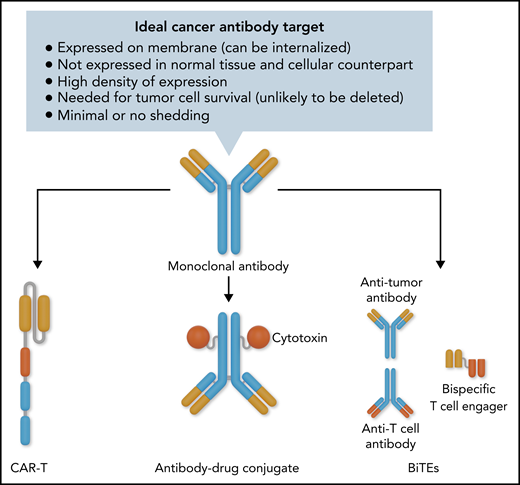
In this issue of Blood, Anderson et al identified a new membrane associated antigen, semaphorin-4A (SEMA4A), which is a strong candidate for immunotherapy in myeloma. They used an interesting strategy to prioritize potential candidate targets for immunotherapy1: mass spectrometry-based plasma membrane profiling of cells from myeloma patient and myeloma cell lines coupled with a novel vector-based prioritization score of antigens detected.
Immunotherapy is a broad term encompassing different therapeutic strategies that helps the patient’s immune system fights cancer. It has had a great impact in treating cancers and comes in 7 different formats:
- 1.
Monoclonal antibodies that engage with natural killer cells or macrophages to induce antibody-dependent cellular cytotoxicity or macrophage-dependent cellular cytotoxicity, respectively. Examples include rituximab (anti-CD20) and daratumumab (anti-CD38).
- 2.
Antibody drug conjugates (ADCs), in which the antibody homes to the tumor cells to selectively deliver conjugated cytotoxic drugs to the tumor. This requires the antigenic target to be internalized so that the drug carried by the antibody can be internalized to kill the tumor cells. An example is belantamab mafodotin, which targets the B-cell maturation antigen (BCMA). A related format is radioimmunotherapy, in which radioisotopes are delivered to tumor cells. An example is 90Y ibritumomab tiuxetan targeting CD20.
- 3.
Bispecific T-cell engagers, which have 2 binding domains, 1 targeting the antigen on the tumor cells and the other targeting T cell, so that the T cells are brought close to the tumor cells and may be activated to eradicate the tumor cells. An example is blinatumomab targeting CD19.
- 4.
Chimeric antigen receptor T cells (CAR-T), in which T cells from donors (autologous or allogeneic) are modified with the introduction of a CAR construct compromising a targeting scFV (similar to antibody-binding sequences) and intracellular T-cell signaling and activation domains. When these modified T cells bind to the protein targets, they are activated to kill the tumor cells. Examples include tisagenlecleucel (targeting CD20) and idecabtagene vicleucel (targeting BCMA).
The above 4 formats are based on antibody binding and require a membrane-associated protein that binds to the therapeutic agent (see figure).
- 5.
Cancer vaccine, in which tumor-specific antigens are used to help the immune system learn to recognize and react to these antigens and destroy the cancer cells that contain them.
- 6.
Checkpoint inhibitors, which are drugs that block the immune checkpoint proteins to increase the activity of T cells that may be exhausted in cancers. An example is the PD1 inhibitor
- 7.
Cytokines, such as interferons and interleukins, which can activate immune response against cancer.
Ideal cancer target for antibody-based immunotherapy with the various formats available once a good target and antibody is developed. Professional illustration by Somersault18:24.
Ideal cancer target for antibody-based immunotherapy with the various formats available once a good target and antibody is developed. Professional illustration by Somersault18:24.
In myeloma, several of these immunotherapies are now in the clinic and have improved outcomes. These include daratumumab, an anti-CD38 antibody, and elotuzumab, a monoclonal antibody targeting SLAMF7.2 More recently, BCMA has been identified as a key target in myeloma and used as a platform for different immunotherapy formats.3 Belantamab mafodotin was the first BCMA-targeting agent approved for myeloma. BCMA CAR-T has shown remarkable activity, with the first, idecabtagene vicleucel, approved for clinical use.4 BCMA bispecific T-cell engagers have also shown activity in myeloma and several are in clinical trials. Other targets, GPRC5D and FcRH5, have also shown promise.5
However, none of these treatments is currently curative. Loss of therapeutic activity eventually happens through various mechanisms, including antigen loss through shedding or even genetic deletions,6 as well as interference by soluble ligands.7 Therefore, the identification of additional targets is important for salvage therapy following relapse from existing immunotherapy. The use of combinations of targets may also provide broader antigen coverage that may improve outcomes.
In this context, the current work is important for a few reasons. The authors identified a new immunotherapeutic target, SEMA4A, that has higher expression than BCMA and SLAMF7 in myeloma cells and is rapidly internalized, making it a good target for ADCs. ADCs are worth further exploration in myeloma because they do not rely on the immune system of the patients, which tend to be impaired from treatment and disease. SEMA4A may also be a good target because it has minimal shedding and seems to be essential to the survival of myeloma cells, making it less likely to be genetically deleted. In addition, the techniques used by the authors can be replicated in other cancers. Their vector-based scoring system to prioritize candidate proteins is flexible, allowing for adjustment of the parameters used and the weightage assigned to each parameter. In the current study, the 3 parameters used included high expression in myeloma cells, low expression in normal tissues, and the size of ectodomain as a surrogate for the number of unique B-cell epitopes. Despite the use of this scoring system, SEMA4A is also expressed in normal plasma cells and may be a marker of late B-cell differentiation. It is also expressed to some extent on monocytes. Although it is true that plasma cell and monocyte depletion may not lead to significant short-term toxicities but there may be long-term immunosuppression, including inadequate immune response to vaccination, particularly with long-term continuous use or persistence in the case of CAR-T. This has been highlighted by current studies of response to COVID vaccination in patients with myeloma treated with daratumumab and BCMA targeting therapies.8
The identification of membrane antigens or epitopes that differentiate malignant from normal cells remains an important challenge.9 Most, if not all, antibody-based immunotherapeutic targets are antigens that are also expressed on normal cellular counterparts. Therefore, although effective in eradicating the blood cancer cells, these immunotherapies also significantly affect normal immune cells, resulting in significant immunosuppression with increased risk of infections. We may need to look beyond proteins but also cancer-specific modifications on the proteins.10
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal