Abstract
18F-fluorodeoxyglucose positron emission tomography-computed tomography (PET-CT) is now established as the gold-standard imaging modality for both staging and response assessment in follicular lymphoma (FL). In this Perspective, we propose where PET can, and cannot, guide clinicians in their therapeutic approach. PET at diagnosis and pretreatment is important for staging, with greater sensitivity compared with standard CT, and consequent improved outcomes in truly limited-stage FL. Small data sets suggesting that a high baseline standardized uptake value (SUVmax) identifies de novo histologic transformation (HT) have not been corroborated by data from GALLIUM, the largest prospective study to examine modern therapies for FL. Nonetheless, the role of baseline quantitative PET measures requires further clarification. The median survival of patients with newly diagnosed FL is now potentially >20 years. Treatment of symptomatic FL aims to achieve remission and optimize quality of life for as long as possible, with many patients achieving a “functional cure” at the cost of unwanted treatment effects. Several studies have identified end-of-induction (EOI) PET after initial chemoimmunotherapy in patients with a high tumor burden as strongly predictive of both progression-free and overall survival, and EOI PET is being evaluated as a platform for response-adapted treatment. Unmet needs remain: improving the inferior survival for patients remaining PET positive and quantifying the progression-free survival and time to next treatment advantage, and additional toxicity of anti-CD20 maintenance in patients who achieve complete metabolic remission. In the absence of an overall survival advantage for frontline antibody maintenance, the question of using PET to guide the therapeutic approach is more important than ever in the context of the COVID-19 pandemic.
Introduction
18F fluorodeoxyglucose (FDG) positron emission tomography combined with computed tomography (PET-CT, hereafter cited as PET) is an important imaging modality in a range of FDG-avid lymphomas. Initially, with a focus on diffuse large B-cell lymphoma (DLBCL) and Hodgkin lymphoma, PET was not considered central to staging and response assessment of the “incurable” indolent follicular lymphoma (FL). However, it has become apparent that FL is universally, albeit not uniformly, FDG avid.1-4 In this Perspective, we consider the current role of PET in the management of FL at key points in the patient pathway. We also consider the potential role of PET in identifying or predicting high-grade transformation and/or selecting optimal biopsy sites. We seek to assist clinicians in deciding how to integrate PET scanning in their own practices for each patient.
Search strategy and selection criteria
References for this perspective were identified through searches of Medline and EMBASE with the search terms “positron emission tomography” and “follicular lymphoma” from January 1, 2000 to December 31, 2019. Articles were also identified in searches of the authors’ files. Only papers published in English were reviewed. The final reference list was generated on the basis of originality and relevance to the broad scope of this Perspective.
Staging and prognostication in newly diagnosed patients
Although there are differences across health care systems, in the authors’ experience, a contrast-enhanced computed tomography (CT) scan is usually performed when the diagnosis of FL is made. Notwithstanding this, the 2014 Lugano Classification recommends PET for staging of all FDG-avid lymphomas based on the greater sensitivity of PET in detecting lymphoma in small nodes and extranodal sites compared with CT.5,6 This guidance is applicable to FL, which is FDG avid in almost all cases, albeit with variable FDG uptake between and within patients.1-4 Additional sites are detected by PET in approximately two-thirds of cases, with upstaging estimated in 10% to 60%, especially in patients with apparently limited-stage disease identified by CT.2,7-9 Retrospective reports suggest that PET scanning alters management in 5% to 25% of patients with lymphoma.10-12 In FL, more sensitive staging could lead to better informed treatment decisions, influencing both choice and timing of therapy.
Interestingly, the application of PET to clinical staging affects the performance of FL prognostic scoring systems.7 In a US SEER-Medicare database study of 5712 patients diagnosed from 2000 through 2009, the use of PET for staging was associated with more favorable overall survival (OS) (hazards ratio [HR], 0.75; 95% confidence interval [CI], 0.68-0.83) and lymphoma-specific survival (HR, 0.69; 95% CI, 0.58-0.82).13 An analysis of the distribution of treatment strategies suggested that PET affects clinical staging, prognostic evaluation, and treatment decisions. This observation was echoed in a National Comprehensive Cancer Network (NCCN) database analysis of 953 patients with grade 1 and 2 FL: the 532 (56%) patients who underwent staging by PET were more likely to receive early treatment and anthracycline-based chemotherapy.14
In keeping with this finding, a recent retrospective analysis from Memorial Sloan Kettering Cancer center (MSKCC), validated in a cohort from the Italian FOLL05 trial, compared patients staged by CT vs those staged by PET. Those staged by CT had an inferior OS, despite a similar rate of progression-free survival (PFS) at 24 months.15 The improvement in outcomes associated with PET staging most likely results from multiple factors, including the Will Rogers phenomenon associated with stage migration, as well as the recent availability of more effective treatments. In the discovery cohort, patients with progression of disease (POD) within 24 months of R-CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone+rituximab) had a 5-year OS of 57.6% for CT-staged patients compared with 70.6% for PET-staged patients. In the validation cohort, the corresponding percentages were 53.9% and 100%. Among patients with POD within 1 year of initiating therapy, the rate of histologic transformation (HT) was higher in CT-staged patients than in those staged by PET (16.7% vs 6.3%). The explanation for the observed association between PET imaging and reduced rates of HT in this retrospective study is unclear, but may reflect confounding variables.
Directly demonstrating the clinical benefit of new imaging modalities presents major challenges related to the many confounding factors that influence, or are influenced by, their application. Consequently, they are often adopted into routine practice based on improved sensitivity alone. The application of PET scanning to FL staging creates the specific challenge of knowing how best to treat patients with FL who have advanced-stage (AS) disease by PET and limited-stage (LS) disease by CT. Because the clinical course and optimal treatment of such patients have not been defined, caution should be exercised when extrapolating from data generated in patient cohorts staged by CT. Nonetheless (and abscopal effects notwithstanding), it is reasonable to assume that localized radiotherapy (RT) is unlikely to be curative in this setting. Conversely, in patients with LS disease defined by both imaging modalities, it is reasonable to assume that local RT would achieve better long-term disease control if the radiation field is based on PET rather than CT. In keeping with this idea, a recent study reported excellent outcomes in patients with LS in whom PET was used to select for radiotherapy.16 Five-year freedom from progression (FFP) was 68.9% (95% CI, 63.9-73.4) and 5-year OS was 96% (95% CI, 93.2-97.6), better than in earlier series from the pre-PET era.17 Similarly, in the MSKCC cohort cited earlier, patients with stage I or II disease defined by PET demonstrated superior OS compared with those with stage I or II disease defined by CT. For patients with stage I disease, 10-year OS was 93% and 82%, respectively (log-rank P = .005), whereas the corresponding figures for patients with stage II disease were 89% and 68%, respectively (log-rank P = .048).15 In contrast, the clinical impact of identifying more disease sites in patients who already have evidence of AS on conventional CT- and bone marrow biopsy (BMB)–based staging is limited, with only 4% of patients upstaged from stage III to IV in one series and no effect on management.2
In summary, in the patient with apparently localized disease on CT, PET may detect additional sites of disease with beneficial implications for therapeutic decision making. In contrast, for patients with asymptomatic AS disease identified on CT who do not meet criteria for commencing therapy, a PET scan can be deferred until treatment is needed.
An important, but conceptually distinct, use of PET staging in treatment-naive patients just prior to therapy is to provide a map of disease distribution to facilitate end-of-induction (EOI) response evaluation.5,6
Role of bone marrow biopsy in the context of PET staging
In DLBCL, PET has effectively rendered the BMB redundant, owing to its greater sensitivity in detecting bone marrow involvement (BMI).18-20 PET is less sensitive in detecting BMI in FL, which is usually more subtle with widespread but low-level paratrabecular involvement.2 That said, focal lesions are more likely to be detected by PET, therefore PET and BMB provide complementary information. Although both are required for accurate staging, the presence of BMI in the GALLIUM trial, which was demonstrated by BMB in 613 of 1190 (51.5%) of patients, had no impact on PFS.21 In a recent retrospective study from MSKCC performed in 261 patients with newly diagnosed FL,22 BMI was found in 46 patients by both modalities, 35 by BMB only, and 32 by PET only. The BMB was positive in 4 of 74, 2 of 26, 26 of 73, and 49 of 88 patients with PET stages I, II, III, and IV disease, respectively. Conversely, PET upstaged 24 patients to stage IV, including 10 from stages I and II. BMI detected by PET but not BMB was an independent predictor of PFS and OS. Consequently, it may be reasonable to defer BMB in FL in patients who do not require immediate treatment or to omit the pretreatment BMB altogether where the results do not influence the therapeutic approach.
Quantitative baseline PET measurements in FL
The maximal standardized uptake value (SUVmax) is a semiquantitative measure of 18F-FDG metabolism that describes the radioactivity in a lesion corrected for dose of FDG and patient weight at a given time after FDG injection. Patients with aggressive B-cell lymphoma generally have a higher baseline SUVmax (bSUVmax) than patients with FL, and earlier small, single-institution studies have suggested that bSUVmax can identify large-cell histologic transformation (HT).23,24 A recent large, single-institution analysis of bSUVmax in 346 patients with AS grade 1 to 3a FL was reported. The median bSUVmax was 11 (range, 1.5-42), with a defined optimal cutoff of >18 for predicting PFS. A biopsy of the most FDG-avid node in all 52 patients with an SUVmax >18 did not identify HT. In patients treated with chemoimmunotherapy (CIT), SUVmax >18 correlated with an inferior OS.25 In large, prospective, multicentre studies, the bSUVmax in newly diagnosed FL likewise ranged considerably, reflecting the biological heterogeneity of this lymphoma, where FDG uptake was likely to be affected not only by the lymphoma cells but also by nonmalignant cells in the tumor microenvironment. In the follicular lymphoma collaboration (FOLLCOLL) combined analysis, involving 181 patients from 3 prospective studies,3,26-28 the median bSUVmax was 10 (range, 3-35; interquartile range, 7-14) with no correlation between bSUVmax and histological grade. Surprisingly, the 47% of patients with a bSUVmax ≤9.4 had an inferior 5-year PFS (47.4% vs 62.4%; HR, 1.62; P = .032). This finding was confirmed (HR, 1.81; P = .044) on a multivariate analysis that took into account age, longest diameter of largest involved lymph node (LodLin) >6 cm and positive BMB and β2 microglobulin.28 Although counterintuitive at first sight, it is possible that higher bSUVmax reflects an immune microenvironment that is more conducive to rituximab-induced, antibody-dependent cellular cytotoxicity.29 In support of this notion, a recent correlation between lesional SUVmax and CD4 and CD8A gene expression suggested a strong influence of the intratumoral T-cell component on bSUVmax.30
The largest prospective data set of bSUVmax31 comes from the phase III GALLIUM study,32 in which patients with high tumor burden (HTB) FL were treated with induction CIT containing either obinutuzumab or rituximab followed by antibody maintenance. Among 549 patients for whom PET data were available, bSUVmax ranged from 3.1 to 64.4. After a median follow-up of 5 years, biopsy-confirmed HT occurred in 15 patients (2.7%). Median bSUVmax was 12.4 (range, 8.1-28.0) in those developing HT vs 11.8 (range, 3.1-64.4) in those without HT. The SUVrange (difference between bSUVmax of the most and least 18F-FDG–avid lymphoma sites) was similar in both groups (median, 8.0 [range, 1.08-23.91] vs 7.1 [range, 0.00-59.81]). Seventy-four of 549 (13.5%) patients had a bSUVmax >20, with only 1 of 74 (1.4%) undergoing documented HT. No association with HT was observed with any specific chemotherapy regimen (CHOP; cyclophosphamide, vincristine, prednisone [CVP]; or bendamustine) or antibody treatment, and SUVmax did not predict subsequent HT in patients treated with any specific regimen.31 Furthermore, baseline SUVmax did not correlate with PFS in the GALLIUM study.43 It is important to note that this is the only prospective data charting quantitative PET metrics in a bendamustine-treated population. Table 1 summarizes the key studies in which SUVmax correlated with outcome in FL. The data suggest that there is no clear benefit in performing a biopsy or a second biopsy of lesions on the basis of SUVmax alone, even if the area of maximum FDG uptake was not sampled. One possible caveat to this conclusion is that some patients with high SUVmax may have been excluded from the GALLIUM trial because of concerns about HT on the pretreatment PET. However, there was a similarly low rate (25 of 653; 3.8%) of documented HT in the subset of GALLIUM patients in whom no baseline PET was performed, arguing against such a selection bias. Nonetheless, to directly investigate this possibility, the PETReA (PET-Guided; Response-Adapted therapy) study (EudraCT number: 2016-004010-10) will include the collection of screening logs for all patients diagnosed with FL at participating institutions, irrespective of trial enrollment. In this way, it should be possible to evaluate the clinical significance of bSUVmax without the potential confounding effects of patient exclusion due to high SUVmax. Notwithstanding these data, the limited reproducibility of SUV measurements with a higher SUVmax charted by more modern PET scanners, will make standardization challenging in future trials.
Key studies relating baseline SUVmax with outcome in FL
| Reference . | Patients, n . | Median baseline SUVmax (range) . | HT . | PFS . |
|---|---|---|---|---|
| PET in PRIMA (retrospective)41 | 58 | 11.7 (4.6-35.6) | No patients with HT | No association of bSUVmax with PFS (P = 0.53). ROC analysis did not identify an optimal pretreatment SUVmax cutoff with a significant impact on PFS |
| FOLLCOLL (retrospective)28 | 181 | 10 (3-35; IQR 7-14). No correlation with histologic grade, P = 0.66. Best cutoff on ROC and X-tile analysis SUVmax 9.4 | 2 patients with HT | SUVmax > 9.4: 5-y PFS 62%, median PFS 78.7 mo. SUVmax <9.4: 5-y PFS 47%, median PFS 48.7 mo. P = 0.0318. No difference in OS, 93.7% vs 88.4%; P = .15 |
| GALLIUM (prospective)31 | 549 | Range, 3-64; median, 12.4 (8.1-28.0) in HT; median 11.8 (3.1-64.4) in non-HT | 15 patients (2.7%) with HT at 5 y | No association of bSUVmax with PFS, Q1 vs Q4; HR, 1.14 (95% CI, 0.72-1.81), P = 0.58 |
| Strati et al (retrospective)25 | 346 | 11 (1.5-42) 52 patients (15%) with SUVmax >18 | HT excluded from study population | No effect on PFS if treated with R-CHOP or other CIT. Inferior 8-y OS if SUVmax >18 (65% vs. 89%; P = 0.001) |
| Reference . | Patients, n . | Median baseline SUVmax (range) . | HT . | PFS . |
|---|---|---|---|---|
| PET in PRIMA (retrospective)41 | 58 | 11.7 (4.6-35.6) | No patients with HT | No association of bSUVmax with PFS (P = 0.53). ROC analysis did not identify an optimal pretreatment SUVmax cutoff with a significant impact on PFS |
| FOLLCOLL (retrospective)28 | 181 | 10 (3-35; IQR 7-14). No correlation with histologic grade, P = 0.66. Best cutoff on ROC and X-tile analysis SUVmax 9.4 | 2 patients with HT | SUVmax > 9.4: 5-y PFS 62%, median PFS 78.7 mo. SUVmax <9.4: 5-y PFS 47%, median PFS 48.7 mo. P = 0.0318. No difference in OS, 93.7% vs 88.4%; P = .15 |
| GALLIUM (prospective)31 | 549 | Range, 3-64; median, 12.4 (8.1-28.0) in HT; median 11.8 (3.1-64.4) in non-HT | 15 patients (2.7%) with HT at 5 y | No association of bSUVmax with PFS, Q1 vs Q4; HR, 1.14 (95% CI, 0.72-1.81), P = 0.58 |
| Strati et al (retrospective)25 | 346 | 11 (1.5-42) 52 patients (15%) with SUVmax >18 | HT excluded from study population | No effect on PFS if treated with R-CHOP or other CIT. Inferior 8-y OS if SUVmax >18 (65% vs. 89%; P = 0.001) |
IQR, interquartile range; ROC, receiver operating characteristic.
Total metabolic tumor volume (TMTV) was conceived as a way of measuring the overall tumor burden and, in doing so, integrating several elements of the Follicular Lymphoma International Prognostic Index 1 (FLIPI1) and FLIPI2: longest diameter of the largest involved node (LODLIN), number of nodal sites, lactate dehydrogenase, and stage. It was originally measured by using a semiautomated method, with lesions initially identified by visual assessment of PET images scaled to a fixed SUV display and color table, followed by calculation of TMTV using 41% of the SUVmax as a threshold. A cutoff of ≥510 cm3 was confirmed as predictive of inferior PFS in the FOLLCOLL analysis.33 However, irrespective of whether the tumor threshold was set at 41% SUVmax or a fixed SUVmax of ≥2.5, TMTV did not clearly correlate with either PFS or OS in 522 patients with baseline PET data in the GALLIUM study.34 A possible explanation of these contradictory findings is that the adverse prognostic effect of high TMTV was overcome by the more intensive therapeutic approach in GALLIUM, where most patients received induction of bendamustine, and all were assigned to antibody maintenance for 2 years.32 In a later effort to simplify TMTV measurement, the GALLIUM investigators developed a fully automated method, using a novel, deep-learning–based approach to calculate whole-body TMTV in <5 minutes and achieving excellent correlation with the manually calculated TMTV.35 Applying this new method to a cohort of 541 patients from the GALLIUM study showed that the 193 (35.7%) patients with high TMTV had an inferior PFS (HR, 1.59; P = .05). The improved predictive value of automated TMTV requires further validation. Other aspects of TMTV measurement requiring additional work include the criteria for defining the splenic involvement, the optimization and standardization of TMTV measurement in clinical trials, and the development of simple software solutions suitable for clinical practice.36 Until this work has been done and the prognostic value of TMTV validated in other trials, it is premature to use TMTV for prognostication or patient stratification, either on its own or in combination with clinical features and/or metabolic/molecular response to therapy.
Response assessment by PET-CT
PET has also emerged as the imaging modality of choice for response evaluation at EOI. The 2014 ICML (Lugano)5 response criteria recommend assessing metabolic response according to a 5-point scale (5-PS) that measures residual FDG uptake relative to that of the mediastinum and liver (Table 2). To mitigate the possibility of optical illusion, the reporter’s qualitative assessment is confirmed by documenting the SUVmax of lymphoma lesions relative to that of these reference organs.37,38 There is ongoing clarification in the distinction between scores 4 and 5, which require lesional SUVmax to be moderately (score 4) and markedly (score 5) higher than that of the liver. It has been suggested that a score of 5 be assigned, not just to the occurrence of new lesions, but also where the lesional SUVmax is >2 or 3 times (depending on the research group) that of the liver.39 In FL, it has been demonstrated that applying a cutoff score ≥4, rather than ≥3, provides both better reporter concordance and greater separation of PFS and OS curves.4,40 Therefore, scores of 1 to 3 are considered to represent a complete metabolic response (CMR) and scores of 4 or 5 to represent an inadequate EOI response. Unlike in DLBCL and Hodgkin lymphoma, there are no published data in FL that show that a score of 5 is associated with a worse outcome than that associated with a score of 4.
Lugano response criteria based on the 5-point Deauville scale
| Deauville score . | Definition . |
|---|---|
| 1 | No uptake |
| 2 | Uptake ≤ mediastinum |
| 3 | Uptake > mediastinum but ≤ liver |
| 4 | Uptake moderately higher than liver |
| 5 | Uptake markedly higher than liver and/or new lesions* |
| X | New areas of uptake unlikely to be related to lymphoma |
| Deauville score . | Definition . |
|---|---|
| 1 | No uptake |
| 2 | Uptake ≤ mediastinum |
| 3 | Uptake > mediastinum but ≤ liver |
| 4 | Uptake moderately higher than liver |
| 5 | Uptake markedly higher than liver and/or new lesions* |
| X | New areas of uptake unlikely to be related to lymphoma |
Score of 4 assigned to FDG uptake > liver, and score of 5 to uptake 2 or 3 times higher than liver (according to research group) or the presence of new lesions.
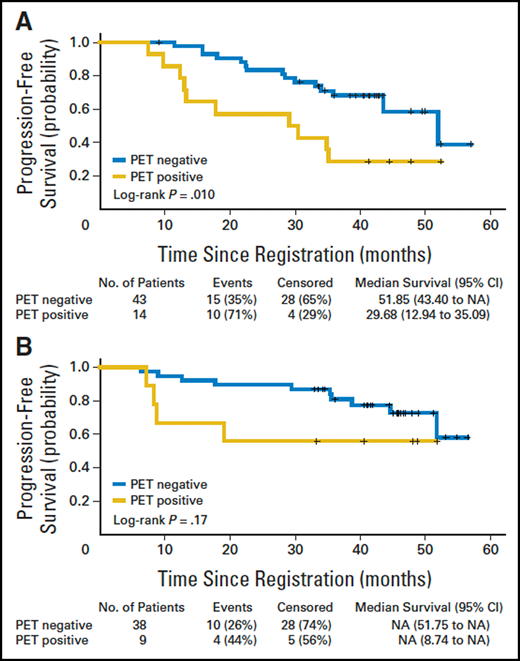
The prognostic value of EOI PET in prospective clinical trials is summarized in Table 3. An exploratory analysis performed as part of the PRIMA trial26 provided the first hypothesis-generating data suggesting that PET is better at prognostication than CT, based on therapeutic response to CIT (Figure 1). The substudy showed that 32 of 122 (26%) patients remaining EOI-PET positive after rituximab chemotherapy had a significantly (P < .001) inferior 42-month PFS of 32.9% (95% CI, 17.2-49.5) compared with 70.7% (95% CI, 59.3-79.4) in those who became PET negative. The risk of death was also increased in EOI-PET–negative patients (HR, 7.0; P < .001).1 The same findings were obtained when the PET scans were centrally reviewed using the 5-PS with a cutoff of ≥4, with an HR for progression or death in the PET-positive group of 3.1 (95% CI, 1.2-7.8; P = .01).41 This study was followed by a similar analysis of EOI-PET scans in 202 patients with HTB FL in the Fondazione Italiana Linfomi FOLL05 trial. Forty-nine (24%) had positive EOI-PET scans7 with a 3-year PFS of 35%, compared with 66% in patients with negative scans (P < .001). EOI PET predicted an outcome independent of anatomical response, FLIPI, and treatment arm (HR, 2.57; 95% CI, 1.52-4.34; P < .001). In the LYSA prospective PET-Folliculaire study,3 121 patients with previously untreated HTB FL were treated with 6 cycles of R-CHOP plus 2 additional cycles of rituximab induction. PET was performed before treatment, after 4 cycles of R-CHOP (interim PET), and at the end of treatment. With a median follow-up of 23 months, 2-year PFS rates were 51% for EOI-PET–positive patients vs 87% for EOI-PET–negative patients (P < .001). Two-year OS also differed significantly, at 88% vs 100%, respectively (P = .0128).
Prognostic value of EOI PET in prospective clinical trials
| Study . | Patients, n . | PET response criteria . | PET+ or non-CMR patients n (%) . | Median follow-up, mo . | PFS PET+ or non-CMR vs PET− or CMR (95% CI) . | OS PET+ or non-CMR vs PET− or CMR (95% CI) . |
|---|---|---|---|---|---|---|
| PET in PRIMA1 | 122 | Local assessment | 32 (26) | 42 | 3.5-y PFS 32.9% (17.2-49.5) vs 70.7% (59.3-79.4); HR, 3.3 (1.9-5.9); P < 0.001 | 3.5-y OS 78.5% (57.6-89.9) vs 96.5% (89.7-98.9) HR, 7.0 (1.8-27.0); P = 0.0011 |
| PET in PRIMA (central review)41 | — | 5 PS with cutoff ≥4 | — | 42 | 3.5-y PFS 25.0%, (3.7-55.8) vs 61.4% (45.4-74.1). P = 0.01; HR, 3.1 (1.2-7.8) | — |
| FOLL057 | 202 | Local assessment | 49 (24) | 34 | 3-y PFS 35% (18-52) vs 66% (57-74); HR, 2.59 (1.59-4.24); P < 0.001 | Overall 3-y OS 99% (94-100); 3 deaths in both PET-positive and PET-negative groups |
| PET Folliculaire3 | 121 | DS ≥ 4 | 15 (12) | 23 | 2-y PFS 61% vs 86%; P = 0.0046 | 2-y OS 88% vs 100%; P = 0.0128 |
| FOLLCOLL, (central review of PRIMA, FOLL05 and PET Folliculaire patients)40 | 246 | DS ≥ 4 | 41 (17) | 55 | 4-y PFS 23.2% (11.1-37.9) vs 63.4% (55.9-70.0), HR, 3.9 (2.5-5.9); P < 0.0001 | 4-y OS 87.2% (71.9-94.5) vs 97.1% (93.2-98.8); P < 0.0001 |
| GALLIUM4 | 508 | Lugano 2014 criteria (incorporating DS ≥4) | 58 (25) | 43 | 2.5-y from EOI, 87.3% (83.7-90.2) vs 54.9% (40.5-67.3), HR, 5.0 (3.3-10)* | 2.5-y from EOI, 84·0% (95% Cl 72·9-90·8) vs 96·6% (95% CI, 94·4-97·9), HR, 5 (2.0-10.0)*; P < 0.0001 |
| Study . | Patients, n . | PET response criteria . | PET+ or non-CMR patients n (%) . | Median follow-up, mo . | PFS PET+ or non-CMR vs PET− or CMR (95% CI) . | OS PET+ or non-CMR vs PET− or CMR (95% CI) . |
|---|---|---|---|---|---|---|
| PET in PRIMA1 | 122 | Local assessment | 32 (26) | 42 | 3.5-y PFS 32.9% (17.2-49.5) vs 70.7% (59.3-79.4); HR, 3.3 (1.9-5.9); P < 0.001 | 3.5-y OS 78.5% (57.6-89.9) vs 96.5% (89.7-98.9) HR, 7.0 (1.8-27.0); P = 0.0011 |
| PET in PRIMA (central review)41 | — | 5 PS with cutoff ≥4 | — | 42 | 3.5-y PFS 25.0%, (3.7-55.8) vs 61.4% (45.4-74.1). P = 0.01; HR, 3.1 (1.2-7.8) | — |
| FOLL057 | 202 | Local assessment | 49 (24) | 34 | 3-y PFS 35% (18-52) vs 66% (57-74); HR, 2.59 (1.59-4.24); P < 0.001 | Overall 3-y OS 99% (94-100); 3 deaths in both PET-positive and PET-negative groups |
| PET Folliculaire3 | 121 | DS ≥ 4 | 15 (12) | 23 | 2-y PFS 61% vs 86%; P = 0.0046 | 2-y OS 88% vs 100%; P = 0.0128 |
| FOLLCOLL, (central review of PRIMA, FOLL05 and PET Folliculaire patients)40 | 246 | DS ≥ 4 | 41 (17) | 55 | 4-y PFS 23.2% (11.1-37.9) vs 63.4% (55.9-70.0), HR, 3.9 (2.5-5.9); P < 0.0001 | 4-y OS 87.2% (71.9-94.5) vs 97.1% (93.2-98.8); P < 0.0001 |
| GALLIUM4 | 508 | Lugano 2014 criteria (incorporating DS ≥4) | 58 (25) | 43 | 2.5-y from EOI, 87.3% (83.7-90.2) vs 54.9% (40.5-67.3), HR, 5.0 (3.3-10)* | 2.5-y from EOI, 84·0% (95% Cl 72·9-90·8) vs 96·6% (95% CI, 94·4-97·9), HR, 5 (2.0-10.0)*; P < 0.0001 |
The HRs for the GALLIUM study are presented as the reciprocal of the values originally reported (PFS 0.2; 95% CI, 0.1-0.3, and OS 0.2; 95% CI, 0.1-0.5) to align its directionality with that of the other studies.
Impact of postinduction PET-CT on PFS in 104 randomly assigned patients in the PRIMA study. (A) Observation arm (n = 57). PET negative designates patients (n = 43) with a negative PET-CT after induction therapy, and PET positive designates those (n = 14) with a positive PET-CT. Log-rank P = .010. (B) Rituximab maintenance arm (n = 47). PET negative designates patients (n = 38) with a negative PET-CT after induction therapy, and PET positive designates those (n = 9) with a positive PET-CT. Log-rank P = .17. N/A, not applicable; PET-CT, positron emission tomography–computed tomography; PFS, progression-free survival; PRIMA, Primary Rituximab and Maintenance. Reprinted from Trotman et al.1 with permission.
Impact of postinduction PET-CT on PFS in 104 randomly assigned patients in the PRIMA study. (A) Observation arm (n = 57). PET negative designates patients (n = 43) with a negative PET-CT after induction therapy, and PET positive designates those (n = 14) with a positive PET-CT. Log-rank P = .010. (B) Rituximab maintenance arm (n = 47). PET negative designates patients (n = 38) with a negative PET-CT after induction therapy, and PET positive designates those (n = 9) with a positive PET-CT. Log-rank P = .17. N/A, not applicable; PET-CT, positron emission tomography–computed tomography; PFS, progression-free survival; PRIMA, Primary Rituximab and Maintenance. Reprinted from Trotman et al.1 with permission.
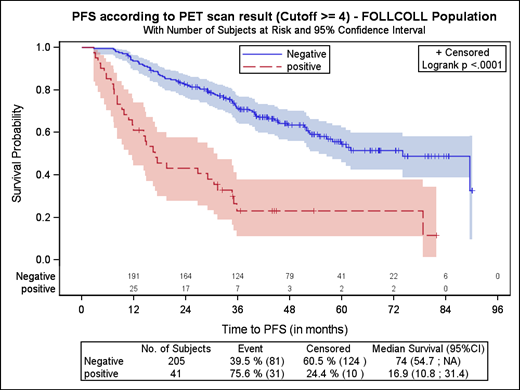
To provide more robust survival estimates and longer term follow-up using the 5-PS as the emerging standard for PET-based response assessment, a pooled analysis of the combined PET data from all 3 studies was conducted (Figure 2). EOI-PET scans available for central review were scored independently by 3 reviewers, with 41 of 246 (17%) scans remaining PET positive (score, ≥4) after CIT induction. With a median follow-up of 54.8 months, the HR was 3.9 (95% CI, 2.5-5.9; P < .0001) for PFS and 6.7 (95% CI, 2.4-18.5; P = .0002) for OS. Among patients with a positive EOI PET, 23.2% (95% CI, 11.1-37.9) were progression free at 4 years, compared with 63.4% (95% CI, 55.9-70.0) of those with a negative EOI-PET scan (P < .0001); 4-year OS was 87.2% (95% CI, 71.9-94.5) vs 97.1% (95% CI, 93.2-98.8), respectively (P < .0001). In contrast, conventional CT-based response (ie, complete response or unconfirmed complete response vs partial response) was only weakly predictive of PFS (HR, 1.7; 95% CI, 1.1-2.5; P = .017).
Effect on PFS and OS of achieving CMR, according to the Kaplan-Meier analysis. Reprinted from Trotman et al40 with permission.
Effect on PFS and OS of achieving CMR, according to the Kaplan-Meier analysis. Reprinted from Trotman et al40 with permission.
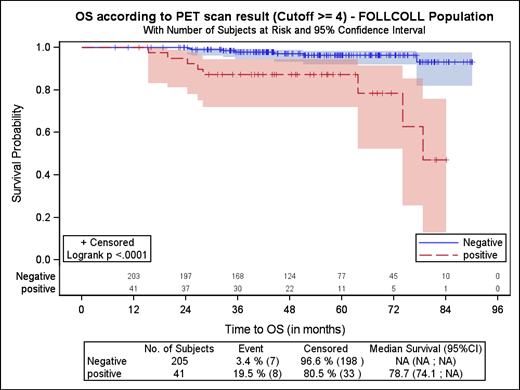
A landmark analysis of 508 patients with advanced FL in the GALLIUM study (in which all patients received CIT induction followed by antibody maintenance) confirmed that EOI PET was superior to CT for response assessment.4 Patients obtaining CMR (PET score, 1-3) had a PFS at 2.5 years of 87.4% (95% CI, 83.7-90.2) compared with 54.9% (95% CI, 40.5-67.3; HR, 0.2; 95% CI, 0.1-0.3; P < .0001) for patients who did not achieve CMR. Crucially, achieving CMR was the only independent predictor of OS (HR, 0.2; 95% CI, 0.1-0.5; P < .0001) on a multivariate analysis that included FLIPI score, type of chemotherapy, and antibody administered. In a recent update with a median follow-up of 77 months, patients who remained PET positive at EOI had a 5-year PFS of 29.4% (95% CI, 17.8-42.0) vs 70.0% (95% CI, 65.2-74.2) for those in CMR (HR, 3.40; 95% CI, 2.33-4.97; P < .0001). Five-year OS was 79.6% (95% CI, 68.0-87.4) and 92.0% (95% CI, 89.0-94.2), respectively (HR, 3.34; 95% CI, 1.81-6.17; P < .0001)42 (Figure 3). Furthermore, updated abstract data from GALLIUM43 showed that POD24 occurred in 31 of 69 (44.9%) patients in the PET-positive group compared with 38 of 450 (8.4%) patients obtaining a CMR (odds ratio, 8.84; 95% CI, 4.96-15.78).
Long-term follow-up PFS and OS Km curves from the GALLIUM study. Reprinted from Nielsen et al.42 with permission.
Long-term follow-up PFS and OS Km curves from the GALLIUM study. Reprinted from Nielsen et al.42 with permission.
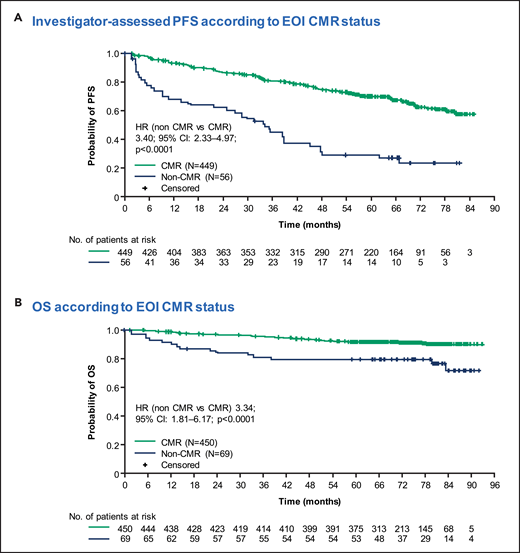
Persistent BMI on BMB was clearly identified in only 2.3% (5 of 213) of patients obtaining CMR at EOI in the GALLIUM study, suggesting limited additional value of EOI BMB in those patients (Figure 4).21 In patients failing to achieve a CMR, repeat BMB adds even less relevant information for therapeutic decision making and is likely to be redundant outside of clinical trials. There is a paucity of data on the clinical significance of changes in quantitative PET measurements (SUVmax and TMTV) in FL response assessment, as well as likely challenges in analyzing such data, given the often low baseline SUV in FL lesions and the heterogeneity of FDG uptake within individual patients.
Lack of impact of BMI on BM biopsy on PFS in GALLIUM. Reprinted from supplemental Data in Rutherford et al21 with permission.
Lack of impact of BMI on BM biopsy on PFS in GALLIUM. Reprinted from supplemental Data in Rutherford et al21 with permission.
These EOI-PET data have set the scene for testing response-adapted approaches in FL: exploring the balance between the beneficial and unwanted effects of maintenance therapy in patients who achieve CMR after CIT and studying treatment escalation in patients with an inadequate response. Preliminary data from the first trial to address these questions, FOLL12,44 suggest that rituximab maintenance prolongs PFS, even in patients who achieve a CMR. This finding is not unexpected, given the magnitude of the PFS advantage of rituximab maintenance in the PRIMA trial,26 and highlights the need for even more sensitive measures of complete response than PET alone. The current UK/Australian PETReA study45 is seeking to directly quantify both the beneficial effect of rituximab maintenance (in terms of PFS and time to next lymphoma treatment) and its unwanted effects in patients who achieve a CMR after frontline CIT. The current COVID-19 pandemic highlights the importance of balancing safety, quality of life, and long-term disease control in patients with FL and of moving beyond the restricted paradigm of focusing on PFS as the sole end point of importance. In addition to addressing risk/benefit considerations of anti-CD20 maintenance in good-risk patients who achieve CMR, it is also important to evaluate approaches to improving the poor outcome in the minority who remain EOI-PET positive (eg, by adding (90)Y ibritumomab tiuxetan [FOLL12]44 or lenalidomide [PETReA]).45 It is also important to appreciate that the prognostic value of EOI PET may depend on the therapeutic context and that EOI-PET–based outcomes after treatment with rituximab and lenalidomide have not yet been reported in a large cohort. To that end, data from the RELEVANCE study46 are important.
Use of PET for interim response assessment and remission surveillance
An interim PET scan after 4 cycles of R-CHOP was shown to be predictive of response in the PET Folliculaire study,3 but did not discriminate between responders and nonresponders, as effectively as the EOI PET. Similarly, as with all lymphoma histologies, a role for surveillance PET imaging has not been demonstrated in FL. Indeed, surveillance imaging is discouraged, owing to a risk of false-positive scans leading to unnecessary biopsies, expense, patient anxiety, and radiation exposure. Nonetheless, we acknowledge that in patients with residual abdominal disease, concerns for asymptomatic progression may warrant judicious use of surveillance CT scanning, dependent on the likely therapeutic approach in the event of significant progression. Importantly, studies in FL have shown a significant delay between PFS (a primarily CT-based end point) and time to next lymphoma therapy (TNLT), with an interval of 2 years in patients who were observed in the PRIMA study.47 Although PFS is a key end point for drug development phase 3 trials in FL, TNLT is the end point arguably of greatest significance to patients in an era in which an OS advantage is not easily demonstrated. Consequently, imaging is not necessary to confirm disease progression outside of a clinical trial, unless further treatment is indicated. Once there are concerns for symptomatic relapse requiring therapy, we recommend restaging with PET and repeat biopsy to exclude HT before retreatment. As is the case at diagnosis, the relationship between SUVmax and subsequent HT is unclear. Consequently, although it is reasonable to use PET to select a representative biopsy site, it should be noted that the SUVmax of abdominal disease in FL is commonly higher than that of involved peripheral lymph nodes38 and that obtaining a large biopsy from an accessible site may be more informative than attempting a technically challenging biopsy from the site with the highest SUVmax.
Future directions
With the prolonged survival of most patients with FL in the modern era and with median PFS after frontline FL therapy and maintenance estimated to be ∼10 years,47 PFS is becoming an increasingly impractical end point in clinical trials. With EOI-PET status predictive of both PFS and OS, it is appropriate that the Follicular Lymphoma Analysis of Surrogacy Hypothesis (FLASH) consortium have begun analyzing individual patient data from a few clinical trials to conduct a meta-analysis to evaluate EOI PET as a surrogate end point after first-line therapy, but a larger data set is likely necessary. Such an analysis would be essential before EOI PET could be accepted by licensing agencies as an early surrogate end point beyond early phase trials to accelerate testing of novel approaches. Furthermore, given the potential for novel agents to affect FDG uptake independent of cytoreduction, the correlation between PET response and PFS would have to be validated for specific classes of drugs before PET status could be considered the primary outcome measure.
Testing of response-adapted approaches after CIT in FL will determine whether EOI PET can be used to guide subsequent therapy.44,45 This testing is particularly important in the current pandemic when the balance between the beneficial effects (improved PFS but not OS) and unwanted effects (increased susceptibility to infection) of continued therapy with anti-CD20 maintenance is being reevaluated. In the meantime, although patients who do not achieve a CMR have a sufficiently poor prognosis to warrant testing of PET-guided intensification of treatment, improvements in the PPV of EOI-PET imaging are needed. To this end, analysis of the characteristics of residual FDG uptake and outcomes in patients who do not achieve CMR in the GALLIUM study is ongoing. Likewise, further studies are necessary to confirm whether EOI-PET status in the modern therapeutic era overrides the prognostic value of pretreatment risk scores. Furthermore, if TMTV is confirmed to have prognostic value, standardization and optimization of TMTV measurement is needed, with software solutions for semiautomated measurements that will be suitable for everyday practice. Finally, if additional studies confirm that the findings in GALLIUM that EOI PET and PCR-based EOI minimal residual disease (MRD) status (with a clonal t(14;18) translocation and/or immunoglobulin variable domain rearrangement detectable in 75% of patients) are each independently predictive of outcome,48 a platform may be created for striving for both CMR and MRD negativity as a necessary first step in a potentially curative approach for younger patients with FL who may not otherwise achieve a “functional cure.” Improvements in MRD sensitivity and the evolution of next-generation sequencing techniques using universal primers will advance circulating tumor DNA as another biomarker in FL that reflects intratumor spatial heterogeneity. Harmonization of techniques to detect the often low levels of circulating tumor DNA found in FL and panel consensus may in the future be combined with PET to enhance our prognostic modeling and response assessment for patients.
Conclusions
This Perspective charts the role of PET as the gold-standard imaging modality for staging and response assessment of FL. The sensitivity of PET supports a PET-guided approach to initial therapy with supplementary BMB in selected cases. There is no confirmed correlation between high bSUVmax and risk of HT or inferior PFS, and exposing patients to repeat biopsy in search of HT should not be prompted solely by this semiquantitative measure of FDG uptake. After first-line CIT, EOI-PET status is strongly predictive of outcome. Achieving CMR provides patients with greater confidence in a prolonged first remission and can assist patients and clinicians in making decisions on the trade-off between the PFS advantage and the toxicity of further treatment with antibody maintenance, a dilemma that has been thrown into sharp focus by the current COVID pandemic. There are currently no data to support preemptive intervention in patients who remain PET positive, and particularly for this poor risk population, the results of current trials involving EOI PET-adapted approaches are awaited with interest.
Authorship
Contribution: J.T. performed the literature search, interpreted the data, and wrote the first draft of the manuscript; A.R.P. edited the manuscript; and both authors approved the manuscript.
Conflict-of-interest disclosure: J.T. reports institutional research funding from Beigene, Roche, Takeda, Celgene, Janssen and PCYC, outside the submitted work. A.R.P. reports institutional research funding from Celgene, Gilead, GSK/Novartis, Chugai, Napp, and Roche outside the submitted work.
Correspondence: Judith Trotman, Haematology Department, Concord Hospital, Hospital Rd, Concord 2139, NSW Australia; e-mail: judith.trotman@health.nsw.gov.au.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal