In this issue of Blood, Saha et al1 have identified a CD45-targeted antibody-drug conjugate (CD45 ADC) as a safe and highly effective conditioning agent for hematopoietic stem cell transplantation (HSCT) in a mouse model. Broad application of HSCT as curative treatment of blood disorders is limited by both donor incompatibility and the damage caused by current protocols using nonselective and genotoxic conditioning agents needed to make space for donor cells in the recipient hematopoietic stem cell (HSC) niche. Successful translation of this new CD45 ADC concept to the clinic has the potential to flatten the risk-benefit ratio in allotransplantation, turning the fantasy of a universal bone marrow donor into a reality and expanding transplantation applications from malignancy and inherited diseases of the blood to acquired diseases, including HIV/AIDS and autoimmunity. Possibly even solid organ allotransplantation might be included, because establishing a state of bone marrow chimerism has been known since the 1940s to establish a state of donor-specific solid organ allograft tolerance.2 In sum, work in this area may augur a second and even broader transformation of medical practice by bone marrow transplantation.
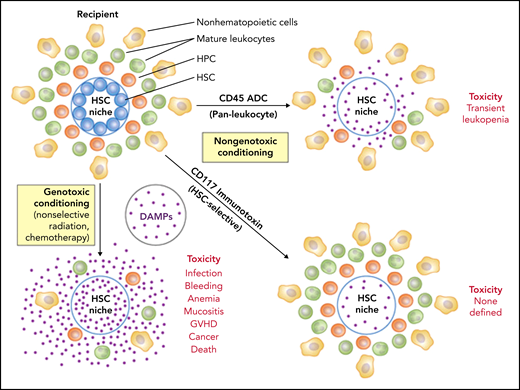
The investigators tested a conditioning concept that is both simple and logical and builds on accomplishments in cancer: to precisely deliver a cytotoxic but nongenotoxic drug payload once and as a single agent to the cells that matter most in allotransplantation, HSCs, and T cells, using the antigen specificity of a monoclonal antibody (mAb) to which the drug has been chemically conjugated. Untargeted genotoxic agents may indiscriminately kill any dividing cell and thereby drive acute toxicity through cell loss and massive release of proinflammatory danger-associated molecular patterns (DAMPs) that promote rejection and graft-versus-host disease. Genotoxic agents also induce mutations that increase the risk of posttransplantation malignancies. The ADC used by Saha et al instead selectively targets cells expressing the pan-leukocyte and HSC marker CD45, thereby sparing nonhematopoietic cells while hitting both HSCs and T cells, obviating the need for treatment with additional immunosuppressive agents after transplantation to prevent graft rejection (see figure). The antibody was designed to lack mouse Fc receptor binding activity, which accelerates in vivo clearance, thereby limiting cytotoxicity to incoming donor cells. The payload is tesirine, a pyrrolobenzodiazepine dimer that kills cells by its antimitotic activity.3 CD45 ADC builds on an earlier immunotoxin concept in which the plant-derived ribosome-inactivating protein (RIP) saporin was conjugated to a mouse CD45 mAb.4 This agent was highly effective for conditioning congenic transplantation in mice.
Conditioning approaches in bone marrow transplantation. The cell target area and mechanism of cytotoxicity are major determinants of safety. Toxicity is a function of cell loss per se, the release of DAMPs from dying cells, and the introduction of mutations in cells that survive. DAMPs can drive unregulated immune responses and facilitate graft-versus-host disease (GVHD). Antigen-targeted mAbs that deliver cytotoxic but nongenotoxic payloads to specific cell types important for engraftment of donor cells after transplantation mitigate key safety concerns and have great potential for replacing X-irradiation and chemotherapeutic agents for bone marrow conditioning. Additional therapeutic antibody strategies, for example, targeting CD47 to enhance phagocytosis of HSCs, have also been described. HPC, hematopoietic progenitor cell. Professional illustration by Patrick Lane, ScEYEnce Studios.
Conditioning approaches in bone marrow transplantation. The cell target area and mechanism of cytotoxicity are major determinants of safety. Toxicity is a function of cell loss per se, the release of DAMPs from dying cells, and the introduction of mutations in cells that survive. DAMPs can drive unregulated immune responses and facilitate graft-versus-host disease (GVHD). Antigen-targeted mAbs that deliver cytotoxic but nongenotoxic payloads to specific cell types important for engraftment of donor cells after transplantation mitigate key safety concerns and have great potential for replacing X-irradiation and chemotherapeutic agents for bone marrow conditioning. Additional therapeutic antibody strategies, for example, targeting CD47 to enhance phagocytosis of HSCs, have also been described. HPC, hematopoietic progenitor cell. Professional illustration by Patrick Lane, ScEYEnce Studios.
Remarkably, in the current work, the team identified a dose of CD45 ADC that as a single agent without additional immunosuppression allowed 100% donor chimerism in a complete major histocompatibility complex (MHC) mismatched strain combination (Balb/c→C57BL/6). This punch line was sweetened by demonstrations of efficacy and safety in congenic transplantation, and in allotransplantation across both a minor MHC mismatch and a major MHC mismatch at lower (ie, subtherapeutic) doses of CD45 ADC that were augmented by combination with Jak inhibitors, anti-CD40L, rapamycin, cyclophosphamide, or low doses of X-irradiation. CD45 ADC was nonmyeloablative at the doses used, causing only transient leukopenia, including neutropenia, but infections were not observed.
There are some limitations of the study impacting the odds of successful translation to the clinic. Only 10 mice were studied in the marquee major MHC mismatch part of the study, and all were very young. Engraftment durability was not defined beyond 22 weeks. A few mice died of undefined causes. Although this level of mortality may not have been greater than for control transplants, future studies need to be conducted through necropsies of all animals dying during the study. Clinical translation will require a new agent that successfully targets human CD45 without serious toxicity. Fortunately, therapeutic antibodies are now a boom industry driven by disease applications for naked antibodies in cancer and autoimmune disease. Moreover, there are now 11 ADCs approved by the Food and Drug Administration (FDA) for applications in cancer, so the development path of this platform is well traveled.
CD45 ADC is joined in the conditioning/transplantation space race by mAbs to CD117 (c-kit or stem cell factor receptor), which is selectively expressed by hematopoietic stem and progenitor cells and not by most mature leukocytes. This approach has been successful in congenic transplantation in immunodeficient but not immunocompetent mice using a naked antagonistic CD117 mAb as a single agent,5 and in both congenic and complete MHC mismatched allogeneic transplantation in immunocompetent mice using a CD117-saporin immunotoxin.6,7 Moreover, CD117-saporin conditioning of bone marrow allotransplants resulted in sufficient hematopoietic chimerism to allow robust donor-specific skin allograft tolerance.7 A fourth conditioning concept that has shown preclinical promise is naked CD117 mAb in combination with anti-CD47 mAb.8 Anti-CD47 blocks a “don’t eat me” signal mediated by CD47 binding to SIRPα (signal regulatory protein α) on macrophages, rendering anti-CD117–bound HSCs susceptible to macrophage phagocytosis.
CD117 strategies have moved to the clinic with Magenta Therapeutics opening phase 1 clinical trials of a CD117 immunotoxin coupled to the RIP amanitin, first in acute myeloid leukemia and myelodysplastic syndrome, and Gilead testing magrolimab, a naked CD47 mAb, first in multiple cancer indications. Naked humanized CD117 mAb AMG 191/JSP 191 (Amgen) is also being explored in trials in various blood and immune diseases.
The era of therapeutic antibodies was launched in 1981 with the discovery by Waldmann and colleagues at the National Cancer Institute (NCI) of the NIH of anti-Tac, an mAb specific for the interleukin-2 receptor α.9 After 17 years of development by PDL BioPharma, anti-Tac was approved by the FDA in 1997 as Daclizumab for the prevention of renal transplant rejection, the first approved humanized mAb. Starting in the early 1980s, Pastan and colleagues at the NCI developed the immunotoxin concept over 3 decades to create Lumoxiti (AstraZeneca and Medimmune), a CD22 immunotoxin for the treatment of relapsed/refractory hairy cell leukemia.10 In 2018, Lumoxiti became the first immunotoxin approved by the FDA. Given the manifest utility of therapeutic antibodies and the well-paved route from concept through optimization to completed clinical trials by these pioneers, the time for successful translation of antibody-based conditioning in HSC transplantation may be sooner rather than later, unleashing the full potential of this curative treatment, limited only by cost.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal