Abstract
Chronic graft-versus-host disease (cGVHD) is a major immunologic complication of allogeneic hematopoietic cell transplantation. cGVHD involves multiple organs, reduces quality of life, and often requires prolonged therapy with glucocorticoids, causing severe side effects. After 4 decades of testing multiple therapeutic approaches, ibrutinib, belumosudil, and ruxolitinib were US Food and Drug Administration approved for cGVHD in the last 4 years. Here we put a spotlight on their mechanisms of action, studies that led to approval, and their future role in cGVHD.
Introduction
Chronic graft-versus-host disease (cGVHD) occurs in 30% to 70% of all patients undergoing allogeneic hematopoietic cell transplantation (allo-HCT)1 receiving nonmanipulated donor grafts and standard calcineurin/antimetabolite GVHD prophylaxis. cGVHD is a chronic inflammatory disease that can lead to serious and debilitating tissue injury and render patients at high risk of death because of infections. The multiple pathomechanistic steps in cGVHD involve B-cell and plasma-cell activation, naive T-cell differentiation into Th17/Tc17 and T-follicular helper cells, failure to delete autoreactive cells, ineffective regulatory mechanisms, macrophage and fibroblast activation, and fibrosis-promoting factors.2 Clinical cGVHD can involve the entire digestive tract, liver, skin, lung, eyes, musculoskeletal system, joint, fascia, hair and nails, lymphohematopoietic system, and genital tract, as well as other organs. Because of the pleiotropic organ manifestations, the diagnosis, scoring, and response assessment of cGVHD have been difficult. A major advancement in the field was the development of the National Institutes of Health consensus criteria3 to define a clinical framework that could be rigorously applied in clinical studies, allowing assessment of therapeutic response.4 The first-line therapy for cGVHD is glucocorticoids, which leads to a response in 40% to 60% of patients. Commonly used treatments after glucocorticoid failure include extracorporeal photopheresis (ECP), antimetabolites, antibodies directed against cytokines or cell surface antigens, interleukin-2 (IL-2), small molecule inhibitors, proteasome inhibitors, and cellular therapies.
US Food and Drug Administration–approved therapies for cGVHD in 2022
Ibrutinib
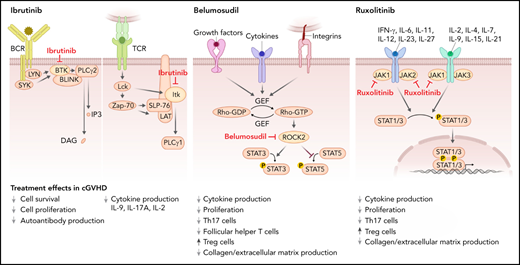
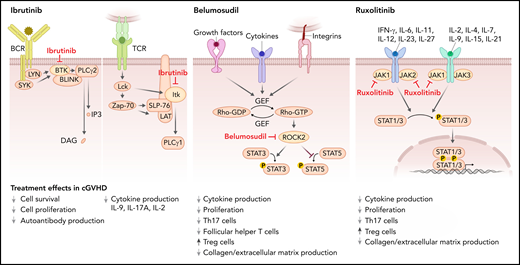
Ibrutinib is a selective and irreversible inhibitor of Bruton’s tyrosine kinase (BTK), which is a nonreceptor tyrosine kinase that belongs to the Tec family of kinases. BTK is predominantly expressed in B cells but not T cells or natural killer cells.5 Ibrutinib inhibits signal transduction downstream from the B-cell receptor (BCR) and thereby blocks activation of B cells (Figure 1). Increased BCR responsiveness was observed in B cells from patients with cGVHD,6 which provided a rationale for targeting BTK. Additionally, ibrutinib blocks IL-2 inducible kinase (ITK), which shares significant sequence and functional homology with BTK and belongs to the Tec kinase family (Figure 1).7 ITK contributes to proximal T-cell receptor (TCR) signaling, activating phosphoinositide phospholipase C (PLC)γ1, which leads to activation of nuclear factor of activated T-cells, nuclear factor κB, and MAPK pathways, ultimately leading to T-cell activation, cytokine release, and proliferation.8 Based on the contribution of T cells to cGVHD,9 ITK inhibition on ibrutinib treatment supports the use of ibrutinib for cGVHD.
Mode of action of the 3 FDA-approved cGVHD therapies. Ibrutinib inhibits BTK, which mediates signal transduction downstream from the BCR including PLCγ2 activation and thereby blocks activation of B cells. Ibrutinib also blocks ITK, which contributes to proximal TCR signaling, activating PLCγ1, which then activates nuclear factor of activated T-cells, nuclear factor κB, and MAPK pathways, ultimately leading to T-cell activation, cytokine release, and proliferation. Belumosudil (KD025) inhibits rho-associated ROCK2, which is downstream of cytokine and growth factor receptors and integrins. ROCK2 activation promotes the production of the proinflammatory cytokines IL-21 and IL-17, inhibits Tregs, and promotes fibrosis. Ruxolitinib inhibits JAK1 and 2. JAK1 and 2 mediate downstream effects of multiple cytokines such as IL-6, IFN-γ, and common γ chain cytokine receptors. Reduced STAT1 and STAT3 activation on ruxolitinib treatment results in increased Treg numbers and lower levels of collagen deposition.
Mode of action of the 3 FDA-approved cGVHD therapies. Ibrutinib inhibits BTK, which mediates signal transduction downstream from the BCR including PLCγ2 activation and thereby blocks activation of B cells. Ibrutinib also blocks ITK, which contributes to proximal TCR signaling, activating PLCγ1, which then activates nuclear factor of activated T-cells, nuclear factor κB, and MAPK pathways, ultimately leading to T-cell activation, cytokine release, and proliferation. Belumosudil (KD025) inhibits rho-associated ROCK2, which is downstream of cytokine and growth factor receptors and integrins. ROCK2 activation promotes the production of the proinflammatory cytokines IL-21 and IL-17, inhibits Tregs, and promotes fibrosis. Ruxolitinib inhibits JAK1 and 2. JAK1 and 2 mediate downstream effects of multiple cytokines such as IL-6, IFN-γ, and common γ chain cytokine receptors. Reduced STAT1 and STAT3 activation on ruxolitinib treatment results in increased Treg numbers and lower levels of collagen deposition.
Ibrutinib was first shown to improve established cGVHD in murine models, including bronchiolitis obliterans syndrome, as measured by pulmonary function tests.10 Histologic analysis of the lungs showed that germinal center reactions, tissue immunoglobulin deposition, and fibrosis were normalized and similar to the control group that received only bone marrow and does not develop cGVHD.10 Ibrutinib given as prophylaxis in murine cGVHD models was also effective in preventing cGVHD.11
Based on these promising preclinical results, ibrutinib was tested in adult patients with cGVHD after failure of first-line systemic therapy within a phase 1b/2, open-label, multicenter trial (#NCT02195869).12 The phase 1b part of the study was conducted to evaluate the safety of daily oral ibrutinib and to determine the phase 2 dose. The primary efficacy end point for phase 2 was the best overall (complete plus partial) cGVHD response rate. Of note, inflammatory skin or mouth involvement was an enrollment requirement. Forty-two patients with cGVHD were enrolled. The most common organs involved were mouth (86%), skin (81%), and gastrointestinal tract (36%). The overall response rate was 67%, with a complete response rate of 21% and a partial response rate of 45%.10 Patient-reported symptoms were also improved, and biomarker analysis showed rapid reductions in inflammatory and lymphocyte activation markers. Most adverse events (AEs) were grade 1 or 2, and the most common grade 3 or higher AEs were pneumonia, fatigue, and diarrhea.10 Based on these promising results, ibrutinib 420 mg daily was approved by the Food and Drug Administration (FDA) in the United States in August 2017 after failure of 1 or more lines of systemic therapy for cGVHD.13 Longer-term follow-up of study participants showed sustained responses >44 weeks in 16 of 29 (55%) responders.14 The results of a randomized, placebo-controlled phase 3 study to evaluate the role of ibrutinib in combination with corticosteroids in treating patients with newly diagnosed moderate-to-severe cGVHD (NCT02959944) was recently reported.15 The primary end point of the study did not meet statistical significance as 41% (39 of 95) of the patients that had received ibrutinib and prednisone had a response at 48 weeks compared with 37% (36 of 98) of patients receiving placebo and prednisone (P = .54).15
Belumosudil
Belumosudil (KD025) is a first-in-class selective inhibitor of rho-associated coiled-coil–containing protein kinase-2 (ROCK2). ROCK2 activation is downstream of cytokine and growth factor receptors and integrins (Figure 1), and it promotes the production of the proinflammatory cytokines IL-21 and IL-17,16 suggesting its inhibition may be effective for cGVHD treatment. ROCK2 inhibition was shown to reduce cGVHD in preclinical murine models where treatment with belumosudil normalized pulmonary function abnormalities and decreased antibody and collagen deposition in the lungs and decreased frequency of T-follicular helper cells and increased frequency of T-follicular regulatory cells in the spleen.17 Activation of STAT3 declined on treatment with belumosudil, whereas STAT5 phosphorylation increased.17 STAT5 activation on ROCK2 inhibition was previously shown to promote regulatory T cells.16 Additionally, in vitro belumosudil inhibited the secretion of IL-21, IL-17, and interferon-γ (IFN-γ) in human peripheral blood mononuclear cells derived from patients with active cGVHD.17
To test the efficacy of ROCK2 inhibition in patients, a phase 1/2a, open-label, dose-finding study of belumosudil enrolled 54 patients with cGVHD, who had received 1 to 3 prior lines of therapy (#NCT02841995).18 The overall response rate with belumosudil 200 mg daily, 200 mg twice daily, and 400 mg daily was 65% (38%-86%), 69% (41%-89%), and 62% (38%-82%), respectively. The failure-free survival rate, defined as the absence of relapse, death, or addition of another systemic immunosuppressive agent, was 76% (62%-85%) and 47% (33%-60%) at 6 and 12 months, respectively. Belumosudil was well tolerated, with low rates of cytopenias.18 A subsequent phase 2, randomized, multicenter study (#NCT03640481) was performed in which patients with cGVHD who had received 2 to 5 prior lines of therapy were treated with belumosudil 200 mg daily (n = 66) or 200 mg twice daily (n = 66).19 The best overall response rate of belumosudil 200 mg daily and 200 mg twice daily was 74% (62%-84%) and 77% (65%-87%), respectively, and response rates were high in all subgroups, including patients previously treated with ibrutinib and ruxolitinib. Patient-reported symptoms were also improved in 59% and 62%, respectively. The median time until addition of another systemic immunosuppressive agent, relapse, or death was 54 weeks in responders, and 44% received belumosudil for more than a year. Sixteen subjects (12%) discontinued belumosudil because of possible drug-related AEs.19 Based on these promising results, belumosudil 200 mg daily was approved by the FDA in July 2021 for adult and pediatric patients 12 years and older with cGVHD after failure of at least 2 prior lines of systemic therapy.20
Ruxolitinib
Ruxolitinib is a tyrosine kinase inhibitor that targets the Janus-associated kinases (JAKs) 1 and 2. JAK1 and 2 mediate downstream effects of multiple cytokines such as IL-6, IFN-γ, IL-7, IL-15 (Figure 1), and others.21 In mice, ruxolitinib reduced the polarization of CD4+ T cells into IFN-γ– and IL-17A–producing cells.22 Additionally, T-regulatory cell (Treg) frequencies increased in the spleens and intestinal tract on ruxolitinib treatment in allo-HCT recipients.22 Besides the impact of ruxolitinib on T cells, it also reduced MHC-II expression on antigen-presenting cells including dendritic cells23 and neutrophil granulocytes.24 In a murine model of cGVHD, ruxolitinib treatment reduced collagen deposition in the lungs and improved pulmonary function.25
To test ruxolitinib for cGVHD in patients, a randomized, open-label, multicenter clinical trial of ruxolitinib compared with best available therapy (BAT) for corticosteroid-refractory or -dependent cGVHD (REACH-3, #NCT03112603) was performed.26 The trial randomized 329 patients (1:1) to receive either ruxolitinib 10 mg twice daily or 1 of 10 investigator-selected BATs. Overall response rate at week 24 was greater in the ruxolitinib group than in the control group (49.7% vs 25.6%; odds ratio, 2.99; P < .001). Median failure-free survival was longer in the ruxolitinib group compared with the control group (>18.6 vs 5.7 months; hazard ratio, 0.37; P < .001), and a higher symptom response (24.2% vs 11.0%; odds ratio, 2.62; P = .001) was observed in the ruxolitinib group.26
In September 2021, the FDA approved ruxolitinib 10 mg twice daily for cGVHD after failure of 1 or 2 lines of systemic therapy for cGVHD in adult and pediatric patients 12 years and older.27
Future directions
All 3 FDA-approved oral cGVHD drugs have in common that they are kinase inhibitors, a class of compounds that reduces the dissemination of growth signals and activation of key cellular proteins that are involved in cell activation, migration, and proliferation, and all 3 therapies were based on preclinical murine studies. Although ibrutinib and ruxolitinib are approved for use after 1 or more prior lines of therapy, the approval for belumosudil is after 2 or more prior lines of therapy. A weakness in the currently available evidence is the lack of knowledge about which patient subgroup may benefit more from 1 therapy vs another. Also, all 3 drugs were approved in open label, nonblinded trials, with only 1, ruxolitinib, having a comparator arm of best available therapy.
Although all 3 have shown activity against cGVHD, there is still a large number of patients that did not respond or that lost response over time. Future studies are needed to identify the underlying mechanisms for cGVHD resistance to ibrutinib, belumosudil, and ruxolitinib. A potential approach to overcome resistance could be the combination with each other or other therapies (eg, the combination of ruxolitinib with ECP for patients with refractory cGVHD has shown promising activity in a retrospective case study).28 Also combination therapy of kinase inhibitors with antibodies directed against colony stimulating factor 1 receptor29 or small molecule inhibitors of wingless/integrated signaling30 besides many others could be tested in preclinical studies for synergism. Complete responses were rare in the studies that led to approval, and therefore, it is appropriate to test new agents or combinations of these approved agents earlier in the disease course. For example, testing belumosudil or ruxolitinib upfront with corticosteroids or even head-to-head against corticosteroids given the morbidity of long-term steroid therapy would be important to improve the outcome of patients with early cGVHD.31 For patients with advanced manifestations, improving the treatment of highly morbid forms of cGVHD such as deep sclerosis and bronchiolitis obliterans syndrome likely requires additional therapeutic approaches and novel study designs and end points.32
Although the improvement in treatment of cGVHD had been tremendous, the most important goal is to develop strategies to more effectively prevent moderate to severe cGVHD. Toward this goal, the 2020 National Institutes of Health Consensus Conference Reports recommended the use of basic studies to understand mechanisms causing cGVHD development,33 attentiveness to early diagnosis features,34 and testing preemptive therapy such as with the relatively well-tolerated agents described here.35 Finally, although the 3 drugs are approved and available in the United States, the approval in other countries is still under consideration; for example, the European Medicines Agency has not approved any of these agents. In addition, high drug costs will limit access to patients with sufficient insurance coverage. Nevertheless, the accelerating number of approvals and clinical trial activity in cGVHD means current and future patients afflicted with this iatrogenic disease will have more effective and less toxic treatment options.
Acknowledgments
R.Z. was supported by the Deutsche Forschungsgemeinschaft (SFB-1479; project 441891347) and S.J.L. is supported by National Institutes of Health grants CA118953 and CA236229.
Authorship
Contribution: R.Z. and S.J.L. reviewed the literature, wrote the manuscript, and designed the figure.
Conflict-of-interest disclosure: R.Z. received honoraria from Novartis, Incyte, and Mallinckrodt. S.J.L. received honoraria from Mallinckrodt, Kadmon, and Pfizer and research funding from Kadmon, Novartis, Millennium, Amgen, Pfizer, Syndax, and AstraZeneca and is on the Steering Committee for the REACH3 trial.
Correspondence: Robert Zeiser, Department of Hematology, Oncology and Stem Cell Transplantation, University Medical Center Freiburg, D-79106 Freiburg, Germany; e-mail: robert.zeiser@uniklinik-freiburg.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal