defined neutrophil specification (proNeu) and commitment cell states (preNeu to immNeu).24 Downregulation of cell cycle–related genes Mcm2, Aurkb, and Cdkn2c, especially in ERP5 and immNeu, confirms that these populations consist of terminally differentiated erythroid and granulocytic cells.
CD71+ cells that were fluorescence-activated cell sorted from EBIs generated 5 clusters of erythroid precursors (ERP1-5) confirmed to be erythroblasts by the high expression of well-known erythroid genes in a pattern demonstrating the continuum of terminal erythropoiesis24-26 (Figure 3D). CD11b+ sorted cells segregated into 3 transcriptionally distinct clusters that represent granulocytic precursors.27 The first stage, proNeu, is marked by genes with expression largely restricted in myeloblasts (Ctsg, Mpo, Elane, Gfi1). A gradual increase of Slpi along with persistent expression of S100a8 and Camp mark the later stages of neutrophil maturation.28 The last cluster is marked by the peak of Mmp9 expression, a tertiary granule protein indicating the terminal stages of granulopoiesis29 (Figure 3D). The identification of sequential states of granulocytic maturation by scRNA-seq analysis is consistent with our flow cytometry finding of maturing granulocytic precursors enriched in the EBI clusters (Figure 2C-D).
The F4/80+ sorted cells were composed of multiple clusters with extensive heterogeneity and low representation (∼20%) in the unsorted data set. The major lineage (monocytic and macrophage) exhibited expression of expected macrophage genes such as Csf1R, Irf8, and Klf428 along with tissue-resident macrophage genes such as Fn1 and Fsp1/S100A4.30-32 The remaining F4/80+ cells contained gene signatures of several miscellaneous cell populations, including multilineage progenitors, megakaryocytes, T cells, and B cells, which may be present in other BM clusters cosedimenting along the EBIs/EMBIs in the 3% BSA fraction. This result is consistent with substantial heterogeneity within the F4/80+ EBI macrophage population,14 and also points to the difficulty isolating EBI macrophages from other cells for studies based solely on immunophenotype.13,33
The EBI balances erythropoiesis and granulopoiesis
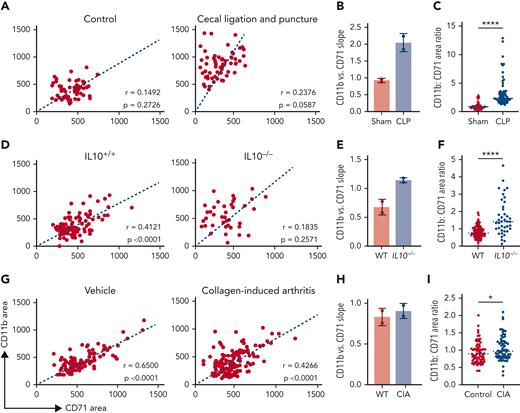
Based on our observation of granulocytic maturation within EBIs, we hypothesized that this common niche might regulate the balance between these 2 lineages. To test this hypothesis, we used biological models of altered granulopoiesis to evaluate concurrent changes in erythropoiesis within EBIs/EMBIs using IFC. EBIs collected from mice injected with GCSF to induce granulocyte production exhibited a dose-dependent increase in the ratio of CD11b+ to CD71+ cells within the EBI population specifically (Figure 4A-C). On the contrary, in Gfi1−/− mice, a model of severe congenital neutropenia,27,34 we observed the reverse effect, with a decreased ratio of CD11b+ to CD71+ area within the islands (Figure 4D-F), reflecting impaired granulopoiesis in these mice.
Changing the balance of granulocyte and erythrocyte production within EBIs. Each point represents an EBI observed by IFC; all EBIs observed in one biological replicate are shown. (A) Administration of GCSF leads to an increase in CD11b+ cells within the EBIs along with a decrease in the number of BM EBIs that contain 2 or more erythroblasts per cluster. Plots of CD11b+ area vs CD71+ area measured by IFC for all EBIs in a representative experiment for each condition are shown. In the case of 250 μg/kg GCSF treatment which dramatically suppressed medullary erythropoiesis, clusters with 3 CD71+ cells were rare because of the overall paucity of erythroblasts in the BM, so clusters with just 2 CD71+ cells were considered as EBIs in this analysis. Spearman correlation coefficient r values are shown on the graphs (P < .0001). (B) Quantification of the slope of CD11b+ vs CD71+ area in control and GCSF-injected (n = 3, mean ± SD is shown in the bar graphs; ∗∗P < .01 based on unpaired Student t test). (C) Ratio of CD11b:CD71 area within each EBI, with the line representing the median CD11b+:CD71+ area ratio (∗∗∗∗P < .0001 by Mann-Whitney test). (D) EBIs from Gfi1−/− mice, which have an arrest in early granulopoiesis, show the reverse trend as imaged by IFC, with fewer CD11b+ cells and a predominance of CD71+ cells within the EMBIs. Of note, these mice were analyzed at 8 to 9 weeks of age because of the early mortality associated with complete deficiency of Gfi1. (E) Quantification of the slope of CD11b+ vs CD71+ area in control and Gfi1−/− mice (n = 3-4 biologic repeats per condition as shown, mean ± SD is shown in the bar graphs; ∗P = .0306 based on unpaired Student t test). (F) Ratio of CD11b+:CD71+ area within each EBI, with the line representing the median CD11b:CD71 area ratio (∗∗∗∗P < .0001 by Mann-Whitney test).
Changing the balance of granulocyte and erythrocyte production within EBIs. Each point represents an EBI observed by IFC; all EBIs observed in one biological replicate are shown. (A) Administration of GCSF leads to an increase in CD11b+ cells within the EBIs along with a decrease in the number of BM EBIs that contain 2 or more erythroblasts per cluster. Plots of CD11b+ area vs CD71+ area measured by IFC for all EBIs in a representative experiment for each condition are shown. In the case of 250 μg/kg GCSF treatment which dramatically suppressed medullary erythropoiesis, clusters with 3 CD71+ cells were rare because of the overall paucity of erythroblasts in the BM, so clusters with just 2 CD71+ cells were considered as EBIs in this analysis. Spearman correlation coefficient r values are shown on the graphs (P < .0001). (B) Quantification of the slope of CD11b+ vs CD71+ area in control and GCSF-injected (n = 3, mean ± SD is shown in the bar graphs; ∗∗P < .01 based on unpaired Student t test). (C) Ratio of CD11b:CD71 area within each EBI, with the line representing the median CD11b+:CD71+ area ratio (∗∗∗∗P < .0001 by Mann-Whitney test). (D) EBIs from Gfi1−/− mice, which have an arrest in early granulopoiesis, show the reverse trend as imaged by IFC, with fewer CD11b+ cells and a predominance of CD71+ cells within the EMBIs. Of note, these mice were analyzed at 8 to 9 weeks of age because of the early mortality associated with complete deficiency of Gfi1. (E) Quantification of the slope of CD11b+ vs CD71+ area in control and Gfi1−/− mice (n = 3-4 biologic repeats per condition as shown, mean ± SD is shown in the bar graphs; ∗P = .0306 based on unpaired Student t test). (F) Ratio of CD11b+:CD71+ area within each EBI, with the line representing the median CD11b:CD71 area ratio (∗∗∗∗P < .0001 by Mann-Whitney test).
To further test this hypothesis, we examined the balance of these lineages within EBIs in AoI, a common cause of anemia in patients with acute or chronic immune activation. We tested 3 different mouse models of AoI: (1) the cecal ligation and puncture model, broadly used to study sepsis and infection-mediated AoI35,36 (Figure 5A-C); (2) the IL10−/− mice, which develop chronic colitis and are used as a mouse model of inflammatory bowel disease37 (Figure 5D-F); and (3) the collagen-induced arthritis model (Figure 5G-I).38 In all 3 models, the ratio of CD11b+ to CD71+ area in the EBIs increased in the inflamed mice vs their corresponding controls; the expansion of CD11b+ cells within the EBIs occurred at the expense of the erythroblasts, resulting in compromised terminal erythropoiesis within the BM and anemia.
The balance between CD71+ erythroblasts and CD11b+ granulocytes in the EBIs is altered in AoI. EBI analysis was performed for 3 different models of AoI. Representative plots of CD11b+ area vs CD71+ area for each of the 3 models of AoI and corresponding controls are shown in order of decreasing acuity and consequently decreasing CD11b vs CD71 slope. Each point represents an EBI observed by IFC; all EBIs observed in one biological replicate are shown. Spearman correlation coefficient r values along with corresponding P values for correlation are shown on the graphs. (A) Cecal ligation and puncture (CLP) model resembles sepsis and shows the most dramatic increase in CD11b+ vs CD71+ slope. (B) Quantification of the slope of CD11b+ vs CD71+ area in control and CLP (n = 2, mean ± SD is shown in the bar graphs). (C) Ratio of CD11b+:CD71+ within each EBI (median shown in graph; ∗∗∗∗P < .0001 by Mann-Whitney test). (D) IL10−/− mice, when they develop inflammatory bowel disease, show a moderate increase in CD11b vs CD71 slope. (E) Quantification of the slope of CD11b vs CD71 area in control and IL10−/− (n = 2; mean ± SD is shown in the bar graphs). (F) Ratio of CD11b+:CD71+ within each EBI (median shown in graph; ∗∗∗∗P < .0001 by Mann-Whitney test). (G) Collagen-induced arthritis (CIA) models show mild inflammation relative to the CLP and IL10−/− with colitis models associated with a mild increase in CD11b+ vs CD71+ slope. (H) Quantification of the slope of CD11b vs CD71 area in control and CIA (n = 2, mean ± SD is shown in the bar graphs). (I) Ratio of CD11b+:CD71+ within each EBI (median shown in graph, ∗P < .05 by Mann-Whitney test). No statistical test was performed for the comparison of slopes in B, E, and H, in which 2 samples for each experimental and each control mouse model of AoI were analyzed.
The balance between CD71+ erythroblasts and CD11b+ granulocytes in the EBIs is altered in AoI. EBI analysis was performed for 3 different models of AoI. Representative plots of CD11b+ area vs CD71+ area for each of the 3 models of AoI and corresponding controls are shown in order of decreasing acuity and consequently decreasing CD11b vs CD71 slope. Each point represents an EBI observed by IFC; all EBIs observed in one biological replicate are shown. Spearman correlation coefficient r values along with corresponding P values for correlation are shown on the graphs. (A) Cecal ligation and puncture (CLP) model resembles sepsis and shows the most dramatic increase in CD11b+ vs CD71+ slope. (B) Quantification of the slope of CD11b+ vs CD71+ area in control and CLP (n = 2, mean ± SD is shown in the bar graphs). (C) Ratio of CD11b+:CD71+ within each EBI (median shown in graph; ∗∗∗∗P < .0001 by Mann-Whitney test). (D) IL10−/− mice, when they develop inflammatory bowel disease, show a moderate increase in CD11b vs CD71 slope. (E) Quantification of the slope of CD11b vs CD71 area in control and IL10−/− (n = 2; mean ± SD is shown in the bar graphs). (F) Ratio of CD11b+:CD71+ within each EBI (median shown in graph; ∗∗∗∗P < .0001 by Mann-Whitney test). (G) Collagen-induced arthritis (CIA) models show mild inflammation relative to the CLP and IL10−/− with colitis models associated with a mild increase in CD11b+ vs CD71+ slope. (H) Quantification of the slope of CD11b vs CD71 area in control and CIA (n = 2, mean ± SD is shown in the bar graphs). (I) Ratio of CD11b+:CD71+ within each EBI (median shown in graph, ∗P < .05 by Mann-Whitney test). No statistical test was performed for the comparison of slopes in B, E, and H, in which 2 samples for each experimental and each control mouse model of AoI were analyzed.
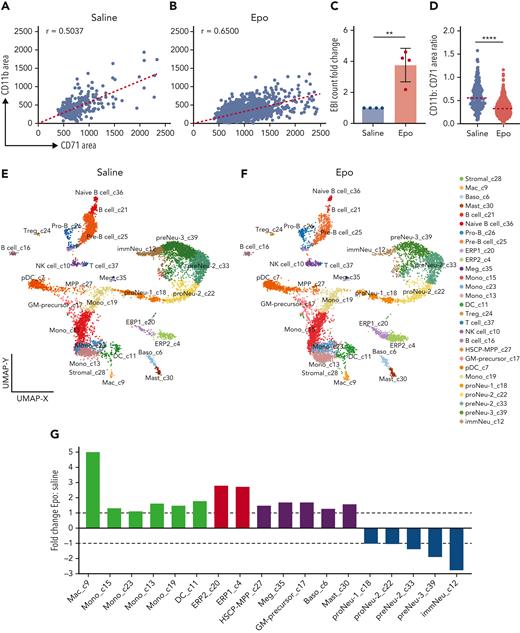
We next asked how this balance within EBIs changes during stress erythropoiesis, such as that induced by Epo administration. After Epo or saline solution (control) administration for 10 days, mouse BM EBIs were quantified using IFC. Increased CD71+ area and decreased slope of CD11b+ vs CD71+ indicated an increased number of erythroblasts per EBI following Epo treatment. There was also a significant increase in the number of EBIs, indicating that stress erythropoiesis increases not only the number of erythroblasts but also the number of EBI macrophages (Figure 6A-D).
EBIs and EBI macrophages are increased following administration of Epo, permitting characterization of EBI macrophages by CITE-seq. IFC analysis of EBIs from saline solution–treated (A) vs Epo-treated (B) mouse BM demonstrates an increase not only in the size of EBIs (increased CD71+ area) but also in the number of EBIs after Epo stimulation, indicating a parallel increase in the number of EBI macrophages. Each point represents an EBI observed by IFC; all EBIs observed in one biological replicate are shown. Spearman correlation coefficient r values are shown on the graphs (P < .0001). (C) The number of EBIs after Epo stimulation increased approximately 4-fold by IFC evaluation (n = 4 biologic repeats, mean ± SD shown in the bar graph; ∗∗P = .002 comparing raw values with unpaired Student t test). (D) Ratio of CD11b+:CD71+ area within each EBI (median shown in graph; ∗∗∗∗P < .0001 by Mann-Whitney test). (E,F) ICGS2 (Iterative Clustering and Guide-gene selection version 2) analysis and CellHarmony of single-cell CITE-seq data collected from the BM clusters enriched in EBIs of saline solution–treated (E) and Epo-treated (F) BM reveals 28 distinct clusters. Clusters 4 and 20 are composed of early erythroblasts remaining despite Ter119+ depletion; clusters 18, 22, 33, 39, and 12 are granulocyte precursors that were also not completely removed despite depletion for Ly6G; and clusters 9, 13, 15, 23, and 19 have a transcriptome compatible with macrophage/monocyte lineage. Cluster 7 demonstrates transcriptomic characteristics of plasmacytoid dendritic cells, such as Siglech, Bst2, and Ly6d, and therefore is not considered a macrophage subset. (G) Fold change in relative frequencies of the cells in the macrophage/monocyte, erythroid, and granulocytic clusters at baseline vs with Epo treatment as indicated by percentage of captured cells in each sample.
EBIs and EBI macrophages are increased following administration of Epo, permitting characterization of EBI macrophages by CITE-seq. IFC analysis of EBIs from saline solution–treated (A) vs Epo-treated (B) mouse BM demonstrates an increase not only in the size of EBIs (increased CD71+ area) but also in the number of EBIs after Epo stimulation, indicating a parallel increase in the number of EBI macrophages. Each point represents an EBI observed by IFC; all EBIs observed in one biological replicate are shown. Spearman correlation coefficient r values are shown on the graphs (P < .0001). (C) The number of EBIs after Epo stimulation increased approximately 4-fold by IFC evaluation (n = 4 biologic repeats, mean ± SD shown in the bar graph; ∗∗P = .002 comparing raw values with unpaired Student t test). (D) Ratio of CD11b+:CD71+ area within each EBI (median shown in graph; ∗∗∗∗P < .0001 by Mann-Whitney test). (E,F) ICGS2 (Iterative Clustering and Guide-gene selection version 2) analysis and CellHarmony of single-cell CITE-seq data collected from the BM clusters enriched in EBIs of saline solution–treated (E) and Epo-treated (F) BM reveals 28 distinct clusters. Clusters 4 and 20 are composed of early erythroblasts remaining despite Ter119+ depletion; clusters 18, 22, 33, 39, and 12 are granulocyte precursors that were also not completely removed despite depletion for Ly6G; and clusters 9, 13, 15, 23, and 19 have a transcriptome compatible with macrophage/monocyte lineage. Cluster 7 demonstrates transcriptomic characteristics of plasmacytoid dendritic cells, such as Siglech, Bst2, and Ly6d, and therefore is not considered a macrophage subset. (G) Fold change in relative frequencies of the cells in the macrophage/monocyte, erythroid, and granulocytic clusters at baseline vs with Epo treatment as indicated by percentage of captured cells in each sample.
CITE-seq highlights the immunophenotypic and transcriptomic heterogeneity of EBI macrophages
The macrophage populations captured in our scRNA-seq experiments, out of unsorted EBI constituent cells and F4/80+ sorted EBI cells, were rather small in terms of the number of cells to permit further identification studies of the EBI macrophages. To evaluate the EBI macrophages on a comprehensive level, we employed a multimodal single-cell analysis of the transcriptome and diverse cell surface epitopes with CITE-seq.39 CITE-seq circumvents compensation issues seen in traditional flow cytometry, which can become increasingly complex on highly autofluorescent cells such as macrophages. Because EBI macrophages increase after Epo administration, we proceeded to use EBIs from mouse BM after Epo administration for 10 days along with control BM EBIs from a mouse following saline solution administration. Moreover, the cells isolated from the 3% BSA fraction were subjected to red cell lysis and negative selection against erythroid, lymphoid, granulocytic, and eosinophilic lineages, leading to significant enrichment of EBI macrophages and other rare populations (supplemental Figure 6A). Accumax solution was added to further disrupt tightly associated EBIs, which can remain intact after magnetic separation if this dissociation step is not implemented (supplemental Figure 6B-F).
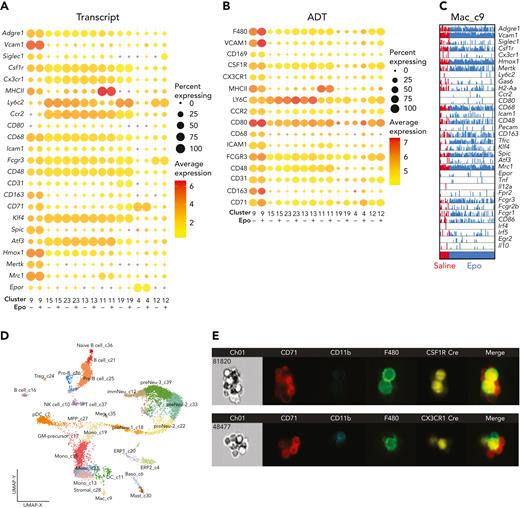
Joint unsupervised analysis of CITE-seq transcriptomes from control and Epo-stimulated EBIs revealed 28 transcriptionally distinct populations, with representation of both captures in all cell populations (Figure 6E-F). Cluster 9 contained classically defined EBI macrophages expressing Hmox1, Mertk, and Siglec1 transcripts and carrying F4/80 and Vcam1 antigens.12,40 Clusters 13, 15, 19, and 23 were defined by well-known monocytic markers such as Ly6c2 and Ccr2, but also found to express Fn1, S100a4, and F13a1, compatible with monocyte-derived BM macrophages (online viewer: http://www.altanalyze.org/ICGS/Public/Mm_EBMI-r2/User.php). Although we cannot fully exclude contamination from other cell types, a few of the macrophages in cluster 9 also express Ly6c2 and Ccr2, more so in the Epo-treated sample rather than the saline solution–treated sample, suggesting heterogeneity among the EBI macrophages and the possibility that monocyte-derived cells may fill the need for more EBI macrophages in stress erythropoiesis (Figure 7A-C). Identified populations were further characterized by immunophenotypic cell surface–specific ADTs (supplemental Figure 7).
CITE-seq of EBI constituent cells coupled with IFC demonstrates EBI macrophage heterogeneity and plasticity. (A,B) Bubble plots of mRNA (A) and ADTs (B) comparing classically defined EBI macrophage cluster 9 with the other macrophage/monocyte clusters in the CITE-seq capture, along with erythroid cluster 4 and granulocyte cluster 12 for comparison. The size of the bubble indicates the percentage of cells from each treatment within the cluster expressing the gene or being positive for the ADT, and the color of the bubble represents the average expression level. (C) Comb plot of cells in cluster 9 demonstrates the heterogeneity within EBI macrophage populations in saline solution– and Epo-treated mice. (D) Uniform Manifold Approximation and Projection (UMAP) plot of CITE-seq as a reference for the clusters included in the bubble plot. (E) IFC demonstrates positivity of EBI macrophages for Csf1R and Cx3cr1 using the tdTomato-reporter of the corresponding cre models.
CITE-seq of EBI constituent cells coupled with IFC demonstrates EBI macrophage heterogeneity and plasticity. (A,B) Bubble plots of mRNA (A) and ADTs (B) comparing classically defined EBI macrophage cluster 9 with the other macrophage/monocyte clusters in the CITE-seq capture, along with erythroid cluster 4 and granulocyte cluster 12 for comparison. The size of the bubble indicates the percentage of cells from each treatment within the cluster expressing the gene or being positive for the ADT, and the color of the bubble represents the average expression level. (C) Comb plot of cells in cluster 9 demonstrates the heterogeneity within EBI macrophage populations in saline solution– and Epo-treated mice. (D) Uniform Manifold Approximation and Projection (UMAP) plot of CITE-seq as a reference for the clusters included in the bubble plot. (E) IFC demonstrates positivity of EBI macrophages for Csf1R and Cx3cr1 using the tdTomato-reporter of the corresponding cre models.
As expected, the CITE-seq of Epo-stimulated BM captured a higher percentage of erythroid cells, whereas the late stages of granulocyte precursors were decreased (Figure 6G). Notably, there was a 5-fold higher number of the cluster 9 macrophages in the Epo- vs the saline solution–stimulated sample, supporting the notion that these are indeed EBI macrophages. Comprehensive differential expression analyses between Epo-stimulated and control using cellHarmony yielded hundreds of differentially expressed genes, which largely localize to distinct stages of erythropoiesis and granulopoiesis (supplemental Figure 7A). Interestingly, there were no differentially expressed genes between treatment groups in cluster 9 macrophages. This may indicate the specialized, optimal nature of the EBI macrophage in supporting terminal erythropoiesis, necessitating an increase in the number of macrophages rather than altering their characteristics during stress-erythropoiesis in the marrow.
We observed a clear increase in the number of cells with transcriptional and ADT characteristics of EBI macrophages following Epo administration, as demonstrated in bubble plots depicting the average frequency and level of a given transcript or ADT in each treatment within a given cluster (Figure 7A-D) or within a given cluster (supplemental Figure 8A-B). IFC provides evidence of macrophage fragmentation on CD71+ and CD11b+ cells, perhaps explaining the presence macrophage specific epitopes on erythroblast and granulocyte clusters (supplemental Figure 8C). Additionally, there were notable differences in intrapopulation levels of each transcript and ADT and the number of cells expressing a gene or carrying an antigen, which can be visualized through the variable size and color of bubbles in a column, indicating a marked degree of heterogeneity between clusters and within clusters. This can be visualized in higher resolution using a comb plot of EBI macrophage-related genes in the cluster 9 population (Figure 7C) or all the macrophage and monocyte populations (online viewer). Although some transcripts, such as Adgre1 and Vcam1, are expressed in most cells, others such as Fcgr1, Cd163, and Siglec1 are present at variable levels in a fraction of the cells in cluster 9, suggesting differences in function and/or origin at baseline and in stress-erythropoiesis (Figure 7C). IFC of EBIs from Csf1r-Cre or Cx3cr1-Cre mice with tdTomato reporter of Cre expression demonstrates tdTomato fluorescence in the majority of EBI macrophages, indicating lineage tracing from Csf1r- or Cx3cr1-expressing cells, respectively (Figure 7E). Nevertheless, our CITE-seq data demonstrate that Csfr1 is a sensitive but not specific marker for the cluster 9 population whereas Cx3cr1 is heterogeneous and also nonspecific for this cluster (online viewer). Similarly, Epo receptor (Epor) is sparsely expressed in macrophages of cluster 9 as well as in a few macrophages of the other clusters, in contrast to a recently published study considering Epor as a transcript defining EBI macrophages.24
M1 and M2 markers were also queried within each cluster (supplemental Figure 8B). No distinct pattern of M1 and M2 markers emerged, in agreement with the observations that M1 and M2 macrophage responses typically intermix41,42 in order for a macrophage population to serve the complicated role of the terminal erythropoiesis and granulopoiesis niche. Of note, we did observe high expression in the cluster 9 macrophages of the canonical M2 markers Mrc1 (Cd206) and Cd163 (Figure 7A-C; supplemental Figure 7B). These receptors are known to be involved in phagocytosis (along with Mertk, even though this is not an M2 marker), consistent with a requirement of the EBI macrophages to phagocytose extruded nuclei and the increased demand for this function under stress.43,44 However, we also noted increased representation of M1 markers such as Cd86, Cd38, and several Fcγ receptors in the same population (supplemental Figure 7B). Thus, it appears that M1 and M2 characteristics are involved in EBI macrophage function. Overall, EBI macrophages are a heterogeneous population at the transcriptomic and immunophenotyping level, clearly seen in the interactive online viewer.
Discussion
We previously demonstrated that CD11b is expressed on cells within the EBI, but not by the EBI macrophage itself.14 In the present study, we identified these CD11b+ cells as granulocyte precursors maturing in close association with the EBI Mφ. We used density gradient sedimentation to collect native BM clusters in the densest fraction, enriching for EBIs independent of immunophenotype, while single cells remain in the lightest fraction. Not all EBIs remain intact through the process of BM extraction, sedimentation, staining, and IFC processing, but many do, allowing relative quantification, ie, comparisons between an experimental and a control condition performed in parallel, when the technique is standardized. Erythroblast and granulocyte precursors are increased within the EBI fraction compared with total BM. Consistent with IFC data, confocal microscopy of BM in situ revealed CD11b+ cells intimately associated with the Mφ in EBIs. We performed EBI reconstitution experiments to exclude the possibility that the presence of these cells within the EBIs is a mere artifact. Our finding appears in contrast with recent work in which CD11b+ cells observed in EBIs analyzed by IFC were described as peripherally associated bystanders.19 However, the same group later reported that CD11b+ cells can have membrane remnants from EBI macrophages (positive for F4/80, VCAM1, and CD169) on their cell surface,33 as we also see (supplemental Figure 8C), indicating an intimate association in vivo compatible with the conclusions of the present study. Such mixed hematopoietic clusters containing erythroid and granulocytic precursors were previously observed in the 1970s and ‘80s20,21; however, the recently developed advanced methods and technology we employed here allowed for further investigation of these clusters and their pathophysiological functions.
scRNA-seq of EBI component cells shows maturing erythroblasts and granulocyte precursors along with macrophages as the 3 predominant cell types within the EBI. Further supporting the notion that the EBI acts as the niche for erythroid and granulocytic precursors, we found that CD11b+ cells within the EBI increased compared with CD71+ cells with administration of GCSF and in mouse models of AoI. GCSF was previously stated to cause a collapse of EBIs in the BM.19 Our study indicates a decrease in number of EBIs satisfying their conventional definition by IFC, ie, a macrophage with 3 or more surrounding erythroblasts.14,19 Granulocyte precursors proliferate excessively in response to GCSF administration and outcompete the erythroblasts in their common niche of terminal erythropoiesis and granulopoiesis, leaving few macrophages surrounded by 3 or more erythroblasts. In a mouse model of neutropenia, we found a shift toward CD71+ area indicating fewer granulocytic cells and an increased proportion of erythroblasts within the EBI. Induction of stress erythropoiesis by Epo administration resulted in an increase not only of the number of erythroblasts within the EBIs, but also the overall number of EBIs, and thus EBI macrophages, demonstrating the plasticity of the EBI macrophages and their ability to act as a fulcrum in the balance between erythropoiesis and granulopoiesis. We anticipate that our findings will help answer critical questions in the fields of normal and disordered erythropoiesis, and more specifically for AoI. Indeed, although it has been acknowledged that emergency granulopoiesis takes place in humans during inflammation, the origin of the associated anemia remains unclear. Our recent studies11,36 have begun to explore these phenomena, identifying a block of Epo signaling during inflammation. Future work will entail testing of the different proinflammatory cytokines on cultured EMBI from human BM and measurement of the changes in the island composition.
The mechanisms by which the Mφ supports and regulates erythropoiesis are still not clear enough to be manipulated for therapeutic purposes in vivo or in vitro, at least in part because of inconclusive characterization of the EBI macrophages. Previous studies have used single or combination immunophenotypic cell-surface markers,12,13,40 fate-mapping reporters,45,46 or transgenic fluorescent proteins17,18 to track and manipulate macrophages, providing valuable insights. However, these markers are neither specific nor all-inclusive for the population of EBI macrophages, as can be seen by our CITE-seq data, which demonstrates a significant heterogeneity. By comparing CITE-seq captures on Epo- and saline solution–treated mouse BM EBIs, we identified a macrophage population (cluster 9) with transcriptional and immunophenotypic features consistent with classically described EBI macrophages that increased 5-fold upon Epo administration, consistent with our IFC observations that total EBIs increase approximately 4-fold. The expansion of EBI macrophages in response to Epo appears to be in agreement with a recently published study18 indicating that EBI macrophages express the Epo receptor (Epor). However, we found that Epor expression was sparse within this population and not a defining feature, suggesting that the EBI macrophage response to Epo could be only for a subset of the cluster 9 cells or be non–cell-autonomous, resulting from signaling within the niche. Alternatively, it may be mediated via membrane swapping between erythroblasts and macrophages, donating Epor functionality to the latter, in a similar manner to that noted by Millard et al33 between granulocyte precursors and macrophages.
This heterogeneity in EBI macrophage populations may reflect the heterogeneity of EBIs. We observed that, in homeostatic BM, approximately 25% of EBIs are pure EBIs whereas 75% also host granulocytic precursors. In this context, one may suggest that the Mφs serving as niches for terminal hematopoiesis are also heterogeneous to support a variety of combinations of differentiating erythroid and granulocytic precursors and to adapt to different pathophysiologic needs of blood cell production. Our CITE-seq analysis revealed additional populations of monocytes and/or monocyte-derived macrophages expressing fibronectin, which may serve as niches for early erythroblasts binding to them via the α5β1 integrin (VLA-5)47,48 or may support hematopoietic progenitors, additional granulocytic precursors, or lymphoid cells also found within the BM clusters collected at the 3% BSA fraction by gravity sedimentation. One limitation of our study resides in the technical challenges of the process to enrich EBIs and then isolate EBI macrophages. Contamination from different cell types is unavoidable, and we cannot completely exclude that this may interfere with clustering of the different populations, despite robust bioinformatics analysis. Further work is needed to identify BM clusters coprecipitating in the 3% BSA fraction using IFC and delineate the functions of these various macrophage populations identified by CITE-seq.
It was recently shown that resident macrophages are highly prone to fragmentation, precluding capture in scRNA-seq experiments.33 Even though there may be significant fragmentation, the prominent reason for lack of recovery is more likely improper dissociation. EBIs are difficult to dissociate and, as a cluster, would be impassable through the 10x chromium controller. Extensive dissociation measures and depletion of more abundant cell types increased recovery in CITE-seq experiments. Evidence of contamination possibly resulting from membrane switching or fragmentation may still be a confounding factor in these studies. Transcripts from recently phagocytosed erythroblasts may also contaminate the gene expression profiles of the macrophages. Nevertheless, coupling CITE-seq with IFC brings forward potential EBI macrophage markers and relevant model systems for studying EBI macrophages. In summary, our studies from CITE-seq analysis of the macrophage and monocyte populations involved in EBIs and other hematopoietic BM clusters lay the foundation for a better understanding of the regulation of blood cell production within these niches in normal and pathological states.
Acknowledgments
This work was funded by National Institutes of Health grants R01HL152099, R01HL122661, and U54 DK126108. The authors thank Matthew Flick for contributing collagen-induced arthritis mice and Michael J. Rosen for contributing IL10−/− mice, Andrew Volk for recommendation of the Accumax dissociation solution, and Annie Song for her advice on the CITE-seq protocol and ADT titration. The authors acknowledge the Research Flow Cytometry Core in the Division of Rheumatology at Cincinnati Children’s Hospital Medical Center for support and maintenance of the flow cytometry and IFC instruments. Single-cell and CITE-seq captures were acquired using equipment provided by the Single Cell Characterization and Procurement Core of the Cincinnati Center of Excellence in Molecular Hematology, and sequencing was performed in the DNA Core at Cincinnati Children’s Hospital Medical Center. Illustrations in figures were created with BioRender.
Authorship
Contribution: L.R. and K.G.S. are joint first authors, listed alphabetically; L.R. and K.G.S. designed, performed, analyzed the research and drafted the manuscript; J.P., D.E.M., and D.K. performed experiments and analyzed data; N.S. performed bioinformatics analyses of scRNA-seq and CITE-seq with assistance from K.C., K.S., and L.R.; D.E.M. and A.O. consulted on analysis of myeloid populations and preparation for scRNA-seq respectively; B.J.B., N.M., J.A.C., Y.Z., and H.L.G. provided valuable scientific insight and advised on protocols and experiment design; and L.B. and T.A.K. designed experiments, analyzed data, and edited the manuscript. All authors reviewed and contributed to the manuscript.
Correspondence: Theodosia A. Kalfa, Cancer and Blood Diseases Institute, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave, MLC 7015, Cincinnati, OH 45229-3039; e-mail: theodosia.kalfa@cchmc.org.
References
Author notes
∗L.R. and K.G.S. are joint first authors.
†L.B. and T.A.K. contributed equally to this study.
The scRNA-seq and CITE-seq datasets have been deposited into Gene Expression Omnibus with the accession number GSE196347.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal