Key Points
In patients with KHE, prednisolone plus sirolimus led to significantly faster improvement of KMP than sirolimus alone.
At month 24, significantly lower residual disease levels were observed in patients with KMP treated with sirolimus plus prednisolone.
Abstract
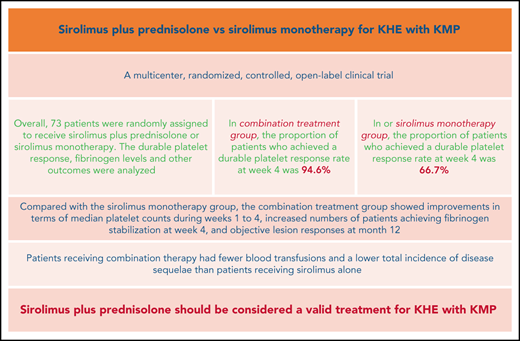
The Kasabach-Merritt phenomenon (KMP) in kaposiform hemangioendothelioma (KHE) is characterized by life-threatening thrombocytopenia and consumptive coagulopathy. This study compared the efficacy and safety of sirolimus plus prednisolone vs sirolimus monotherapy as treatment strategies for KHE with KMP in the largest cohort to date. Participants were randomized to receive either sirolimus in combination with a short course of prednisolone or sirolimus monotherapy for at least 12 months. The primary outcome was defined as achievement of a durable platelet response (platelet count >100 × 109/L) at week 4. Participants completed efficacy assessments 2 years after the initial treatment. At week 4, a durable platelet response was achieved by 35 of 37 patients given sirolimus and prednisolone compared with 24 of 36 patients given sirolimus monotherapy (difference 27.9%; 95% confidence interval, 10.0-44.7). Compared with the sirolimus monotherapy group, the combination treatment group showed improvements in terms of measures of durable platelet responses at all points during the initial 3-week treatment period, median platelet counts during weeks 1 to 4, increased numbers of patients achieving fibrinogen stabilization at week 4, and objective lesion responses at month 12. Patients receiving combination therapy had fewer blood transfusions and a lower total incidence of disease sequelae than patients receiving sirolimus alone. The frequencies of total adverse events and grade 3-4 adverse events during treatment were similar in both groups. The responses seen in patients with KHE with KMP were profound and encouraging, suggesting that sirolimus plus prednisolone should be considered a valid treatment of KHE with KMP. This trial was registered at www.clinicaltrials.gov as #NCT03188068.
Introduction
Kaposiform hemangioendothelioma (KHE) is a rare vascular neoplasm with locally aggressive characteristics. Approximately 70% of KHE patients have life-threatening thrombocytopenia and consumptive coagulopathy, known as the Kasabach-Merritt phenomenon (KMP).1,2 In patients with KHE, the presence of KMP is typically associated with aggressive tumor progression and poorer outcomes. The primary goal of therapy for patients with KMP is to keep the risk of bleeding to a minimum by normalizing hematologic parameters. However, there are no medications approved by the US Food and Drug Administration for treating KHE. Medical treatments with corticosteroids and/or vincristine have been recommended by consensus treatment statements.3 However, many patients do not improve with corticosteroid monotherapy.4 In addition, vincristine has been shown to be ineffective in some patients with severe KHE.5,6
Since 2010, an increasing number of studies have reported the exceptional effectiveness of sirolimus in treating KHE.6-14 Sirolimus for the management of KMP has resulted in a reduction in mortality in these patients over the past decade. However, some patients still have incompletely controlled disease, which can progress after treatment.15,16 We have previously demonstrated that treatment with sirolimus plus prednisolone led to rapid responses in patients with KHE and KMP. Remarkably, patients who were previously resistant to conventional approaches (eg, embolization, vincristine, steroids) and sirolimus monotherapy showed dramatic responses to sirolimus plus prednisolone.17 Based on these previous studies, there is a very good rationale for sirolimus combination therapy in patients with KMP. To determine whether corticosteroids in addition to sirolimus provide added benefits, we conducted this multicenter, randomized clinical trial to assess the efficacy, safety, and tolerability of sirolimus plus prednisolone vs sirolimus monotherapy in patients with KHE and KMP.
Methods
Study design
This was a multicenter, randomized, controlled, open-label clinical trial comparing sirolimus plus prednisolone and sirolimus for the management of KHE with KMP. The study was designed to establish whether sirolimus plus prednisolone was superior to sirolimus monotherapy. The trial was conducted in collaboration among 5 tertiary referral centers. Data were collected from each participating site and sent to the principal investigation site at the West China Hospital of Sichuan University for analysis. The study was approved by the ethics committee on medical research of each participating site and was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. Patients’ parents or guardians gave written, informed consent before enrollment.
Study patients
According to previously published criteria, the diagnosis of KHE was based on clinical features, magnetic resonance imaging (MRI), and/or histopathologic data.18-20 Patients were enrolled if they were between 0 and 14 years of age and had KHE with KMP. KMP was defined as a platelet count <100 × 109/L, with consumptive coagulopathy and hypofibrinogenemia.1,2 Patients were required to have adequate liver, renal, and bone marrow function and an absence of active infection. Key exclusion criteria were as follows: patients with contraindications for the administration of sirolimus (eg, those with an allergy to sirolimus or other rapamycin analogs); patients who had a history of treatment with sirolimus or other mTOR inhibitors; patients previously treated with the following KHE therapies within 1 week before enrollment: embolization, sclerotherapy, corticosteroids, propranolol, and chemotherapy; patients who had a history of a major surgery within 2 weeks before enrollment; patients who had concurrent severe and/or uncontrolled medical disease that could compromise participation in the study (eg, severe infection, severe malnutrition, chronic liver or renal disease, immunodeficiency, active upper gastrointestinal tract ulceration); and patients who were unwilling or unable to comply with the protocol. Detailed definitions and inclusion and exclusion criteria are provided in the trial protocol.
Randomization and masking
We used a computer-generated random sequence to allocate patients (1:1) to either the sirolimus plus prednisolone group or the sirolimus monotherapy group. The families and the investigators who treated and followed the patients were not blinded to the treatment. The investigators who measured the outcomes were blinded to the patients’ treatment allocations.
Study intervention
Treatment was continued for at least 12 months in patients who showed disease stabilization or improvement. Patients were followed for a total of 2 years. Treatment consisted of sirolimus monotherapy or sirolimus in combination with a short course of prednisolone. The starting dose of sirolimus was 0.8 mg/m2, which was administered orally twice daily and subsequently titrated to achieve trough levels of 10 to 15 ng/mL.11,21 Blood sirolimus levels were checked weekly for 2 weeks, then every other week for 2 weeks, then monthly for 2 months, and then every 3 months thereafter or as clinically indicated. The target sirolimus range remained unchanged throughout the study. In both treatment groups, sirolimus was tapered and discontinued after complete or nearly complete resolution of KHE or if no further improvement of tumor lesion and symptoms was observed after month 12 (for 12 weeks of observation). Concurrent prednisolone was administered at 2 mg/kg orally once daily. An attempt at tapering and discontinuing prednisolone was required within 4 to 6 weeks when satisfactory responses ensued (eg, platelet count ≥100 × 109/L for at least 1 week). Major criteria for the discontinuation of the study drug included evidence of clinically and radiologically progressive disease, parent request, and toxicities precluding further therapy. In the case of disease progression, the study drug was withdrawn and the choice of alternative therapy was left at the discretion of the treating investigators.
Fresh frozen plasma and/or cryoprecipitate were given to patients to treat consumption coagulopathy and hypofibrinogenemia in the following circumstances: (1) active bleeding; (2) platelet count <10 × 109/L; and/or (3) fibrinogen <0.5 g/L. Packed red blood cell transfusion was recommended for patients who had symptomatic anemia and/or hemoglobin concentrations <60 g/L. The anticipated length of treatment with sirolimus was 12 months; however, patients with a favorable response were given the option to continue treatment with serial follow-up to monitor treatment efficacy and toxicity. Prophylactic treatment of Pneumocystis jirovecii pneumonia (PJP) (trimethoprim-sulfamethoxazole, 10 mg/kg twice daily, 3 times per week) was required for all patients at least during the first 6 months. A requirement for any additional KHE- or KMP-specific intervention was considered treatment failure.
Definitions
According to previous studies, KHE lesions were classified into 3 groups: superficial, mixed, and deep.2 Hypofibrinogenemia was defined as a fibrinogen level <1.6 g/L. Severe anemia was defined as a hemoglobin concentration <60 g/L.2 Consumptive coagulopathy was defined as a low fibrinogen level (<1.6 g/L) and an elevated D-dimer level (>0.5 μg/mL). Decreased range of motion (ROM) was defined as ≥10° of extension loss, flexion <120°, or both.22 Chronic pain was defined as daily pain for at least 3 months. Stemmer sign was defined as the inability to pinch the skin of the dorsal side or the base of the second toe.23
Study outcomes
All outcome measures were prospectively defined before the start of the study. The primary outcome was the difference between the 2 groups in the proportion of patients who had a durable platelet response at week 4. A durable platelet response was defined as a platelet count ≥100 × 109/L lasting at least 4 consecutive weeks. The evaluation of the primary outcome was performed through the measurement of blood counts every 2 days until the stabilization of platelet counts was reached.
Secondary outcome measures included mean changes in platelet counts during weeks 0 through 4, weekly durable platelet responses during weeks 1 through 3, the proportion of patients achieving fibrinogen stabilization at week 4, lesion responses, quality of life (QOL), KMP rebound rate, the incidence of disease sequelae, safety, and tolerability. Fibrinogen stabilization was defined as a fibrinogen level >1.6 g/L lasting at least 4 consecutive weeks.
Sequential volumetric MRI was performed at months 6 and 12 and every 12 months thereafter. The volumetric measurements at 12 months after treatment were compared with the baseline volumetric measurements. Changes in KHE size were classified as further growth (increase ≥20%), no change (<20% increase and <20% decrease), partial involution (decrease ≥20% and <75%), nearly complete involution (decrease ≥75% and <100%), or complete involution (100%).21 Lesion responses were the overall lesion response rate and a good lesion response rate. The overall lesion response comprised complete, nearly complete, and partial involutions. A good lesion response comprised complete and nearly complete involutions.
The QOL measurements included the Pediatric Quality of Life Inventory 4.0 Generic Core (3-18 years) and Infant Scales (0-24 months). In these questionnaires, standardized response choices consisted of 5 categories scored from 0 to 4. The items were then linearly transformed to a 0 to 100 scale (0 = 100, 1 = 75, 2 = 50, 3 = 25, 4 = 0), with higher scores indicating better QOL.11,24 The questionnaires were completed by parents at baseline and at 12 months after treatment. QOL improvement was defined as a total score increase ≥4.5 points at 12 months compared with baseline.
KMP rebound was defined as a platelet count decrease to <100 × 109/L after a durable platelet response was achieved. Disease sequelae (eg, chronic pain, lymphedema, decreased ROM, leg-length discrepancy, scoliosis) were assessed by site investigators at month 24. The site investigators assessed patients’ extremity swelling (if any), general physical activity, and exercise levels. The diagnosis of lymphedema was based on physical examination (eg, Stemmer sign) and lymphoscintigraphic findings. Residual KHE lesions were not considered disease sequelae. The proportion of patients needing blood transfusion (during severe KMP) and the frequency of blood transfusion were also recorded by a site investigator between baseline and month 12.
The evaluation of safety included vital signs, adverse events, and laboratory assessments. The incidence and severity of all adverse events were recorded during in-house monitoring at the scheduled visits (baseline; at 1, 2, 4, 8, 12, 24 36, 48, 60, 72, and 84 weeks; and 2 years) by physical examination (body weight, height, and vital signs) and clinical laboratory tests (eg, full blood count, liver function). The site investigators also asked the parents if there were any new concerns since the last evaluation. All adverse events were collected and graded according to the Common Terminology Criteria for Adverse Events, version 4.0.
Detailed descriptions of these outcome measures are provided in the trial protocol.
Statistical analysis
We postulated that sirolimus plus prednisolone would be more effective than sirolimus monotherapy, with a primary outcome of 70% vs 55% for sirolimus monotherapy at week 4.25 With ∼32 patients per treatment group, this study had ∼80% power to detect a difference of 15% between sirolimus or sirolimus plus prednisolone for the primary endpoint. We calculated the power for the planned sample size with 2-group χ2 tests of equal proportions.
We used descriptive statistics to compare baseline characteristics between the 2 groups: frequencies with percentages for qualitative variables and means with ranges and/or medians for continuous variables. Comparisons of baseline characteristics were constructed with Student t test or the nonparametric Mann-Whitney U test for continuous variables where appropriate and Fisher's exact test or a χ2 test for categorical variables.
We analyzed efficacy and safety outcomes in the intention-to-treat population, defined as all randomly assigned patients treated with at least 1 dose of their allocated treatment. The primary outcome compared the percentage of patients having a durable platelet response at week 4 in the sirolimus plus prednisolone group and in the sirolimus monotherapy group. The overall type I error was 5% (2-sided). Generalized linear mixed models were used to compare the difference between the 2 groups in platelet count changes from baseline to 4 weeks. Other efficacy variable comparisons were performed with Fisher's exact test or a χ2 test, except for blood transfusion, which was analyzed with the nonparametric Mann-Whitney U test. For categorical efficacy analyses, patients were considered nonresponders at each visit if they were not responders at that visit; permanently discontinued study treatment at any time before that visit for any reason; required the initiation (sirolimus monotherapy group) or reintroduction of prednisolone after randomization; or had missing data at that visit. No imputation was needed with mixed models repeated measures for analyzing the continuous efficacy endpoints.
Adverse events were inclusive of the treatment period and up to 30 days after treatment. We used Fisher's exact test or a χ2 test for all adverse events, discontinuations, and other categorical safety data. We analyzed continuous safety data using analyses of covariance, adjusting for baseline value and treatment. Statistical analyses were conducted using SPSS 23.0 for Windows (SPSS Inc., Chicago, IL). All analyses were assessed at a 2-sided α of 0.05.
Results
Baseline characteristics
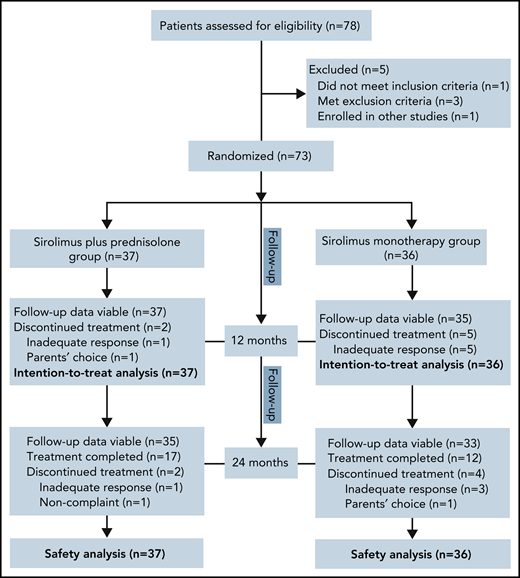
Of the 78 patients screened for this study between July 2017 and June 2019, 73 were randomly assigned to receive sirolimus plus prednisolone (n = 37) or sirolimus monotherapy (n = 36). The reasons for ineligibility are shown in Figure 1. Baseline demographics and disease activity were similar among groups (Table 1). In total, 35 patients were female, and the mean age at baseline was 4.1 months (range, 0.3-25.0 months). The age at the onset of KMP ranged from the first day of life to 22.0 months (mean, 2.1 months). At baseline, the mean duration of KMP was 2.1 months (range, 0.0-17.5 months). Retroperitoneal and intrathoracic lesions were detected in 6.8% of patients. Twenty-four (32.9%) patients had been treated previously with at least 1 therapy for KHE. The most common previous therapy in all patients was corticosteroids (Table 2). Biopsies were available for 39 patients before enrollment. All pathological examinations of lesions confirmed the clinical diagnosis of KHE. The remaining patients were diagnosed based on clinical findings, laboratory studies, and MRI.
Comparison of patient demographic and clinical characteristics between the combination treatment group and sirolimus monotherapy group
| Parameters . | SIR plus PRE (n = 37) . | SIR (n = 36) . | P value . |
|---|---|---|---|
| Patients | |||
| Sex* | .729† | ||
| Female | 17 (45.9) | 18 (50) | |
| Age at discovery of tumor lesion (m) | .395‡ | ||
| Mean (range) | 1.5 (0.0-13.0) | 1.7 (0.0-20.0) | |
| Median (IQR) | 0.0 (0.0-1.8) | 0.0 (0.0-1.0) | |
| Age at onset of KMP (m) | .359§ | ||
| Mean (range) | 2.1 (0.0-13.0) | 2.0 (0.0-22.0) | |
| Median (IQR) | 1.3 (0.0-3.0) | 1.0 (0.0-2.0) | |
| Age at baseline (m) | .678§ | ||
| Mean (range) | 4.1 (0.3-15.0) | 4.2 (0.3-25) | |
| Median (IQR) | 3.0 (1.5-6.0) | 2.6 (1.5-4.4) | |
| Weight (kg) | .554§ | ||
| Mean (range) | 6.2 (3.5-12.2) | 6.2 (3.4-13.5) | |
| Median (IQR) | 6.0 (4.5-7.5) | 5.7 (4.5-7.0) | |
| Height (cm) | .800§ | ||
| Mean (range) | 58.6 (47.8-80.0) | 58.8 (47.7-92.5) | |
| Median (IQR) | 56.0 (51.7-63.5) | 56.3 (51.6-61.4) | |
| Prior treatment* | .562† | ||
| Yes | 11 (29.7) | 13 (36.1) | |
| KHE | |||
| Morphology* | .576ǁ | ||
| Superficial | 2 (5.4) | 3 (8.3) | |
| Mixed | 31 (83.8) | 30 (83.3) | |
| Deep | 4 (10.8) | 3 (8.3) | |
| Maximal tumor dimension (cm) | .599‡ | ||
| Mean (range) | 10.0 (4.0-21.0) | 10.8 (3.5-25.0) | |
| Median (IQR) | 9.0 (6.0-14.5) | 10.5 (6.0-13.8) | |
| Retroperitoneal or intrathoracic location* | 3 (8.1) | 2 (5.6) | 1.000§ |
| Bone and/or joint involvement* | 4 (10.8) | 5 (13.9) | .736§ |
| KMP | |||
| Duration of KMP (m) | .693‡ | ||
| Mean (range) | 2.0 (0.0-9.0) | 2.2 (0.0-17.5) | |
| Median (IQR) | 1.3 (0.5-2.4) | 1.7 (0.5-3.0) | |
| Platelet level (10 × 109/L) | .851‡ | ||
| Mean (range) | 15.6 (3.0-47.0) | 16.3 (3.0-46.0) | |
| Median (IQR) | 14.0 (9.0-21.0) | 13.0 (7.0-24.8) | |
| Platelet level <10 × 109/L* | 13 (35.1) | 13 (36.1) | .931† |
| Fibrinogen level (g/L) | .623‡ | ||
| Mean (range) | 0.8 (0.1-1.58) | 0.8 (0.2-1.4) | |
| Median (IQR) | 0.8 (0.4-1.2) | 0.9 (0.6-1.0) | |
| Fibrinogen level <0.5 g/L* | 10 (27.0) | 7 (19.4) | .443† |
| Hemoglobin (g/L) | .787‡ | ||
| Mean (range) | 97.1 (55.0-152.0) | 97.9 (59.0-156.0) | |
| Median (IQR) | 89.0 (79.5-113.0) | 98.0 (79.3-112.0) | |
| Hemoglobin <60 g/L* | 3 (8.1) | 1 (2.8) | .615ǁ |
| Parameters . | SIR plus PRE (n = 37) . | SIR (n = 36) . | P value . |
|---|---|---|---|
| Patients | |||
| Sex* | .729† | ||
| Female | 17 (45.9) | 18 (50) | |
| Age at discovery of tumor lesion (m) | .395‡ | ||
| Mean (range) | 1.5 (0.0-13.0) | 1.7 (0.0-20.0) | |
| Median (IQR) | 0.0 (0.0-1.8) | 0.0 (0.0-1.0) | |
| Age at onset of KMP (m) | .359§ | ||
| Mean (range) | 2.1 (0.0-13.0) | 2.0 (0.0-22.0) | |
| Median (IQR) | 1.3 (0.0-3.0) | 1.0 (0.0-2.0) | |
| Age at baseline (m) | .678§ | ||
| Mean (range) | 4.1 (0.3-15.0) | 4.2 (0.3-25) | |
| Median (IQR) | 3.0 (1.5-6.0) | 2.6 (1.5-4.4) | |
| Weight (kg) | .554§ | ||
| Mean (range) | 6.2 (3.5-12.2) | 6.2 (3.4-13.5) | |
| Median (IQR) | 6.0 (4.5-7.5) | 5.7 (4.5-7.0) | |
| Height (cm) | .800§ | ||
| Mean (range) | 58.6 (47.8-80.0) | 58.8 (47.7-92.5) | |
| Median (IQR) | 56.0 (51.7-63.5) | 56.3 (51.6-61.4) | |
| Prior treatment* | .562† | ||
| Yes | 11 (29.7) | 13 (36.1) | |
| KHE | |||
| Morphology* | .576ǁ | ||
| Superficial | 2 (5.4) | 3 (8.3) | |
| Mixed | 31 (83.8) | 30 (83.3) | |
| Deep | 4 (10.8) | 3 (8.3) | |
| Maximal tumor dimension (cm) | .599‡ | ||
| Mean (range) | 10.0 (4.0-21.0) | 10.8 (3.5-25.0) | |
| Median (IQR) | 9.0 (6.0-14.5) | 10.5 (6.0-13.8) | |
| Retroperitoneal or intrathoracic location* | 3 (8.1) | 2 (5.6) | 1.000§ |
| Bone and/or joint involvement* | 4 (10.8) | 5 (13.9) | .736§ |
| KMP | |||
| Duration of KMP (m) | .693‡ | ||
| Mean (range) | 2.0 (0.0-9.0) | 2.2 (0.0-17.5) | |
| Median (IQR) | 1.3 (0.5-2.4) | 1.7 (0.5-3.0) | |
| Platelet level (10 × 109/L) | .851‡ | ||
| Mean (range) | 15.6 (3.0-47.0) | 16.3 (3.0-46.0) | |
| Median (IQR) | 14.0 (9.0-21.0) | 13.0 (7.0-24.8) | |
| Platelet level <10 × 109/L* | 13 (35.1) | 13 (36.1) | .931† |
| Fibrinogen level (g/L) | .623‡ | ||
| Mean (range) | 0.8 (0.1-1.58) | 0.8 (0.2-1.4) | |
| Median (IQR) | 0.8 (0.4-1.2) | 0.9 (0.6-1.0) | |
| Fibrinogen level <0.5 g/L* | 10 (27.0) | 7 (19.4) | .443† |
| Hemoglobin (g/L) | .787‡ | ||
| Mean (range) | 97.1 (55.0-152.0) | 97.9 (59.0-156.0) | |
| Median (IQR) | 89.0 (79.5-113.0) | 98.0 (79.3-112.0) | |
| Hemoglobin <60 g/L* | 3 (8.1) | 1 (2.8) | .615ǁ |
IQR, interquartile range.
Values are presented as number (percentage).
P value was calculated using the χ2 test.
P value was calculated using the Mann-Whitney U test.
P value was calculated with Fisher's exact test.
P value was calculated using the Pearson χ2 test.
Treatment before enrollment
| Prior treatment . | SIR + PRE group (n = 37) . | SIR group (n = 36) . | P values . | OR (95% CI) . |
|---|---|---|---|---|
| Total, no. (%)* | 17 (45.9) | 20 (55.6) | .412† | 0.680 (0.270-1.710) |
| Partial resection | 0 (0.0) | 1 (2.8) | N/A | N/A |
| Chest tube | 1 (2.7) | 0 (0.0) | N/A | N/A |
| Sclerotherapy | 3 (8.1) | 2 (5.6) | 1.000‡ | 1.500 (0.236-9.552) |
| Embolization | 1 (2.7) | 2 (5.6) | .615‡ | 0.472 (0.041-5.449) |
| Compression therapy | 3 (8.1) | 1 (2.8) | .615‡ | 3.088 (0.306-31.170) |
| Medical therapy§ | ||||
| Propranolol | 4 (10.8) | 3 (8.3) | 1.000‡ | 1.333 (0.277-6.427) |
| Corticosteroidsǁ | 8 (21.6) | 10 (27.8) | .542† | 0.717 (0.246-2.091) |
| Vincristine | 2 (5.4) | 2 (5.6) | 1.000‡ | 0971 (0.129-7.294) |
| Supportive care treatments¶ | 8 (21.6) | 6 (16.7) | .591† | 1.379 (0.426-4.467) |
| Prior treatment . | SIR + PRE group (n = 37) . | SIR group (n = 36) . | P values . | OR (95% CI) . |
|---|---|---|---|---|
| Total, no. (%)* | 17 (45.9) | 20 (55.6) | .412† | 0.680 (0.270-1.710) |
| Partial resection | 0 (0.0) | 1 (2.8) | N/A | N/A |
| Chest tube | 1 (2.7) | 0 (0.0) | N/A | N/A |
| Sclerotherapy | 3 (8.1) | 2 (5.6) | 1.000‡ | 1.500 (0.236-9.552) |
| Embolization | 1 (2.7) | 2 (5.6) | .615‡ | 0.472 (0.041-5.449) |
| Compression therapy | 3 (8.1) | 1 (2.8) | .615‡ | 3.088 (0.306-31.170) |
| Medical therapy§ | ||||
| Propranolol | 4 (10.8) | 3 (8.3) | 1.000‡ | 1.333 (0.277-6.427) |
| Corticosteroidsǁ | 8 (21.6) | 10 (27.8) | .542† | 0.717 (0.246-2.091) |
| Vincristine | 2 (5.4) | 2 (5.6) | 1.000‡ | 0971 (0.129-7.294) |
| Supportive care treatments¶ | 8 (21.6) | 6 (16.7) | .591† | 1.379 (0.426-4.467) |
SIR, sirolimus; PRE, prednisolone; N/A, data not available.
One patient may have received more than 1 treatment regimen.
P value was calculated with χ2 test.
P value was calculated with Fisher's exact test.
All drugs were administered in referring institutions (before referral).
The corticosteroid treatment protocols were variable, including oral prednisolone/prednisone (11 patients), intravenous methylprednisolone (4 patients), intravenous dexamethasone (2 patients), and intralesional injection of triamcinolone and betamethasone (1 patient). The average duration of oral prednisolone/prednisone therapy was 21.3 d (range, 6.0-35.0). The mean duration from previous corticosteroid treatment to trial treatment was 28.7 d (range, 9.0-62.0).
Supportive care treatments included fresh frozen plasma, cryoprecipitate, and packed red blood cells.
Efficacy outcomes
No patients were excluded from the efficacy and safety analyses. Sixty-six patients completed the 12-month treatment period. The most common reason for not completing 12 months of treatment was lack of efficacy (2.7% in the combination treatment group vs 13.9% in the monotherapy group; difference, 11.2%; 95% confidence interval [CI], −2.4 to 26.1). In the combination treatment group, the average duration of oral prednisolone treatment was 8.1 weeks (range, 6.0-11.0).
In total, 59 of 73 patients achieved the target platelet count of 100 × 109/L or more (a durable platelet response) at week 4. The proportion of patients who achieved a durable platelet response rate was significantly higher in the sirolimus plus prednisolone group than in the sirolimus monotherapy group (94.6% in the combination treatment group vs 66.7% in the monotherapy group; difference, 27.9%; 95% CI, 10.0-44.7). During weeks 1 to 3, the number of patients achieving a durable platelet response ranged from 9 (24.3%) to 31 (83.8%) in the combination treatment group and from 4 (11.1%) to 20 (55.6%) in the sirolimus monotherapy group (Table 3). Patients in the combination treatment group had higher median platelet counts during weeks 1 to 4 than those in the sirolimus monotherapy group (29.0 × 109/L vs 22.0 × 109/L at week 1 and 270.0 × 109/L vs 208.0 × 109/L at week 4; difference, 38.0 × 109/L; 95% CI, 6.1-69.8) (Figure 2; supplemental Figures 1-4; supplemental Table 1 on the Blood Web site).
Primary and secondary efficacy analyses*
| Outcomes . | SIR + PRE (n = 37) . | SIR group (n = 36) . | OR (95% CI) . |
|---|---|---|---|
| Primary outcome | |||
| Proportion of patients achieving a durable platelet response rate at week 4† | 35 (94.6) | 24 (66.7) | 8.750 (1.794-42.673)‡ |
| Secondary outcome | |||
| Proportion of patients achieving a durable platelet response rate during weeks 1-3§ | |||
| Week 1 | 9 (24.3) | 4 (11.1) | 2.571 (0.713-9.270)ǁ |
| Week 2 | 21 (56.8) | 11 (30.6) | 2.983 (1.140-7.808)‡ |
| Week 3 | 31 (83.8) | 20 (55.6) | 4.133 (1.384-12.340)ǁ |
| Proportion of patients achieving fibrinogen stabilization at week 4¶ | 27 (73.0) | 16 (44.4) | 3.375 (1.268-8.984)‡ |
| Lesion responses | |||
| Overall lesion response rate | 34 (91.9) | 29 (80.6) | 2.736 (0.648-11.551)ǁ |
| Good lesion response rate | 30 (81.1) | 21 (58.3) | 3.061 (1.064-8.804)‡ |
| KMP rebound rate# | 2 (5.4) | 6 (16.7) | 3.500 (0.657-18.648)ǁ |
| Blood transfusions (per patient) | 1.4 | 2.5 | — |
| Improvement in QOL | 33 (89.2) | 28 (77.8) | 2.357 (0.641-8.663)ǁ |
| Disease sequelae** | 4 (10.8) | 12 (33.3) | 0.242 (0.070-0.844)ǁ |
| Lymphedema | 2 (5.4) | 5 (13.9) | 0.354 (0.064-1.958) |
| Decreased range of motion‡ | 1 (2.7) | 4 (11.1) | 0.222 (0.024-2.093) |
| Chronic painǁ | 0 (0.0) | 3 (8.3) | N/A |
| Scoliosis | 1 (2.7) | 2 (5.6) | 0.472 (0.041-5.449) |
| Leg-length discrepancy | 1 (2.7) | 1 (2.8) | 0.972 (0.058-16.158) |
| Outcomes . | SIR + PRE (n = 37) . | SIR group (n = 36) . | OR (95% CI) . |
|---|---|---|---|
| Primary outcome | |||
| Proportion of patients achieving a durable platelet response rate at week 4† | 35 (94.6) | 24 (66.7) | 8.750 (1.794-42.673)‡ |
| Secondary outcome | |||
| Proportion of patients achieving a durable platelet response rate during weeks 1-3§ | |||
| Week 1 | 9 (24.3) | 4 (11.1) | 2.571 (0.713-9.270)ǁ |
| Week 2 | 21 (56.8) | 11 (30.6) | 2.983 (1.140-7.808)‡ |
| Week 3 | 31 (83.8) | 20 (55.6) | 4.133 (1.384-12.340)ǁ |
| Proportion of patients achieving fibrinogen stabilization at week 4¶ | 27 (73.0) | 16 (44.4) | 3.375 (1.268-8.984)‡ |
| Lesion responses | |||
| Overall lesion response rate | 34 (91.9) | 29 (80.6) | 2.736 (0.648-11.551)ǁ |
| Good lesion response rate | 30 (81.1) | 21 (58.3) | 3.061 (1.064-8.804)‡ |
| KMP rebound rate# | 2 (5.4) | 6 (16.7) | 3.500 (0.657-18.648)ǁ |
| Blood transfusions (per patient) | 1.4 | 2.5 | — |
| Improvement in QOL | 33 (89.2) | 28 (77.8) | 2.357 (0.641-8.663)ǁ |
| Disease sequelae** | 4 (10.8) | 12 (33.3) | 0.242 (0.070-0.844)ǁ |
| Lymphedema | 2 (5.4) | 5 (13.9) | 0.354 (0.064-1.958) |
| Decreased range of motion‡ | 1 (2.7) | 4 (11.1) | 0.222 (0.024-2.093) |
| Chronic painǁ | 0 (0.0) | 3 (8.3) | N/A |
| Scoliosis | 1 (2.7) | 2 (5.6) | 0.472 (0.041-5.449) |
| Leg-length discrepancy | 1 (2.7) | 1 (2.8) | 0.972 (0.058-16.158) |
N/A, data not available; PRE, prednisolone; SIR, sirolimus.
Unless otherwise indicated, values are presented as the number (percentage).
The values were calculated using generalized linear mixed models.
The values were calculated using a χ2 test.
A durable platelet response was defined as platelet counts ≥100 × 109/L lasting at least 4 consecutive weeks.
The values were calculated using Fisher's exact test.
Fibrinogen stabilization was defined as fibrinogen levels >1.6 g/L lasting at least 4 consecutive weeks.
KMP rebound was defined as a platelet count decrease to <100 × 109/L after achieving a durable platelet response.
One patient may have had more than 1 disease sequelae.
Median platelet count during the initial 4 weeks in patients treated with sirolimus plus prednisolone or sirolimus alone. Data include all 73 patients with KMP. Error bars indicate the range from the first to the third quartiles.
Median platelet count during the initial 4 weeks in patients treated with sirolimus plus prednisolone or sirolimus alone. Data include all 73 patients with KMP. Error bars indicate the range from the first to the third quartiles.
In both groups, there was an improvement in the fibrinogen level related to the time of evolution. At week 4, fibrinogen increased to a clinically nonsignificant level (>1.6 g/L) in 27 (73.0%) and 16 (44.4%) patients in the combination treatment group and sirolimus monotherapy group, respectively (difference, 28.5%; 95% CI, 6.0-47.4).
The achievement of objective lesion response at month 12 was primarily driven by sirolimus plus prednisolone. The overall lesion response rate was 91.9% in the combination treatment group and 80.6% in the sirolimus monotherapy group (difference, 11.3%; 95% CI, −5.0 to 27.8). The good lesion response rate was higher in the combination treatment group than in the sirolimus monotherapy group (81.1% vs 58.3%; difference, 22.8%; 95% CI, 1.7-41.4) (Table 3; Figure 3; supplemental Figures 1-4).
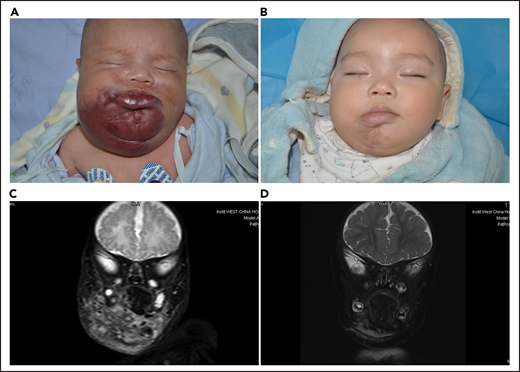
Lesion response assessed by MRI. (A) KHE associated with KMP in a 2-month-old boy (patient 5618). He was treated with a combined therapy of sirolimus and prednisolone. (B) After 12 months of treatment, the reddish skin disappeared and evolved in size. (C) Coronal T2-weighted MRI changes in lesions before treatment. (D) A good lesion response was noted at month 12.
Lesion response assessed by MRI. (A) KHE associated with KMP in a 2-month-old boy (patient 5618). He was treated with a combined therapy of sirolimus and prednisolone. (B) After 12 months of treatment, the reddish skin disappeared and evolved in size. (C) Coronal T2-weighted MRI changes in lesions before treatment. (D) A good lesion response was noted at month 12.
A total of 67 patients achieved a durable platelet response during treatment (supplemental Table 2). The overall KMP rebound rate was 11.0%. The KMP rebound rate was lower in the combination treatment group than in the sirolimus monotherapy group (5.4% vs 16.7%). However, the difference was not significant (difference, 11.3%; 95% CI, −3.9 to 27.0) (Table 3).
For other secondary endpoints, considerably more blood transfusions (per patient) were needed to prevent or treat bleeding in the sirolimus monotherapy group than in the combination treatment group (2.5 vs 1.4; P = .003) (Table 3). Moreover, an improvement in QOL from baseline to month 12 was noted in 33 (89.2%) patients in the combination treatment group and in 28 (77.8%) patients in the sirolimus monotherapy group (difference, 11.4%; 95% CI, −6.0 to 28.6).
At month 24, a total of 68 patients (35 patients in the combination treatment group and 33 in the sirolimus monotherapy group) had follow-up data. Among them, 17 patients in the combination treatment group and 12 patients in the sirolimus monotherapy group had completed treatment. Finally, 16 patients had disease sequelae at month 24, including lymphedema (7 patients), decreased ROM (5 patients), daily pain at the involved site (3 patients), scoliosis (3 patients), and leg-length discrepancy (2 patients) (supplemental Figures 5 and 6). The total incidence of disease sequelae was significantly lower for patients who received sirolimus plus prednisolone than for those who received sirolimus monotherapy (33.3% vs 10.8%; difference, 22.5%; 95% CI, 3.4-40.1) (Table 3). None of the patients died of KMP or other KHE-related complications during the follow-up period in either group.
Safety outcomes
No deaths, malignancies, or cases of interstitial pneumonitis or PJP occurred in the study. No appreciable differences in growth variables were noted between the sirolimus monotherapy group and the sirolimus plus prednisolone group. There was no difference in the overall incidence of adverse events between the 2 groups (3.6 events per patient in the combination treatment group vs 3.4 events per patient in the sirolimus monotherapy group, P = .981) (Table 4; supplemental Table 3). During treatment, the most common adverse events of any grade (in ≥10% of patients in both groups) were upper respiratory tract infection, mucositis, and nausea/vomiting. There were no clinically significant differences in laboratory abnormalities. Laboratory changes were generally of low grade and consistent with those observed for other conditions. Four patients in the combination treatment group and 2 patients in the sirolimus monotherapy group had increases in transaminase concentrations to twice the upper limit of normal. These events were either known side effects of sirolimus or expected in the pediatric population. In total, 21 grade 3-4 adverse events occurred, with no significant difference overall or according to individual events between the sirolimus monotherapy groups and the combination treatment group (supplemental Table 4).
Total adverse events and serious adverse events in the 2 study groups*
| Category . | Total adverse events . | Serious adverse events† . | ||
|---|---|---|---|---|
| SIR + PRE . | SIR . | SIR + PRE . | SIR . | |
| Upper respiratory infection | 20 | 17 | 4 | 3 |
| Mucositis | 15 | 17 | 1 | 2 |
| Nausea/vomiting | 15 | 12 | 0 | 0 |
| Pneumonia‡ | 11 | 8 | 2 | 3 |
| Thrombocytosis | 9 | 10 | 0 | 0 |
| Cough | 6 | 10 | 0 | 0 |
| Eczema | 7 | 8 | 0 | 1 |
| Increased liver enzyme levels | 5 | 5 | 0 | 0 |
| Constipation | 6 | 4 | 0 | 0 |
| Diarrhea | 6 | 3 | 1 | 0 |
| Pain | 4 | 4 | 0 | 0 |
| Gastroenteritis | 2 | 6 | 1 | 1 |
| Rash | 5 | 3 | 0 | 0 |
| Acne | 3 | 4 | 0 | 0 |
| Decreased appetite | 3 | 3 | 0 | 0 |
| Lymphopenia | 4 | 2 | 0 | 0 |
| Lymph gland infection | 2 | 3 | 0 | 0 |
| Hyperlipidemia | 3 | 1 | 0 | 0 |
| Hypercholesterolemia | 2 | 2 | 0 | 0 |
| Urinary tract infection | 2 | 1 | 1 | 0 |
| Neutropenia | 2 | 0 | 0 | 0 |
| Cataract | 1 | 0 | 1 | 0 |
| Total | 133 | 123 | 11 | 10 |
| Category . | Total adverse events . | Serious adverse events† . | ||
|---|---|---|---|---|
| SIR + PRE . | SIR . | SIR + PRE . | SIR . | |
| Upper respiratory infection | 20 | 17 | 4 | 3 |
| Mucositis | 15 | 17 | 1 | 2 |
| Nausea/vomiting | 15 | 12 | 0 | 0 |
| Pneumonia‡ | 11 | 8 | 2 | 3 |
| Thrombocytosis | 9 | 10 | 0 | 0 |
| Cough | 6 | 10 | 0 | 0 |
| Eczema | 7 | 8 | 0 | 1 |
| Increased liver enzyme levels | 5 | 5 | 0 | 0 |
| Constipation | 6 | 4 | 0 | 0 |
| Diarrhea | 6 | 3 | 1 | 0 |
| Pain | 4 | 4 | 0 | 0 |
| Gastroenteritis | 2 | 6 | 1 | 1 |
| Rash | 5 | 3 | 0 | 0 |
| Acne | 3 | 4 | 0 | 0 |
| Decreased appetite | 3 | 3 | 0 | 0 |
| Lymphopenia | 4 | 2 | 0 | 0 |
| Lymph gland infection | 2 | 3 | 0 | 0 |
| Hyperlipidemia | 3 | 1 | 0 | 0 |
| Hypercholesterolemia | 2 | 2 | 0 | 0 |
| Urinary tract infection | 2 | 1 | 1 | 0 |
| Neutropenia | 2 | 0 | 0 | 0 |
| Cataract | 1 | 0 | 1 | 0 |
| Total | 133 | 123 | 11 | 10 |
The safety population included all patients who received at least 1 dose of sirolimus. Adverse events were assessed using the Common Terminology Criteria for Adverse Events, version 4.0. The details of the assessment plan for adverse events are provided in the trial protocol.
A serious adverse event was defined as any of the grade ≥3 toxicities identified during the 12 months of treatment.
Potential causative agents were found in 13 patients. A bacterial agent was found in 9 patients and a respiratory virus was found in 7 patients. In 3 patients, there was evidence of a mixed bacteria/virus infection.
No patients permanently discontinued treatment because of adverse events. Adverse events leading to temporary discontinuation (3-28 days) or a reduction in sirolimus dose, but not withdrawal from the study, occurred in 15 (20.5%) and 7 (9.6%) patients, respectively. The most frequent adverse events causing temporary discontinuation of sirolimus were grade ≥3 upper respiratory tract infections (7 events) and pneumonia (5 events). Intravenous cefoperazone-sulbactam or imipenem-cilastatin was required in some of these patients.
Discussion
Previously, KHE with KMP had a notably high mortality rate (up to 10%-30%) because of a lack of effective treatment.19 Despite treatment advances, KHE remains a disease with unacceptable morbidity. New treatment regimens with an acceptable safety profile that reduce disease activity, reduce flares, and improve long-term outcomes are urgently needed for patients with KMP. The recent introduction of sirolimus and prednisolone combination therapy offers an exciting perspective for high-risk or refractory KHE patients. However, experience with this therapeutic regimen is limited. Therefore, we conducted a randomized trial of sirolimus plus prednisolone vs sirolimus monotherapy to characterize the impact of adding corticosteroids to sirolimus on the time to achieve KMP remission, medium-term outcomes, and safety outcomes. Indeed, our results showed a significantly decreased risk of death after sirolimus therapy, whether used alone or in combination with prednisolone. In addition, we found that combination treatment was associated with significant clinical improvements compared with sirolimus alone. The mechanism or mechanisms through which sirolimus and prednisolone combination therapy induces rapid responses are a point of major interest. Experience with this combination treatment regimen is based on our assumption that KHE with KMP has a neoplastic-inflammatory nature.15 Both sirolimus and corticosteroids exert antineoplastic and anti-inflammatory effects via different mechanisms.26,27 Theoretically, combining antineoplastic treatment with anti-inflammatory treatment may have additive or synergistic effects, enhancing the efficacy of both.28 Future basic studies are needed and could prove useful to elucidate this point.
In the present study, all the enrolled patients had KMP, and their clinical manifestations were comparable to those reported in other cohorts.2,8,9,29 We found that sirolimus combination therapy with corticosteroids could accelerate the time to achieve the complete remission of KMP. As the primary outcome for this clinical trial, we chose the stabilization of the platelet count, because the platelet count is a widely used outcome measure for the study of KHE with KMP.1,30,31 The threshold (a durable platelet count >100 × 109/L) was selected to show unequivocal improvement in platelet counts, although the treatment of patients with KMP is typically started at platelet counts <30 × 109/L.1,2 In fact, severe thrombocytopenia is the most definitive/distinguishing factor impacting the symptoms in patients with KMP. The platelet count is one of the major factors to be considered for patients with KHE to meet the coagulopathy criterion for KMP.
In the present study, platelet count increases could be seen within 1 to 2 weeks and were sustained throughout the 24-month follow-up period. In addition, the >94% durable platelet response for sirolimus and prednisolone combination therapy was higher than that reported for other treatments, such as vincristine, corticosteroid monotherapy, interferon, and aspirin/ticlopidine.25,32,33 This finding is especially striking because the patients in this study were a very refractory group, including 32.9% of patients who did not respond to vincristine and other conventional treatments. However, despite the highly successful response rates, not all patients achieved a durable platelet count at week 4. This finding might reflect the variation in the severity of KHE in the patient population. In fact, retroperitoneal and intrathoracic KHE tend to be more expansive and infiltrative and are more likely to be refractory to treatment.1,5,16,34 In some patients with retroperitoneal KHE, a longer time for the normalization of platelet counts after sirolimus treatment can be seen.35 The reason for this phenomenon remains unexplained, but it could be related to pharmacokinetic differences.
In conjunction with the rise in platelet count, the sirolimus plus prednisolone group also showed a significant improvement in hypofibrinogenemia when compared with the sirolimus monotherapy group. Two other important findings in patients given sirolimus combination therapy were a lower rate of KMP rebound and the reduced use of blood transfusion. In addition, it is not surprising that patients receiving combination treatment did show significant improvements in volume changes at month 12 compared with patients receiving sirolimus monotherapy. However, nonsignificant volume changes between the 2 treatment groups were observed in a previous study, possibly because of the retrospective nature and the small sample size of that study.17 Continued platelet trapping and aggregation in KHE lesions might contribute to worsening thrombocytopenia and coagulopathy (KMP) by promoting critical processes such as neovascularization.15,30 The coexistence of KMP represents aggressive tumor progression with rapidly enlarged KHE lesions. The onset of the effect of sirolimus and prednisolone combination treatment not only shortened the courses of KMP, but also rapidly stabilized KHE growth, potentially leading to a considerable minimization of the residual lesion. Pathologically, uncontrolled KHE and KMP lesions are characterized by progressive fibrosis, leading to chronic degenerative changes (eg, joint contracture) and significant residual lesions.22 In this regard, there is substantial evidence that both sirolimus and corticosteroids can prevent and reduce fibrosis via different mechanisms and therefore attenuate disease progression in different tissues and organs.36-39
Health-related QOL in patients with KHE is poor. The need for treatments that improve QOL and reduce medium-term and long-term sequelae has been articulated by patients and/or their parents.22,40 In this study, as in the previous phase 2 studies, scores for health-related QOL improved over the 12 months of treatment.11,21 Additionally, short-term treatment with prednisolone led to, by physical assessment, a decrease in the total proportion of patients with disease sequelae. KMP and musculoskeletal complications (eg, chronic pain, decreased ROM) are prominent complications in patients with KHE, and improvement in these problems is associated with improvement in QOL.23,41
Because of its limited efficacy, sirolimus is used in combination with corticosteroids rather than as a single agent, which raises the question of whether patients treated with this combination have an increased risk of toxicity. Although our prior experience with sirolimus combined with corticosteroids demonstrated high safety,17,29 the occurrence of opportunistic infections with corticosteroid and/or sirolimus treatment is an area of increased attention and debate.42-45 In this study, close laboratory and clinical monitoring showed that treatment with sirolimus plus a short course of prednisolone were well tolerated by patients. The safety profile of sirolimus plus prednisolone in patients with KMP was consistent with findings in patients receiving sirolimus monotherapy in other published studies46-48 and was also comparable with results from studies of sirolimus plus corticosteroids for other conditions.26 In this trial, no quantitative differences were recorded in the side effect profile (total infection rate and serious infection rate) between the sirolimus monotherapy and combination therapy groups. Our findings confirmed that additional short-term prednisolone treatment was not associated with any notable safety findings compared with results from corticosteroid studies in pediatric populations.49 The rate of infections might not be affected by the short-term administration of prednisolone. The majority of adverse effects could be controlled using conservative treatments or by decreasing the drug dosage. In addition, there were no reports of deaths, malignances, interstitial pneumonitis, or PJP. Nonetheless, slight increases in liver enzymes were noted in patients in both groups, so monitoring liver function is recommended during sirolimus treatment.
Limitations
There are several limitations to the conclusions that can be drawn from this trial. First, because of our open-label design, patients’ parents (or guardians) and treating investigators were aware of the treatment group in which the patients were placed. However, our outcome assessments involved regular follow-up, standardized image analyses, and independent and blind readings. The limitations associated with an open-label trial could be minimized by the objective nature of our trial outcomes. Second, our study results may be limited by the small size of our patient cohort. However, KHE is extremely rare, and our trial is thus far the largest prospective investigation in this disease. Third, although this trial is the first randomized clinical trial evaluating the effects of sirolimus on KHE and shows a positive outcome within 24 months, the timeframe of the study limited the ability to assess 5-year outcomes. Further improvements in efficacy might be seen in future studies. Fourth, we cannot assess overall disease activity improvement with an established composite endpoint because no validated endpoint for studies of KHE exists. However, platelet counts are a widely accepted clinical study and global regulatory endpoint for assessing the severity of KMP. Additional results supporting the finding for the primary outcome were the significant improvements in fibrinogen levels for patients in the combination treatment group. Finally, lower dose sirolimus (3-5 ng/mL serum levels) may be associated with low toxicity and may also be effective for treating patients with KMP.50,51 Ongoing randomized studies comparing different doses of sirolimus in patients with KMP can have a great impact in the clinical disease setting.
Conclusions
Short-term prednisolone treatment plus sirolimus therapy was superior to sirolimus monotherapy in improving the signs and symptoms of active KHE with KMP. Our study provided additional evidence for the use of sirolimus plus prednisolone in KMP patients not only to normalize hematologic parameters and reduce lesion mass promptly, but also to prevent long-term sequelae. This work provides the foundation for future studies of sirolimus plus corticosteroids as a potentially effective oral treatment option for patients with active KMP.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81400862 and 81401606), the Key Project in the Science & Technology Program of Sichuan Province (2019YFS0322), the Science Foundation for The Excellent Youth Scholars of Sichuan University (2015SU04A15), and the 1·3·5 Project for Disciplines of Excellence Clinical Research Incubation Project, West China Hospital of Sichuan University (2019HXFH056, 2020HXFH048, and ZYJC21060).
Authorship
Contribution: Y.J., S.C., K.Y., X.Z., and J.Z. designed the study, followed the patients, analyzed the data, and drafted the manuscript; Y.J., S.C., K.Y., X.Z., J.Z., B.X., T.Q., X.G., X.Z., Y.L., F.H., Q.Q., F.K., and Y.Z. participated in the acquisition, analysis, or interpretation of data; Y.J. and S.C. obtained funding; and all authors were involved in the critical analysis of the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yi Ji, Division of Oncology, Department of Pediatric Surgery, West China Hospital of Sichuan University, 37# Guo-Xue-Xiang, Chengdu 610041, China; e-mail: jijiyuanyuan@163.com; and Siyuan Chen, Pediatric Intensive Care Unit, Department of Critical Care Medicine, West China Hospital of Sichuan University, 37# Guo-Xue-Xiang, Chengdu 610041, China; e-mail: siy_chen@163.com.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal