Although related to a single point mutation, one central characteristic of sickle cell disease (SCD) is the extreme variability in the clinical picture from one patient to another. Genetic variants may account for part of this variability. In this issue of Blood, Dosunmu-Ogunbi et al1 highlighted the importance of a genetic variant affecting the mitochondrial function in the cardiovascular pathophysiology of SCD. Their findings bring important insights and perspectives for the management of patients.
Oxidative stress is a key factor in the SCD pathophysiology. SCD is associated with prooxidant enzyme activation, the hemolysis-induced release of cell-free hemoglobin and heme, red blood cell autooxidation, and reduced nitric oxide bioavailability, all of which promote oxidative stress.2 At the vascular level (eg, red blood cells, platelets, neutrophils, and endothelial cells), oxidative stress induces a proinflammatory state and a cascade of damage and/or dysfunction (eg, cell adhesion, hypercoagulability, and hemorheological disturbances) leading to vasculopathy (eg, cerebral vasculopathy, pulmonary hypertension, and retinopathy).3 To fight against oxidative stress, nonenzymatic and enzymatic antioxidant mechanisms exist, but unfortunately, both pathways are defective in SCD.3 Superoxide dismutase (SOD) belongs to the enzymatic antioxidant category, and its second isoform (SOD2) is located in the mitochondrial matrix.4
In their study, Dosunmu-Ogunbi et al investigated the role of a variant of SOD2 (SOD2V16A) on mitochondrial function. The authors found both mitochondrial complex IV inhibition and higher reactive oxygen species (ROS) production with the variant. The authors also investigated the impact of this variant on different pulmonary and cardiovascular markers in 410 patients with SCD (from the walk-PHaSST trial) among whom 217 were heterozygous and 64 were homozygous for the variant. Dosunmu-Ogunbi et al found that SOD2V16A homozygous patients with SCD displayed (i) increased tricuspid regurgitant velocity, systolic blood pressure, systolic right ventricular area, and hemolytic index; (ii) a strong trend toward decreased hemoglobin concentration; and (iii) lower 6-minute walk distance. Together, these results suggest that the presence of the variant reduces pulmonary and cardiovascular functions, worsens anemia and hemolysis, and decreases exercise capacity/physical ability.
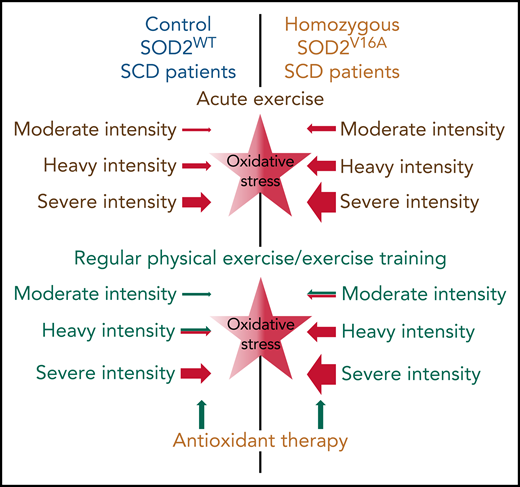
The results of Dosunmu-Ogunbi et al are of paramount importance because, in the classic picture of the role of oxidative stress in SCD pathophysiology, the role of mitochondria is often ignored or at least underestimated, whereas mitochondrial respiration produces a significant amount of ROS. During physical activities, even those of everyday life (eg, walking, climbing the stairs, and shopping), which are already intensive for patients with SCD,5 mitochondria are prone to produce more ROS. Exercise intensity is determinant in that matter. Indeed, mitochondrial ROS production rises with increasing exercise intensity.4 Given the low antioxidant defenses of patients with SCD, extra exercise-related mitochondrial ROS production may further aggravate the prooxidant state of the patients.6 The results of Dosunmu-Ogunbi et al suggest that this situation could be even worse in patients with the SOD2V16A variant (see figure). From that point of view, exercising patients with the SOD2V16A variant may experience higher oxidative stress and, consequently, more severe complications or at least more impact on pulmonary and cardiovascular functions. Of note, oxidative stress can also directly impact muscle function. In their comprehensive review, Powers and Jackson4 explored cellular mechanisms by which an excessive oxidative stress may have deleterious effects on muscle force production and may favor muscle fatigue. Therefore, the lower performance/distance in the 6-minute walk test in patients homozygous for the SOD2V16A variant is likely related to the deleterious impact of oxidative stress on the cardiopulmonary and muscle functions.
Putative basal oxidative stress (central stars) and effects of acute and regular physical exercise of different intensities on oxidative stress in patients with SCD with SOD2WT or homozygous to the SOD2V16A variant. The intensity of color (central stars) indicates the basal level of oxidative stress. Sizes of arrows indicate the putative size effect. Green and red colors indicate putative positive/blunting or negative/aggravating effects, respectively.
Putative basal oxidative stress (central stars) and effects of acute and regular physical exercise of different intensities on oxidative stress in patients with SCD with SOD2WT or homozygous to the SOD2V16A variant. The intensity of color (central stars) indicates the basal level of oxidative stress. Sizes of arrows indicate the putative size effect. Green and red colors indicate putative positive/blunting or negative/aggravating effects, respectively.
Various antioxidant therapies (eg, l-glutamine, l-arginine, and N-acetylcysteine supplementations) have been tested in SCD to reduced oxidative stress. Therefore, one may expect that such therapies might have a greater effect in patients with the SOD2V16A variant, especially during exercise. Nitrate supplementation has been shown to improve exercise capacity in a mice model of SCD.7
Because recent studies have reported beneficial effects of endurance training in patients with SCD,8 there has been growing interest in considering regular physical activity as part of the therapeutic strategy for patients with SCD. Endurance training improves antioxidant defenses, including SOD2 activity.4 However, at the present time (to my knowledge), it is not known if endurance training changes SOD2 activity in patients with SCD. Determining this would be helpful for patients with SCD, especially for those with the SOD2V16A variant. On the other hand, it has been suggested that too much oxidative stress is deleterious for muscle adaptation (see figure). Therefore, it appears essential, especially in patients with the SOD variant, to properly calibrate the training exercises to avoid excessive oxidative stress, which (i) could lead to complications during training sessions and (ii) would be deleterious for the beneficial pulmonary, cardiovascular, and muscular adaptations induced by the physical exercise training. Would a dual antioxidant-endurance training therapy (see figure) be the most appropriate for patients with SCD with SOD2V16A variant? The more we discuss the results of Dosunmu-Ogunbi et al, the more we find perspectives and generate new hypotheses for future research.
As a whole, the work of Dosunmu-Ogunbi et al brings new ideas to explain the clinical variability between SCD patients and opens the way to new research.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal