Abstract
Venous thromboembolism (VTE) is a common complication occurring in 5% to 10% of patients with lymphoma. As the complexity of lymphoma management has increased with novel therapies, so too has the treatment of VTE. Therapeutic options for the treatment of cancer-associated VTE have expanded from only warfarin and low-molecular-weight heparins (LMWHs) to include the direct oral anticoagulants (DOACs) apixaban, edoxaban and rivaroxaban. There have been no head-to-head trials comparing different DOACs in this setting, and randomized trials comparing a DOAC with LMWH dalteparin differ in trial design and results. Drug–drug interactions, drug-specific side effects, and patient selection are important considerations when prescribing anticoagulant therapy. In all patients, the relative risks of thrombosis and bleeding, the availability of the anticoagulant, and the life expectancy of the patient are vital elements in selecting the most appropriate anticoagulant (which can vary over time) for the individual patient. We describe the intricacies and challenges of treating thrombotic complications in patients with lymphoma with an emphasis on evidence and guideline-based care.
Introduction
Venous thromboembolism (VTE) is a common complication of malignancies, occurring at rates of sixfold higher than in patients without cancer.1,2 In those with lymphoma, approximately 5% to 10% will develop VTE during their course of disease.3,4 The risk of an initial VTE is greatest in the first few months after a cancer diagnosis as during this time there is active malignancy, chemotherapy initiation, potential hospitalization, procedures, and immobilization.5 As it is for all patients with cancer-associated VTE, the exact risk of VTE in a patient with lymphoma is dependent on patient-related factors, the histologic type and stage of lymphoma, and the treatment regimen.3,4,6 Rituximab received US Food and Drug Administration approval in 1997 for relapsed and refractory low-grade non-Hodgkin lymphoma and over the ensuing decade moved to frontline therapy.7 In 2002, rituximab added to CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) demonstrated superiority over CHOP alone in patients with diffuse large B-cell lymphoma.8 The following year in cancer-associated VTE research, the Comparison of Low-Molecular-Weight Heparin vs Oral Anticoagulant Therapy trial demonstrated low-molecular-weight heparin (LMWH) dalteparin was superior to warfarin in treating acute VTE and preventing recurrent VTE.9 Recently, the expanded use of direct oral anticoagulants (DOACs) has added more anticoagulant options for the prevention and treatment of cancer-associated VTE.10 Concurrently, therapies such as ibrutinib and bendamustine have transformed the natural history of patients with indolent lymphoma, whereas chimeric antigen receptor T (CAR T)–cell therapy is further advancing therapeutic options in those with aggressive, refractory disease.11-13 Given that the treatments for both VTE and lymphoma have evolved significantly in the last 2 decades, thoughtful and patient-centered approaches to managing patients with both conditions are paramount. We present some common clinical scenarios reflecting current lymphoma and thrombosis management to illustrate our approaches.
Case 1: chronic lymphocytic leukemia/small lymphocytic lymphoma
A 62-year-old man with known chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) presents with swelling of the right leg for the last 4 days. He denies any chest pain but endorses increasing fatigue and new-onset dyspnea on his usual walks. He has no other significant medical history. At presentation with CLL/SLL in 2012, he had night sweats, diffuse lymphadenopathy, and splenomegaly. He responded well to fludarabine, cyclophosphamide, and rituximab and, since that time, he has been in remission. On examination, his right leg is visibly edematous with palpable inguinal adenopathy bilaterally. His vitals and oxygen saturation are normal. Blood work demonstrates a white blood cell count of 28 × 109/L and lymphocyte count of 24 × 109/L, hemoglobin of 93 g/L, and platelet count of 145 × 109/L. Relapsed cancer, an acute deep vein thrombosis (DVT), and possibly pulmonary embolism (PE) are suspected. What is the likelihood or pretest probability of VTE in this scenario and the risk of VTE in patients with lymphoma?
Epidemiology and diagnosis of VTE in patients with lymphoma
Rates of cancer-associated VTE vary by cancer type.1 In a meta-analysis of 18 018 patients with lymphoma, the estimated pooled incidence of VTE was 5.3% (95% confidence interval [CI], 5.0% to 5.7%).3 Including both venous and arterial thrombosis, patients with non-Hodgkin lymphoma had a significantly higher rate (6.5%) than those with Hodgkin lymphoma (4.7%). Patients with high-grade non-Hodgkin lymphoma had a higher risk than those with low-grade disease. Similar results have been reported in recent population-based cohort studies and large registries.6,14 Patients with central nervous system lymphoma or primary mediastinal B-cell lymphoma seem to have particularly high incidences of VTE.3,14,15 Risk factors associated with VTE include advanced stage, high grade, body mass index (BMI) >30 kg/m2, and use of doxorubicin-based therapy.3,6,14,16,17
In a recent study reporting on the incidence of thrombotic events in 5773 patients with Hodgkin lymphoma from the German Hodgkin Study Group HD13-15 trials, 193 thrombotic events occurred for an incidence of 3.3%; 92% of the events were venous, whereas 8% were arterial.4 Similar to earlier literature, the highest risk was found in patients with advanced disease. Most of the venous thrombotic events occurred during chemotherapy (78.9%), and many occurred in association with a catheter (30.6%). Dose-dense therapy, older age, smoking, and the use of oral contraception were associated with a higher risk of thrombosis.
The diagnostic approach in outpatients with suspected VTE is well established, relying on the use of clinical assessment models, D-dimer testing, and objective radiologic imaging.18 In those with a low clinical probability and a normal D-dimer level, VTE can be safely ruled out without the use of ultrasonography, ventilation/perfusion lung scanning, or computed tomography pulmonary angiography. In patients with cancer, imaging remains necessary in the vast majority of patients to confirm or refute a diagnosis of DVT or PE because: (1) risk assessment models do not incorporate features specific to cancer patients that identify them as having a higher risk of VTE and (2) D-dimer levels are frequently elevated in the absence of VTE, thereby rendering the test less useful in ruling out VTE.19
In patients with lymphoma, specific features that are not included in risk assessment models but do increase the likelihood of VTE include the following: stage IV disease, high-grade histology, lymphadenopathy producing venous stasis, requirement for IV immunoglobulin support, and use of high-dose corticosteroid therapy.6,17,20-22 Consequently, clinicians should have a low threshold for ordering investigations to look for VTE in patients with these factors and compatible symptoms. In addition to radiologic imaging, basic blood work needed include a complete blood count and smear (to determine whether there is significant anemia or thrombocytopenia), international normalized ratio and partial thromboplastin time (to look for coagulopathy), creatinine (to assess renal function), and bilirubin and liver enzymes (to detect liver impairment). These results are important to guide anticoagulant options.
Case 1 continued
A compression ultrasound shows a right-sided, nonocclusive proximal DVT involving the common and superficial femoral veins. A computed tomography pulmonary angiography was negative for PE, but there is mediastinal lymphadenopathy. What are the anticoagulant options for treatment of VTE in patients with lymphoma?
Treatment with LMWH vs DOAC
LMWHs have been the guideline-recommended anticoagulant of choice for treatment of cancer-associated VTE.23-25 In the randomized controlled trials and prospective cohort studies that studied LMWH, hematologic malignancies accounted for 8.4% to 10.4% of cancer types included.9,26-29 Results in this subgroup of patients have not been reported. Data published on the efficacy and safety of anticoagulation in patients with lymphoma are scarce. In a retrospective study of 57 patients with different types of lymphoma, fewer episodes of recurrent VTE and major bleeding were observed in those treated with LMWH than with warfarin.30
In 2018, the first randomized controlled trial comparing the use of a DOAC with LMWH for the treatment of cancer-associated VTE was reported. Since then, 3 different inhibitors of factor Xa have been evaluated: apixaban, edoxaban, and rivaroxaban31-34; dabigatran, an oral direct thrombin inhibitor, has not been compared with LMWH in a clinical trial. Similar to the LMWH vs warfarin trials, patients with lymphoma accounted for approximately 5% to 10% of the study patients in the DOAC vs LMWH trials. Table 1 summarizes key design features of the DOAC vs LMWH trials.
Study designs and outcomes of randomized trials that compared a DOAC with a LMWH for the treatment of cancer-associated VTE
| Name of trial . | Hokusai VTE Cancer31 2018 . | SELECT-D34 2018 . | ADAM-VTE33 2019 . | Caravaggio32 2020 . |
|---|---|---|---|---|
| DOAC evaluated | Edoxaban | Rivaroxaban | Apixaban | Apixaban |
| No. of patients | 1050 | 406 | 287 | 1155 |
| Hematologic malignancy, n (%) | 111 (10.6) | 33 (8.1) | 28 (9.3) | 85 (7.4) |
| Lymphoma, n (%) | Not reported | Lymphoma 23 (5.7); CLL 3 (0.7) | 16 (5.3) | Not reported |
| Study design | Noninferiority | Pilot trial | Superiority | Noninferiority |
| Primary outcome | Composite of recurrent VTE or major bleeding | Recurrent VTE | Major bleeding | Recurrent VTE |
| Exclusion criteria* | ||||
| Cancer type | Basal-cell or squamous-cell carcinoma of the skin | Basal-cell or squamous-cell carcinoma of the skin, esophageal or gastro-esophageal cancer | Not specified | Basal-cell or squamous-cell carcinoma of the skin, primary brain tumor known brain mets; acute leukemia |
| Life expectancy | <3 mo | Not specified | <60 d | <6 mo |
| Platelets (×109/L) | <50 | <100 | <50 | <75 |
| Antiplatelet use | Aspirin >100 mg daily, other antiplatelet agents, or chronic NSAIDs | Aspirin >75 mg daily or dual antiplatelet therapy | Not specified | Aspirin >165 mg daily or other antiplatelet agents |
| Drug interaction | P-gp inhibitors | Strong CYP3A4 inhibitors or inducers | Strong CYP3A4 inducers | Strong CYP3A4 or P-gp inhibitors or inducers |
| Other | Not applicable | Weight <40 kg | Not applicable | Recent brain, spinal or ophthalmic surgery |
| Qualifying index event (symptomatic and unsuspected events unless specified) | PE: segmental or more proximal; DVT: lower-limb proximal; other sites: none | PE: type not reported; DVT: lower-limb proximal (symptomatic only); other sites: none | PE: type not reported; DVT: lower or upper-limb; other sites: splanchnic, cerebral vein | PE: segmental or more proximal; DVT: lower-limb proximal; other sites: none |
| Outcome recurrent VTE | PE: subsegmental (symptomatic), segmental or more proximal (symptomatic or unsuspected); DVT: lower-limb distal (symptomatic) or proximal (symptomatic or unsuspected) | PE: vessel greater than 2.5 mm or more proximal (symptomatic or unsuspected); DVT: lower-limb proximal (symptomatic) | Any venous or arterial thromboembolism including (PE, VTE, MI, stroke, TIA, arterial thromboembolism) (symptomatic or unsuspected) | PE: segmental or more proximal (symptomatic or unsuspected); DVT: lower-limb (symptomatic or unsuspected) or upper limb (symptomatic) |
| DOAC regimen | LMWH for ≥5 d followed by edoxaban 60 mg PO daily | Rivaroxaban 15 mg twice daily for 3 wk then 20 mg once daily | Apixaban 10 mg twice daily for 7 d followed by 5 mg twice daily | Apixaban 10 mg twice daily for 7 d followed by 5 mg twice daily |
| DOAC dose reduction | 30 mg daily if CrCl 30-50 mL/min, weight ≤60 kg, or use of P-gp inhibitors | No | 2.5 mg twice daily for the use of strong CYP3A4 and/or P-gp inhibitors | No |
| Duration of anticoagulant therapy | 12 mo | 6 mo | 6 mo | 6 mo |
| Composite rVTE or major bleeding DOAC vs LMWH, n (%) | 67 (12.8) vs 71 (13.5)† | Not reported | Not reported | 51 (8.9) vs 66 (11.4) |
| Recurrent VTE, DOAC vs LMWH, n (%) | 41 (7.9) vs 59 (11.3)† | 8 (3.9) vs 18 (8.9) | 1 (0.7) vs 9 (6.3) | 32 (5.6) vs 46 (7.9) |
| Major bleeding, DOAC vs LMWH, n (%) | 36 (6.9) vs 21 (4)† | 11 (5.4) vs 6 (3) | 0 vs 2 (1.4) | 22 (3.8) vs 23 (4) |
| Any bleeding, DOAC vs LMWH, n (%) | 97 (18.6) vs 73 (13.9)† | 36 (17.7) vs 13 (6.4) | 9 (6.2) vs 9 (6.3) | 70 (12.2) vs 56 (9.7) |
| Death, DOAC vs LMWH, n (%) | 6 mo: 140 (26.8) vs 127 (24.2) 12 mo: 206 (39.5) vs 192 (36.6) | 6 mo: 48 (23.6) vs 56 (27.6) | 6 mo: 23 (16) vs 15 (11) | 6 mo: 135 (23.4) vs 153 (26.4) |
| Name of trial . | Hokusai VTE Cancer31 2018 . | SELECT-D34 2018 . | ADAM-VTE33 2019 . | Caravaggio32 2020 . |
|---|---|---|---|---|
| DOAC evaluated | Edoxaban | Rivaroxaban | Apixaban | Apixaban |
| No. of patients | 1050 | 406 | 287 | 1155 |
| Hematologic malignancy, n (%) | 111 (10.6) | 33 (8.1) | 28 (9.3) | 85 (7.4) |
| Lymphoma, n (%) | Not reported | Lymphoma 23 (5.7); CLL 3 (0.7) | 16 (5.3) | Not reported |
| Study design | Noninferiority | Pilot trial | Superiority | Noninferiority |
| Primary outcome | Composite of recurrent VTE or major bleeding | Recurrent VTE | Major bleeding | Recurrent VTE |
| Exclusion criteria* | ||||
| Cancer type | Basal-cell or squamous-cell carcinoma of the skin | Basal-cell or squamous-cell carcinoma of the skin, esophageal or gastro-esophageal cancer | Not specified | Basal-cell or squamous-cell carcinoma of the skin, primary brain tumor known brain mets; acute leukemia |
| Life expectancy | <3 mo | Not specified | <60 d | <6 mo |
| Platelets (×109/L) | <50 | <100 | <50 | <75 |
| Antiplatelet use | Aspirin >100 mg daily, other antiplatelet agents, or chronic NSAIDs | Aspirin >75 mg daily or dual antiplatelet therapy | Not specified | Aspirin >165 mg daily or other antiplatelet agents |
| Drug interaction | P-gp inhibitors | Strong CYP3A4 inhibitors or inducers | Strong CYP3A4 inducers | Strong CYP3A4 or P-gp inhibitors or inducers |
| Other | Not applicable | Weight <40 kg | Not applicable | Recent brain, spinal or ophthalmic surgery |
| Qualifying index event (symptomatic and unsuspected events unless specified) | PE: segmental or more proximal; DVT: lower-limb proximal; other sites: none | PE: type not reported; DVT: lower-limb proximal (symptomatic only); other sites: none | PE: type not reported; DVT: lower or upper-limb; other sites: splanchnic, cerebral vein | PE: segmental or more proximal; DVT: lower-limb proximal; other sites: none |
| Outcome recurrent VTE | PE: subsegmental (symptomatic), segmental or more proximal (symptomatic or unsuspected); DVT: lower-limb distal (symptomatic) or proximal (symptomatic or unsuspected) | PE: vessel greater than 2.5 mm or more proximal (symptomatic or unsuspected); DVT: lower-limb proximal (symptomatic) | Any venous or arterial thromboembolism including (PE, VTE, MI, stroke, TIA, arterial thromboembolism) (symptomatic or unsuspected) | PE: segmental or more proximal (symptomatic or unsuspected); DVT: lower-limb (symptomatic or unsuspected) or upper limb (symptomatic) |
| DOAC regimen | LMWH for ≥5 d followed by edoxaban 60 mg PO daily | Rivaroxaban 15 mg twice daily for 3 wk then 20 mg once daily | Apixaban 10 mg twice daily for 7 d followed by 5 mg twice daily | Apixaban 10 mg twice daily for 7 d followed by 5 mg twice daily |
| DOAC dose reduction | 30 mg daily if CrCl 30-50 mL/min, weight ≤60 kg, or use of P-gp inhibitors | No | 2.5 mg twice daily for the use of strong CYP3A4 and/or P-gp inhibitors | No |
| Duration of anticoagulant therapy | 12 mo | 6 mo | 6 mo | 6 mo |
| Composite rVTE or major bleeding DOAC vs LMWH, n (%) | 67 (12.8) vs 71 (13.5)† | Not reported | Not reported | 51 (8.9) vs 66 (11.4) |
| Recurrent VTE, DOAC vs LMWH, n (%) | 41 (7.9) vs 59 (11.3)† | 8 (3.9) vs 18 (8.9) | 1 (0.7) vs 9 (6.3) | 32 (5.6) vs 46 (7.9) |
| Major bleeding, DOAC vs LMWH, n (%) | 36 (6.9) vs 21 (4)† | 11 (5.4) vs 6 (3) | 0 vs 2 (1.4) | 22 (3.8) vs 23 (4) |
| Any bleeding, DOAC vs LMWH, n (%) | 97 (18.6) vs 73 (13.9)† | 36 (17.7) vs 13 (6.4) | 9 (6.2) vs 9 (6.3) | 70 (12.2) vs 56 (9.7) |
| Death, DOAC vs LMWH, n (%) | 6 mo: 140 (26.8) vs 127 (24.2) 12 mo: 206 (39.5) vs 192 (36.6) | 6 mo: 48 (23.6) vs 56 (27.6) | 6 mo: 23 (16) vs 15 (11) | 6 mo: 135 (23.4) vs 153 (26.4) |
Primary end points in bold. All trials compared the DOAC to dalteparin 200 IU/kg daily for a month then dose reduced to 150 IU/kg daily.
ADAM-VTE, apixaban and dalteparin in active malignancy-associated venous thromboembolism; CrCl, creatinine clearance as per Crockoff Gault equation; CYP3A4, cytochrome P450 3A4; Hgb, hemoglobin g/L; mets, metastases; MI, myocardial infarction; NSAID, nonsteroidal anti-inflammatory; P-gp, permeability-glycoprotein; Plt, platelet count (× 109/L); rVTE, recurrent VTE; SELECT-D, selected cancer patients at risk of recurrence of venous thromboembolism; TIA, transient ischemic attack.
All trials excluded patients with Eastern Cooperative Oncology Group 3 or 4, impaired liver function, pregnancy, creatinine clearance <30 mL/min, or history of heparin-induced thrombocytopenia. Most trials excluded bacterial endocarditis, uncontrolled hypertension, thrombectomy, vena cava filter insertion, or thrombolysis.
Reported at 12 mo in Hokusai VTE Cancer, whereas other studies reported at 6 mo.
To date, there are no head-to-head comparisons of different DOACs for the treatment of cancer-associated VTE. Indirect comparisons between published DOAC trials are problematic because of differences in design, patient population (in terms of tumor type, qualifying thrombotic events, and overall prognosis), drug adherence, and primary outcomes. For example, the Apixaban and Dalteparin in Active Malignancy-Associated Venous Thromboembolism trial included splanchnic and cerebral vein thrombosis, whereas the other trials did not. Its primary endpoint was major bleeding and the low mortality suggests the patients included were not representative of most patients with cancer-associated VTE.33 The Caravaggio trial excluded patients with primary brain tumors, intracerebral metastases, or acute leukemia.32 The Selected Cancer Patients at Risk of Recurrence of Venous Thromboembolism pilot study excluded patients with esophageal and gastroesophageal cancers during the study because of an excessive number of major bleeding events in these patients who received rivaroxaban.34 In the Hokusai VTE Cancer trial, which used a composite primary endpoint of recurrent VTE or major bleeding, patients with gastrointestinal malignancies had a fourfold higher risk of major bleeding with edoxaban than dalteparin.35
Overall, currently available evidence and guidelines support the selected use of apixaban, edoxaban, or rivaroxaban for the treatment of cancer-associated VTE.23-25 In the absence of head-to-head trials comparing different DOACs, which anticoagulant to use should be based on cancer type, drug–drug interactions, drug availability, and patient and provider preference. Importantly, it is prudent to remember that all DOACs are absorbed primarily or partially in the stomach or duodenum such that any condition that can interfere with drug absorption (eg, Whipple’s, Roux-en-Y gastric bypass, or gastrectomy for malignancy) may pose as a relative or absolute contraindication for DOAC use.
In addition, of particular relevance in patients with lymphoma and other hematologic malignancies, all DOAC trials excluded patients with a platelet count below 50 to 100 × 109/L or a high risk for bleeding. Management of cancer-associated VTE in patients with thrombocytopenia remain uncertain, but expert guidance has been published (Figure 1).36 Concomitant use of antiplatelet therapy varied among the studies (Table 1). It is also noteworthy that patients with a short life expectancy or Eastern Cooperative Oncology Group of 3 or 4 were excluded from all clinical trials in cancer-associated VTE. These exclusions may apply to a significant number of patients with advanced or relapsed lymphoma.
International Society on Thrombosis and Hemostasis Scientific and Standardization Committee, Subcommittee on Hemostasis and Malignancy guidance statements on cancer-associated venous thromboembolism management in the setting of thrombocytopenia. *Acute VTE defined as diagnosis of VTE within the previous 30 days and subacute/chronic greater than 30 days. †High risk of progression includes symptomatic segmental or more proximal pulmonary embolism, proximal deep vein thrombosis, or a history of recurrent/progressive thrombosis. Low risk events include distal deep vein thrombosis, incidental subsegmental pulmonary embolism, and catheter-related thrombosis. Platelet counts are reported in platelets ×109/L. 1If unable to maintain platelets of ≥40 to 50, a reduced dose is reasonable. 2Consider withholding LMWH if platelets are <50 in the low-risk group in subacute/chronic period. 3Consider prophylactic dose if platelets ≥10 during the acute period. UFH, unfractionated heparin. See Samuelson et al.36
International Society on Thrombosis and Hemostasis Scientific and Standardization Committee, Subcommittee on Hemostasis and Malignancy guidance statements on cancer-associated venous thromboembolism management in the setting of thrombocytopenia. *Acute VTE defined as diagnosis of VTE within the previous 30 days and subacute/chronic greater than 30 days. †High risk of progression includes symptomatic segmental or more proximal pulmonary embolism, proximal deep vein thrombosis, or a history of recurrent/progressive thrombosis. Low risk events include distal deep vein thrombosis, incidental subsegmental pulmonary embolism, and catheter-related thrombosis. Platelet counts are reported in platelets ×109/L. 1If unable to maintain platelets of ≥40 to 50, a reduced dose is reasonable. 2Consider withholding LMWH if platelets are <50 in the low-risk group in subacute/chronic period. 3Consider prophylactic dose if platelets ≥10 during the acute period. UFH, unfractionated heparin. See Samuelson et al.36
Case 1 continued
After reviewing the evidence, the patient is started on apixaban. The oncologist confirms that the patient has symptomatic CLL/SLL progression. Ibrutinib is a recommended therapy in this setting, but increased bleeding has been reported with ibrutinib, especially when combined with anticoagulant therapy. What are the mechanisms for bleeding in patients receiving ibrutinib and how do we choose which anticoagulant to use?
Drug–drug interactions with ibrutinib and anticoagulation
Ibrutinib, a Bruton tyrosine kinase (BTK) inhibitor widely used to treat CLL/SLL, is known to contribute to bleeding.37 During a median follow-up of 41 months in the Ibrutinib versus Ofatumumab in Previously Treated Chronic Lymphoid Leukemia trial for relapsed CLL, thrombocytopenia occurred in 8% and major bleeding in 6%.38 Real-world data showed 55% to 56% of patients had bleeding events and 2% to 3% of patients experienced a major bleeding event.39-41 Bruising and epistaxis are common presentations, consistent with a mucosal- or platelet-related bleeding phenotype. BTK inhibitors impair platelet activation that is initiated by collagen binding to the platelet receptor glycoprotein VI, which then leads to tyrosine phosphorylation.42,43 Inhibition of tyrosine phosphorylation thereby results in decreased dense granule secretion, calcium mobilization, and platelet aggregation. BTK is also required for von Willebrand factor and platelet membrane glycoprotein Ib signal transduction for the formation of thrombosis.44 The effect of impaired collagen-mediated platelet aggregation has been confirmed in vivo in patients taking ibrutinib.40,45 Acalabrutinib, a more selective BTK inhibitor with less off-target activity, demonstrates less in vitro platelet inhibition than ibrutinib.46 However, bleeding events remain elevated among patients receiving acalabrutinib compared with non-acalabrutinib arms in clinical trials.47,48 Further evidence is required to determine whether bleeding is reduced with more specific BTK inhibitors than ibrutinib.49
Atrial fibrillation is also a common complication of ibrutinib therapy and is an indication for anticoagulation.38 Consequently, coadministration of an anticoagulant and ibrutinib in patients with CLL/SLL and atrial fibrillation has been reported. Because of the high incidence of major bleeding, including subdural hematoma, associated with warfarin use in early ibrutinib trials, warfarin or other vitamin k antagonists is now avoided in patients on ibrutinib.50
The role of DOACs in this setting is not well studied.51 In addition to the concerns for ibrutinib-induced platelet dysfunction and thrombocytopenia, theoretical drug–drug interactions exist because ibrutinib, apixaban, and rivaroxaban are all substrates of the CYP3A4 enzyme pathway and rely on P-glycoprotein transporter for clearance.52 Therefore, concomitant use of ibrutinib and apixaban or rivaroxaban along with a CYP3A4 or P-glycoprotein inhibitor may increase their circulating levels from saturation and inhibition of these pathways.53,54 Although dabigatran and edoxaban are less likely to have the same degree of drug–drug interaction because they only rely on P-glycoprotein transport, dabigatran is associated with gastrointestinal bleeding, and edoxaban is not widely available.55 Overall, the theoretical risk from drug–drug interaction between ibrutinib and DOACs is thought to have minimal clinical relevance.56 Nonetheless, it might be prudent to avoid the combination by using an alternative lymphoma treatment (if possible) when anticoagulation is required.37
Although direct ibrutinib and DOAC interactions may not be consequential, that may not be the case with other cancer therapies and DOACs. Concomitant administration with potent inhibitors will increase DOAC levels and likely increase the risk of bleeding, whereas strong inducers will reduce DOAC levels and potentially lower efficacy.55 A number of commonly used drugs in lymphoma therapy (eg, dexamethasone) and for antiemetic effects (eg, aprepitant) do have drug–drug interactions with DOACs (Table 2).57 The clinical relevance of interactions with mild or moderate modulators of CYP3A4 or P-glycoprotein is likely minimal, but when multiple drugs are used in combination, the net effect is difficult to predict. In the clinic, we check for potential drug–drug interactions and review with a pharmacist if needed. We inform our patients of the potential risks, review the signs and symptoms of VTE and bleeding before prescribing an oral anticoagulant. We do not use DOAC in patients who may develop severe thrombocytopenia and in those taking strong CYP3A4 or P-glycoprotein modulators.
Cytochrome P450 3A4 (CYP3A4) and permeability-glycoprotein (P-gp) metabolism of commonly used drugs in lymphoma treatment
| . | CYP3A4 . | P-gp . | ||||
|---|---|---|---|---|---|---|
| Substrate . | Inducer . | Inhibitor . | Substrate . | Inducer . | Inhibitor . | |
| Lymphoma drugs with potential interactions | ||||||
| Acalabrutinib | ● | O | O | |||
| Belinostat | O | O | ||||
| Bendamustine | O | |||||
| Bexarotene | O | ◐ | ||||
| Bortezomib | ● | O | ||||
| Brentuximab vedotin | O | O | O | |||
| Carfilzomib | O | O | O | |||
| Cladribine | O | |||||
| Cyclophosphamide | O | |||||
| Dexamethasone | ● | O | O | |||
| Doxorubicin | ● | O | ● | |||
| Etoposide | ● | ● | ||||
| Ibrutinib | ● | ◐ | ||||
| Idarubicin | O | |||||
| Idelalisib | ● | ● | O | |||
| Ifosfamide | ● | O | ||||
| Lenalidomide | O | |||||
| Prednisone | O | O | ||||
| Romidepsin | ● | O | ||||
| Temsirolimus | ● | O | O | |||
| Teniposide | ● | O | ● | |||
| Venetoclax | ● | O | ◐ | |||
| Vinblastine | ● | ● | O | |||
| Vincristine | ● | O | ||||
| Lymphoma drugs with no known interactions | ||||||
| Bleomycin | ||||||
| Fludarabine | ||||||
| Rituximab | ||||||
| Supportive care drugs | ||||||
| Aprepitant | ● | ◐ | ||||
| Clonazepam | ● | |||||
| Fosaprepitant | ● | O | ||||
| Fentanyl | ● | |||||
| Methadone | ● | |||||
| Ondansetron | ● | O | ||||
| Strong CYP3A4 and/or P-gp inhibitors (eg, idelalisib, ketoconazole, itraconazole, voriconazole, posaconazole, fluconazole, ritonavir, lopinavir/ritonavir, indinavir/ritonavir, cyclosporine, tacrolimus, clarithromycin, conivaptan) or inducers (eg, phenytoin, carbamazepine, rifampin, St. John’s wort) are generally contraindicated with direct oral anticoagulant use (DOAC).56,57 | ||||||
| . | CYP3A4 . | P-gp . | ||||
|---|---|---|---|---|---|---|
| Substrate . | Inducer . | Inhibitor . | Substrate . | Inducer . | Inhibitor . | |
| Lymphoma drugs with potential interactions | ||||||
| Acalabrutinib | ● | O | O | |||
| Belinostat | O | O | ||||
| Bendamustine | O | |||||
| Bexarotene | O | ◐ | ||||
| Bortezomib | ● | O | ||||
| Brentuximab vedotin | O | O | O | |||
| Carfilzomib | O | O | O | |||
| Cladribine | O | |||||
| Cyclophosphamide | O | |||||
| Dexamethasone | ● | O | O | |||
| Doxorubicin | ● | O | ● | |||
| Etoposide | ● | ● | ||||
| Ibrutinib | ● | ◐ | ||||
| Idarubicin | O | |||||
| Idelalisib | ● | ● | O | |||
| Ifosfamide | ● | O | ||||
| Lenalidomide | O | |||||
| Prednisone | O | O | ||||
| Romidepsin | ● | O | ||||
| Temsirolimus | ● | O | O | |||
| Teniposide | ● | O | ● | |||
| Venetoclax | ● | O | ◐ | |||
| Vinblastine | ● | ● | O | |||
| Vincristine | ● | O | ||||
| Lymphoma drugs with no known interactions | ||||||
| Bleomycin | ||||||
| Fludarabine | ||||||
| Rituximab | ||||||
| Supportive care drugs | ||||||
| Aprepitant | ● | ◐ | ||||
| Clonazepam | ● | |||||
| Fosaprepitant | ● | O | ||||
| Fentanyl | ● | |||||
| Methadone | ● | |||||
| Ondansetron | ● | O | ||||
| Strong CYP3A4 and/or P-gp inhibitors (eg, idelalisib, ketoconazole, itraconazole, voriconazole, posaconazole, fluconazole, ritonavir, lopinavir/ritonavir, indinavir/ritonavir, cyclosporine, tacrolimus, clarithromycin, conivaptan) or inducers (eg, phenytoin, carbamazepine, rifampin, St. John’s wort) are generally contraindicated with direct oral anticoagulant use (DOAC).56,57 | ||||||
Edoxaban and dabigatran are substrates for P-gp. Apixaban and rivaroxaban are substrates of P-gp and CYP3A4. Their levels are influenced by coadministration of inhibitors and inducers of these metabolic pathways. Interactions are identified from the BC Cancer Drug Index, IBM MicroMedex accessed through DynaMed, Lexicomp, and Product Monographs available through the Government of Canada Drug Product Database.78-81 Clinicians should refer to reference sources for the most updated information and to consult a pharmacist for guidance regarding possible drug–drug interactions whenever new medications are started or stopped. Data are variable between sources and interactions may or may not be clinically relevant. ●, major substrate or strong interaction; ◐, moderate interaction; ○, minor substrate or weak interaction or of uncertain clinical significance.
Case 1 continued
Ibrutinib therapy is started after discussing alternative lymphoma treatments and side effects, including an elevated risk of bleeding with concurrent apixaban use. The patient is counseled to avoid nonsteroidal anti-inflammatory drugs and antiplatelet medications, as well as fish oil, grapefruit juice, and naturopathic products that may further enhance bleeding. No major bleeding occurs, and his CLL/SLL responds with decreased lymphadenopathy and peripheral lymphocyte counts over the following months.
Case 2: diffuse large B-cell lymphoma
A 48-year-old previously healthy woman is admitted to hospital after discovering a large neck mass. She has been unwell for a few weeks with nightly drenching sweats and has a recent 20-lb weight loss (current BMI, 37 kg/m2). Imaging demonstrated diffuse cervical and mediastinal lymphadenopathy. Initial blood work revealed a pancytopenia with a white blood cell count of 1.4 × 109/L, hemoglobin of 90 g/L, and platelet count of 110 × 109/L. A bone marrow and lymph node biopsy diagnosed stage IV diffuse large B-cell lymphoma. A peripherally inserted central catheter was placed for R-CHOP administration because of poor venous access. After the first cycle of chemotherapy, the neck lymphadenopathy improved significantly, and the patient was ready to be discharged home. While in the hospital, she received daily thromboprophylaxis with LMWH. On discharge, she asked if she needed to continue thromboprophylaxis because her mother died of PE at age 44 and her sister had a DVT while on oral contraception. How do we assess her risk of VTE? What is the recommendation for outpatient thromboprophylaxis in patients receiving chemotherapy?
Thrombosis risk assessment in ambulatory patients with lymphoma
The Khorana score is the most widely used validated model to estimate the short-term risk of symptomatic VTE in patients starting chemotherapy (Table 3).58,59 Patients with lymphoma accounted for 12.1% and 13.5% of the patients in the derivation and validation cohorts, respectively. According to the score, patients with lymphoma have at least an intermediate risk of thrombosis and having abnormal complete blood count parameters or obesity will place them in the high-risk category. In a retrospective study evaluating the utility of the Khorana score in 428 patients with either Hodgkin lymphoma or diffuse large B-cell lymphoma, the risk of VTE was 15% and 17% in patients in the intermediate- and high-risk groups, respectively.60
Khorana score to predict thrombosis in outpatients initiating chemotherapy
| Patient characteristic . | Points . |
|---|---|
| Site of cancer | |
| Very high risk (stomach, pancreas) | 2 |
| High risk (lung, lymphoma, gynecologic, genitourinary excluding prostate) | 1 |
| Prechemotherapy platelet count ≥350 × 109/L | 1 |
| Hemoglobin ≤100 g/L or use of red cell growth factors | 1 |
| Prechemotherapy leukocyte count ≥11 × 109/L | 1 |
| Body mass index ≥35 kg/m2 | 1 |
| Patient characteristic . | Points . |
|---|---|
| Site of cancer | |
| Very high risk (stomach, pancreas) | 2 |
| High risk (lung, lymphoma, gynecologic, genitourinary excluding prostate) | 1 |
| Prechemotherapy platelet count ≥350 × 109/L | 1 |
| Hemoglobin ≤100 g/L or use of red cell growth factors | 1 |
| Prechemotherapy leukocyte count ≥11 × 109/L | 1 |
| Body mass index ≥35 kg/m2 | 1 |
Risk category and rate of thrombosis: low (0 points), 0.3% to 0.8%; intermediate (1-2 points), 1.8% to 2%; high (≥3 points), 6.7% to 7.1%. See Khorana et al.58
The Thrombosis Lymphoma (ThroLy) score was developed to predict thromboembolic events specifically in patients with lymphoma (Table 4).61 Patients with Hodgkin lymphoma, non-Hodgkin lymphoma, and CLL were enrolled from time of cancer diagnosis or relapse until 3 months after chemotherapy completion. In the derivation and validation cohorts, 97.6% to 98.5% of patients with a low score did not develop thrombosis, whereas 25.1% to 28.9% of patients with intermediate or high scores developed thrombosis. The sensitivity of the score was 64.7% to 75.4% and specificity was 87.5% to 90.2%. Further validation of the ThroLy score is needed, and prospective comparison of the Khorana and ThroLy scores has not been done.
ThroLy score for predicting the risk of thromboembolism in patients with lymphoma
| Patient characteristic . | Points . |
|---|---|
| Previous VTE/MI/stroke | 2 |
| Obesity (BMI >30 kg/m2) | 2 |
| Mediastinal involvement | 2 |
| Extranodal localization | 1 |
| Reduced mobility (ECOG 2-4) | 1 |
| Neutrophil count <1.0 × 109/L | 1 |
| Hemoglobin level <100 g/L | 1 |
| Patient characteristic . | Points . |
|---|---|
| Previous VTE/MI/stroke | 2 |
| Obesity (BMI >30 kg/m2) | 2 |
| Mediastinal involvement | 2 |
| Extranodal localization | 1 |
| Reduced mobility (ECOG 2-4) | 1 |
| Neutrophil count <1.0 × 109/L | 1 |
| Hemoglobin level <100 g/L | 1 |
Risk category: low (0-1), intermediate (2-3), and high (>3).
VTE, venous thromboembolism; MI, myocardial infarction; BMI, body mass index; ECOG, Eastern Cooperative Oncology Group.
See Antic et al.61
Thromboprophylaxis in ambulatory patients with lymphoma
Thromboprophylaxis with LMWH, apixaban, or rivaroxaban is an option to prevent VTE in ambulatory patients with cancer.25 In a 2016 meta-analysis, it was demonstrated that LMWH compared with no thromboprophylaxis significantly reduced the incidence of symptomatic VTE (RR, 0.54; 95% CI, 0.38-0.75) in patients with cancer without increasing the risk of major bleeding (relative risk, 1.44; 95% CI, 0.98-2.11)62; all 9 trials included only nonhematologic solid tumors. An underpowered randomized trial of 98 patients (7 had lymphoma) with a Khorana score of 3 or greater found no significant difference in VTE rate between patients treated with dalteparin and patients who did not receive thromboprophylaxis (12% vs 21%; P = .37).63 The cost and need for subcutaneous administration are the major barriers to the use of LMWH in the outpatient setting.
Thromboprophylaxis with a DOAC in outpatients starting chemotherapy who had a Khorana score of 2 or more were evaluated in the Apixaban to Prevent Venous Thromboembolism in Patients with Cancer (AVERT) and Rivaroxaban for Thromboprophylaxis in High-Risk Ambulatory Patients with Cancer (CASSINI) trials.64,65 In 563 patients randomized to receive apixaban or placebo for 180 days, AVERT found VTE occurred in 4.2% of patients treated with apixaban 2.5 mg twice daily compared with 10.2% of patients treated with placebo (hazard ratio [HR], 0.41; 95% CI, 0.26-0.65; P < .001). The apixaban group had a higher rate of major bleeding during the study period (3.5% vs 1.8%; HR, 2.00; 95% CI, 1.01-3.95; P = .046), but the difference was not significant while patients are receiving the study drug (HR, 1.89; 95% CI, 0.39-9.24). In this trial, 25.3% of patients had lymphoma; results of this subgroup have not been reported.
Using a similar design, the CASSINI trial compared rivaroxaban 10 mg once daily with placebo in 841 patients. Patients with asymptomatic DVT on a baseline screening ultrasound were excluded. VTE occurred in 6.0% of the rivaroxaban group and 8.8% of the placebo group (HR, 0.66; 95% CI, 0.40-1.09; P = .10). No difference was found in major bleeding at 2% and 1%, respectively. Patients with lymphoma accounted for 7% of patients; 2 of 32 patients with lymphoma assigned to rivaroxaban compared with none of the 25 patients with lymphoma assigned to placebo developed VTE. A possible explanation for the discrepant findings in CASSINI and AVERT trials may be the difference in the early discontinuation rate of the study drugs: 42.1% in patients assigned to rivaroxaban vs 16.4% assigned to apixaban. This is supported by an on-treatment analysis of the CASSINI results. The effectiveness and safety of DOACs for prophylaxis outside of concurrent outpatient chemotherapy administration or beyond 6 months of prophylaxis have not been studied.
Data supporting the use of other antithrombotic agents for thromboprophylaxis in patients with cancer are old and limited. A trial of patients with stage IV breast cancer demonstrated a reduction in thrombotic events in the 156 patients (1 VTE) assigned low-dose warfarin (target international normalized ratio between 1.3 and 1.9) vs placebo in 159 patients (7 VTE; P = .031).66 Long-term thromboprophylaxis with fixed low-dose warfarin up to a median duration of 25 months was not more effective than aspirin in patients with myeloma.67 Aspirin prophylaxis is recommended for patients with myeloma on immunomodulatory therapy, and there are no placebo controlled trials supporting its use in other cancers.62,68 Therefore, current evidence and ease of administration support low dose apixaban or rivaroxaban for VTE thromboprophylaxis in the ambulatory oncology setting, over warfarin, LMWH, or aspirin.
In addition to chemotherapy, the presence of a peripherally inserted central catheter or other indwelling central venous catheters further elevates the risk of VTE.69 Guidelines do not recommend routine anticoagulation prophylaxis for the prevention of catheter-related thrombosis in patients with cancer based on data from randomized controlled trials.24,70,71 A Cochrane review of 13 randomized trials found that LMWH decreases the risk of symptomatic catheter-related thrombosis compared with no LMWH (RR, 0.43; 95% CI, 0.22-0.81).72 The rates of thrombosis were higher in earlier studies, potentially reflecting a lower thrombosis risk with newer generation catheters. Ongoing studies evaluating anticoagulant thromboprophylaxis will be helpful to provide future guidance.
Case 2 continued
This patient has a Khorana score of 3 and a ThroLy score of 5, placing her at intermediate to high risk of developing VTE. Having first-degree relatives with thrombosis likely further increases her risk of VTE.73 She has no risk factors for bleeding. Cyclophosphamide, doxorubicin, vincristine, and prednisone in R-CHOP are substrates and/or modulators of CYP3A4, but the clinical significance of the interactions with rivaroxaban and apixaban are unknown.57 She chooses to start apixaban at 2.5 mg twice daily for the duration of her chemotherapy because she is fearful of VTE from personal experience and accepts the risk of bleeding. She is aware there is currently no evidence that DOAC prophylaxis is effective for preventing catheter-related thrombosis. She is counseled that she should have the catheter removed promptly after her lymphoma treatment is completed. Signs and symptoms of catheter-related thrombosis, DVT, PE, and major bleeding are reviewed, and she will seek urgent medical attention if they develop.
Case 3: indolent lymphoma
A 78-year-old man presents with pancreatitis secondary to choledocholithiasis. He was diagnosed with advanced stage III follicular lymphoma 3 years ago and was treated with bendamustine and rituximab followed by 2 years of maintenance rituximab. He has no symptomatic disease currently. In the hospital, he develops abdominal pain, and portal and splenic vein thrombosis is discovered on computed tomography scan. There was no evidence of lymphadenopathy. LMWH is started for his portal vein thrombosis while his oral intake remains poor. The patient asks how long he needs to stay on anticoagulation.
Duration of anticoagulation in patients with indolent lymphoma
The optimal duration of anticoagulant therapy in patients with cancer-associated VTE has not been adequately evaluated.74 Guidelines recommend a minimum of 3 to 6 months of anticoagulation for the treatment of cancer-associated VTE,24,25,75 after which the decision to continue or stop anticoagulation is guided by a clinical assessment of ongoing patient- and cancer-related risk factors such as chemotherapy, progressive cancer, or metastatic disease. In a retrospective study of consecutive patients with cancer-associated VTE treated at our institution, we found that 8.2% of patients who were still alive at 6 months after their diagnosis of VTE developed recurrent VTE over the following year, although 68.9% of them continued anticoagulant therapy.76 Among patients alive at 6 months, 64 (19.9%) had hematologic malignancies.
In patients with lymphoma, remission status and active therapy are likely important determinants of VTE recurrence. If the lymphoma is in complete remission and the patient is not on active treatment at the time of VTE diagnosis, then the VTE is more likely to be unrelated to the lymphoma and the choice and duration of anticoagulant therapy should be based on non–cancer-related risk factors. If the lymphoma is not in complete remission or if the patient is on active lymphoma therapy, then the thrombotic event is likely attributable to active cancer, and the choice and duration of anticoagulation need to be evaluated at 3 months and regularly thereafter. For example, a patient with CLL Rai stage 0, stable counts, and no CLL-specific treatment who develops VTE in the postoperative period would likely be considered as having a VTE provoked by a major transient risk factor (surgery) and is expected to have a low risk of recurrent thrombosis after completing a 3-month course of anticoagulation.77 However, if after 3 months of anticoagulation, the patient is no longer in remission, then the risk of recurrent VTE is likely sufficiently high to warrant continuing anticoagulant therapy, particularly if the patient needs to resume lymphoma therapy.3,74
Case 3 continued
A thorough physical examination and further imaging did not reveal any concerning lymphadenopathy. His blood work is also unremarkable. The splanchnic vein thrombosis is likely provoked by his pancreatitis, and therefore 3 months of anticoagulation is recommended. A follow-up visit at 3 months is scheduled to review his lymphoma status and tolerance with anticoagulant therapy.
Conclusion
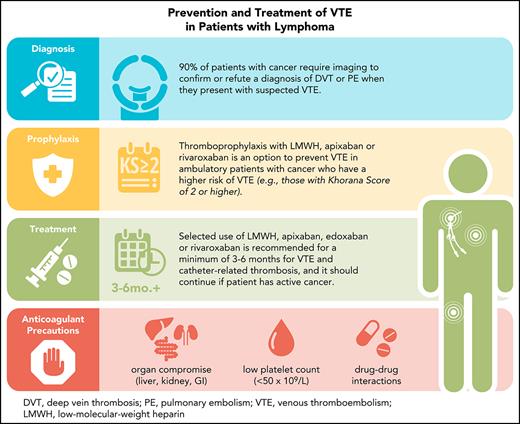
VTE is a common complication among patients with lymphoma. Guideline-directed therapy has expanded from LMWH to include apixaban, edoxaban, and rivaroxaban for the treatment of cancer-associated VTE. Although patients with lymphoma represented a small subgroup of patients in clinical trials evaluating LMWH and DOACs, it is reasonable to use these anticoagulants in preference to warfarin for prevention and treatment given their convenience and acceptable safety profiles. Importantly, drug–drug interactions among anticoagulants, lymphoma therapies and other medications, and risk factors for bleeding (including likelihood of severe thrombocytopenia) need to be reviewed before starting any anticoagulant therapy. Anticoagulation therapy requires frequent assessment for recurrent VTE events, bleeding, and lymphoma status and treatment (Table 5). More research is required in patients with lymphoma and other hematologic malignancies to understand the efficacy and safety of anticoagulant therapy in these unique patients.
Recommendations and reminders in the prevention and treatment of VTE in patients with lymphoma
| Clinical topic . | Recommendations . |
|---|---|
| Diagnosis |
|
| Prophylaxis |
|
| Treatment |
|
| Anticoagulant precautions |
|
| Clinical topic . | Recommendations . |
|---|---|
| Diagnosis |
|
| Prophylaxis |
|
| Treatment |
|
| Anticoagulant precautions |
|
CYP3A4, cytochrome P450 3A4. Recommendations and reminders in the prevention and treatment of VTE in patients with lymphoma.19,23-25,58,70
Acknowledgment
The authors thank Nadine Badry (BC Cancer–Provincial Pharmacy, Victoria, BC, Canada) for assistance in reviewing drug–drug interactions in Table 2.
Authorship
Contribution: R.A.S. and A.Y.Y.L. reviewed literature, wrote the manuscript, and approved the final version.
Conflict-of-interest disclosure: A.Y.Y.L. has received research funding from Bristol-Myers Squibb and consultancy honoraria from Bayer, Bristol-Myers Squibb, LEO Pharma, and Pfizer. R.A.S. declares no competing financial interests.
Correspondence: Agnes Y. Y. Lee, Gordon and Leslie Diamond Health Care Centre, 2775 Laurel St, 10th Floor, Vancouver, BC V5Z 1M9, Canada; e-mail: alee14@bccancer.bc.ca.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal