Abstract
The prognosis of several lymphoid malignancies has improved through development of novel therapies, combination with traditional chemotherapies, and delineation of appropriate therapeutic sequencing. Toxicities that are arising because of prolonged or multiple sequential therapeutic interventions are becoming increasingly impactful. Among the broad spectrum of complications that patients with lymphoid malignancies may experience, cardiovascular toxicities are significant in terms of morbidity and mortality. The entire cardiovascular system can be affected, but cardiomyopathy, heart failure, and arrhythmias remain of greatest concerns with the use of anthracyclines, hematopoietic stem cell transplantation, and radiation therapy in patients with lymphoid malignancies. These aspects will be covered in this article within the framework of case-based discussions. Key to the management of cardiovascular complications in patients with lymphoid malignancies is awareness and preparedness across the cancer continuum. Baseline risk stratification helps to direct surveillance and early intervention efforts before, during, and after cancer therapy, which are paramount for the best possible outcomes. Along these lines, the overall goal is to enable the best possible therapies for lymphoid malignancies without the complications of clinically significant cardiovascular events.
Introduction
Lymphoid malignancies are a heterogeneous group of malignancies that include Hodgkin lymphoma (HL), non-Hodgkin lymphoma (NHL), hairy cell leukemia (HCL), and chronic lymphocytic leukemia (CLL).1 Owing to the advancements of therapeutic and clinical interventions, the prognosis of these malignancies has improved significantly over the last decades; in fact, so much that the clinical impact of toxicities and comorbidities has become increasingly evident. Cardiovascular diseases (CVDs) are a prime example in this regard, and in the current era, patients with lymphoma have a risk of dying from CVDs at least as high as that from the malignancy itself.2 Assessing and addressing CVDs in this patient population is therefore becoming increasingly important.3 Given the broad spectrum of disease types and therapies (Table 1), not all of the aspects of cardiovascular care in this patient population can be covered herein, and the interested reader is refereed to recent reviews.4-7 The focus of this article will be on 3 therapies with greatest concern for inducing or aggravating CVD in patients with lymphoid malignancies: anthracyclines, hematopoietic stem cell transplantation (HSCT), and radiation therapy.
Drug therapies used for the treatment of lymphoid malignancies and common cardiovascular toxicities
| Medication . | Use . | Select cardiovascular toxicities . |
|---|---|---|
| Acalabrutinib | CLL, NHL | Atrial fibrillation (≤5%), atrial flutter (≤5%), hypertension (3%) |
| Alemtuzumab | CLL | Hypotension (16%), cardiac arrhythmia (14%), hypertension (14%) |
| Azacitidine | NHL | Chest pain (16%), hypertension (9%), hypotension (7%), atrial fibrillation (<5%), cardiomyopathy (<5%) |
| Belinostat | NHL | Peripheral edema (20%), QTc prolongation (11%), hypotension (10%) |
| Bendamustine | NHL, HL, CLL | Peripheral edema (13%), tachycardia (7%), chest pain (6%), hypotension (6%), exacerbation of hypertension (3%) |
| Bleomycin | HL | Interstitial pneumonitis (acute or chronic: ≤5% to 10%), pulmonary, ischemic heart disease (<1%), Raynaud’s phenomenon (<1%), pulmonary fibrosis (≤5% to 10%) |
| Bortezomib | NHL | Hypotension (8% to 9%), cardiac disease (treatment emergent; 8%), acute pulmonary edema (≤1%), cardiac failure (≤1%), cardiogenic shock (≤1%), pulmonary edema (≤1%) |
| Brentuximab vedotin | HL | Peripheral edema (11%) |
| Carboplatin | HL, NHL | Arterial and venous thrombotic events, ischemic events, hypertension, hypotension |
| Carmustine | HSCT | Chest pain (5%), arterial occlusive disease, tachycardia |
| Cisplatin | HL, NHL | Arterial and venous thrombotic events, arrhythmias, hypertension, hypotension,vasospasm |
| Cladribine | HCL | (Peripheral) edema, phlebitis, tachycardia |
| Copanlisib | NHL | Hypertension (35%), hypertriglyceridemia (58%) |
| Cyclophosphamide | HL, NHL, CLL, HSCT | Arrhythmias, hemorrhagic myocarditis, pericarditis, pericardial effusion, even tamponade, myocardial infarction, arterial and venous thrombosis |
| Cytarabine (Ara-C) | HL, NHL, HSCT | Thrombophlebitis (common); angina pectoris, chest pain, pericarditis (rare) |
| Darutumumab | NHL | Hypertension (10%), infusion reaction |
| Daunorubicin Doxorubicin | Daunorubicin: NHL, ALL Doxorubicin: HL, NHL | Acute cardiotoxicity: ECG abnormalities including atrioventricular block, bradycardia, bundle branch block, extrasystoles (atrial or ventricular), nonspecific ST or T wave changes on ECG, sinus tachycardia, supraventricular tachycardia, tachyarrhythmia, ventricular tachycardia, decline in cardiac function, heart failure, cardiogenic shock, perimyocarditis Delayed cardiotoxicity: Heart failure, decline in cardiac function, arrhythmias |
| Dexamethasone | HL, NHL, ALL | Bradycardia, cardiac arrhythmia, cardiac failure, cardiomegaly, circulatory shock, edema, embolism (fat), hypertension, hypertrophic cardiomyopathy (premature infants), myocardial rupture (post-MI), syncope, tachycardia, thromboembolism, thrombophlebitis, vasculitis |
| Duvelisib | NHL, CLL | Edema (11% to 14%) |
| Etoposide | NHL, HL, HSCT | Hypotension (1% to 2%; due to rapid infusion) |
| Fludarabine | NHL, CLL | Peripheral edema (8%) |
| Ibrutinib | CLL, NHL | Peripheral edema (35%), hypertension (19%), atrial fibrillation/flutter (8%), ventricular tachycardia (<1%) |
| Idelalisib | CLL, NHL | Peripheral edema (10%) |
| Ifosfamide | HL, NHL | Arrhythmias, cardiac failure, cardiogenic shock, cardiomyopathy, myocardial infarction, myocarditis, pericardial effusion, pericarditis (risk of events especially with high dose therapy, fatal outcomes possible) |
| Lenalidomide | NHL, CLL | Peripheral edema (16% to 20%), hypotension (7%), hypertension (6%), chest pain (5%), palpitations (5%), deep vein thrombosis (2% to 4%), pulmonary embolism (1% to 2%) |
| Melphalan | HSCT | Peripheral edema (33%), vasculitis |
| Methotrexate | NHL | Pericarditis, pericardial effusion, hypotension, and thromboembolic events |
| Mitoxantrone | NHL | Arrhythmia (8%), ECG changes (3%), cardiomyopathy and heart failure |
| Mogamulizumab | NHL | Edema (16%), hypertension 10%), arrhythmia (5%) |
| Moxetumomab pasudotox | HCL | Capillary leak syndrome (34%) including hypotension, hypoalbuminemia, and hemoconcentration plus peripheral edema |
| Nivolumab | HL | Peripheral edema (10%), hypertension (7%), pulmonary embolism (rare), myocarditis (rare) |
| Ofatumumab | CLL, NHL | Peripheral edema (9%), hypertension/hypotension (5%), tachycardia (5%) |
| Oxaliplatin | HL | Edema (15%), prolonged QT interval and ventrical arrhythmias including torsades de pointes (rare) |
| Pegaspargase | ALL | Thromboembolic complications (8%), elevated triglycerides (30%) |
| Pentostatin | HCL, CLL, NHL | Chest pain, facial or peripheral edema, hypotension (3% to 10%) |
| Pembrolizumab | HL, NHL | Peripheral edema (15%), cardiac arrhythmia (11%), acute myocardial infarction (2%), cardiac tamponade (2%), facial edema (10%), ischemic heart disease (2%), pericardial effusion (2%), pericarditis (4%), pulmonary embolism (2%), myocarditis (rare) |
| Procarbazine | HL | Hypotension, syncope, tachycardia |
| Rituximab | NHL, CLL, HCL, ALL | Cardiac disorder (5% to 29%), flushing (5% to 14%), hypertension (6% to 12%), peripheral edema (8% to 16%); chest tightness (7%), hypotension (10%), significant cardiovascular event (2%) (acute myocardial infarction, cardiogenic shock, supraventricular cardiac arrhythmia, ventricular fibrillation, ventricular tachycardia) |
| Romidepsin | NHL | Hypotension (7% to 23%), ECG changes including QTc prolongation (1% to 2%) |
| Tazemetostat | NHL | Elevated triglycerides (36%) |
| Venetoclax | CLL | Edema (22%) |
| Vinblastine | HL | Hypertension (common); myocardial infarction, angina pectoris, transient abnormalities on ECG related to ischemia (rare) |
| Vincristine | HL, NHL, ALL | Hypertension, hypotension (presumed autonomic) |
| Zanubrutinib | NHL | Hypertension (12%), atrial fibrillation/flutter (2%) |
| Medication . | Use . | Select cardiovascular toxicities . |
|---|---|---|
| Acalabrutinib | CLL, NHL | Atrial fibrillation (≤5%), atrial flutter (≤5%), hypertension (3%) |
| Alemtuzumab | CLL | Hypotension (16%), cardiac arrhythmia (14%), hypertension (14%) |
| Azacitidine | NHL | Chest pain (16%), hypertension (9%), hypotension (7%), atrial fibrillation (<5%), cardiomyopathy (<5%) |
| Belinostat | NHL | Peripheral edema (20%), QTc prolongation (11%), hypotension (10%) |
| Bendamustine | NHL, HL, CLL | Peripheral edema (13%), tachycardia (7%), chest pain (6%), hypotension (6%), exacerbation of hypertension (3%) |
| Bleomycin | HL | Interstitial pneumonitis (acute or chronic: ≤5% to 10%), pulmonary, ischemic heart disease (<1%), Raynaud’s phenomenon (<1%), pulmonary fibrosis (≤5% to 10%) |
| Bortezomib | NHL | Hypotension (8% to 9%), cardiac disease (treatment emergent; 8%), acute pulmonary edema (≤1%), cardiac failure (≤1%), cardiogenic shock (≤1%), pulmonary edema (≤1%) |
| Brentuximab vedotin | HL | Peripheral edema (11%) |
| Carboplatin | HL, NHL | Arterial and venous thrombotic events, ischemic events, hypertension, hypotension |
| Carmustine | HSCT | Chest pain (5%), arterial occlusive disease, tachycardia |
| Cisplatin | HL, NHL | Arterial and venous thrombotic events, arrhythmias, hypertension, hypotension,vasospasm |
| Cladribine | HCL | (Peripheral) edema, phlebitis, tachycardia |
| Copanlisib | NHL | Hypertension (35%), hypertriglyceridemia (58%) |
| Cyclophosphamide | HL, NHL, CLL, HSCT | Arrhythmias, hemorrhagic myocarditis, pericarditis, pericardial effusion, even tamponade, myocardial infarction, arterial and venous thrombosis |
| Cytarabine (Ara-C) | HL, NHL, HSCT | Thrombophlebitis (common); angina pectoris, chest pain, pericarditis (rare) |
| Darutumumab | NHL | Hypertension (10%), infusion reaction |
| Daunorubicin Doxorubicin | Daunorubicin: NHL, ALL Doxorubicin: HL, NHL | Acute cardiotoxicity: ECG abnormalities including atrioventricular block, bradycardia, bundle branch block, extrasystoles (atrial or ventricular), nonspecific ST or T wave changes on ECG, sinus tachycardia, supraventricular tachycardia, tachyarrhythmia, ventricular tachycardia, decline in cardiac function, heart failure, cardiogenic shock, perimyocarditis Delayed cardiotoxicity: Heart failure, decline in cardiac function, arrhythmias |
| Dexamethasone | HL, NHL, ALL | Bradycardia, cardiac arrhythmia, cardiac failure, cardiomegaly, circulatory shock, edema, embolism (fat), hypertension, hypertrophic cardiomyopathy (premature infants), myocardial rupture (post-MI), syncope, tachycardia, thromboembolism, thrombophlebitis, vasculitis |
| Duvelisib | NHL, CLL | Edema (11% to 14%) |
| Etoposide | NHL, HL, HSCT | Hypotension (1% to 2%; due to rapid infusion) |
| Fludarabine | NHL, CLL | Peripheral edema (8%) |
| Ibrutinib | CLL, NHL | Peripheral edema (35%), hypertension (19%), atrial fibrillation/flutter (8%), ventricular tachycardia (<1%) |
| Idelalisib | CLL, NHL | Peripheral edema (10%) |
| Ifosfamide | HL, NHL | Arrhythmias, cardiac failure, cardiogenic shock, cardiomyopathy, myocardial infarction, myocarditis, pericardial effusion, pericarditis (risk of events especially with high dose therapy, fatal outcomes possible) |
| Lenalidomide | NHL, CLL | Peripheral edema (16% to 20%), hypotension (7%), hypertension (6%), chest pain (5%), palpitations (5%), deep vein thrombosis (2% to 4%), pulmonary embolism (1% to 2%) |
| Melphalan | HSCT | Peripheral edema (33%), vasculitis |
| Methotrexate | NHL | Pericarditis, pericardial effusion, hypotension, and thromboembolic events |
| Mitoxantrone | NHL | Arrhythmia (8%), ECG changes (3%), cardiomyopathy and heart failure |
| Mogamulizumab | NHL | Edema (16%), hypertension 10%), arrhythmia (5%) |
| Moxetumomab pasudotox | HCL | Capillary leak syndrome (34%) including hypotension, hypoalbuminemia, and hemoconcentration plus peripheral edema |
| Nivolumab | HL | Peripheral edema (10%), hypertension (7%), pulmonary embolism (rare), myocarditis (rare) |
| Ofatumumab | CLL, NHL | Peripheral edema (9%), hypertension/hypotension (5%), tachycardia (5%) |
| Oxaliplatin | HL | Edema (15%), prolonged QT interval and ventrical arrhythmias including torsades de pointes (rare) |
| Pegaspargase | ALL | Thromboembolic complications (8%), elevated triglycerides (30%) |
| Pentostatin | HCL, CLL, NHL | Chest pain, facial or peripheral edema, hypotension (3% to 10%) |
| Pembrolizumab | HL, NHL | Peripheral edema (15%), cardiac arrhythmia (11%), acute myocardial infarction (2%), cardiac tamponade (2%), facial edema (10%), ischemic heart disease (2%), pericardial effusion (2%), pericarditis (4%), pulmonary embolism (2%), myocarditis (rare) |
| Procarbazine | HL | Hypotension, syncope, tachycardia |
| Rituximab | NHL, CLL, HCL, ALL | Cardiac disorder (5% to 29%), flushing (5% to 14%), hypertension (6% to 12%), peripheral edema (8% to 16%); chest tightness (7%), hypotension (10%), significant cardiovascular event (2%) (acute myocardial infarction, cardiogenic shock, supraventricular cardiac arrhythmia, ventricular fibrillation, ventricular tachycardia) |
| Romidepsin | NHL | Hypotension (7% to 23%), ECG changes including QTc prolongation (1% to 2%) |
| Tazemetostat | NHL | Elevated triglycerides (36%) |
| Venetoclax | CLL | Edema (22%) |
| Vinblastine | HL | Hypertension (common); myocardial infarction, angina pectoris, transient abnormalities on ECG related to ischemia (rare) |
| Vincristine | HL, NHL, ALL | Hypertension, hypotension (presumed autonomic) |
| Zanubrutinib | NHL | Hypertension (12%), atrial fibrillation/flutter (2%) |
This table is not intended to be all inclusive. Percentages are listed when available and derived from package inserts available at www.dailymed.nlm.nih.gov. Rare adverse events are reported to the FDA through the Adverse Event Reporting System (AERS) and providers are encouraged to use this system for evaluation of more atypical presentations.
ALL, acute lymphoblastic leukemia.
Case 1
A 57-year-old woman, newly diagnosed with diffuse large B-cell lymphoma (DLBCL) after presenting with progressive widespread lymphadenopathy, is recommended to undergo R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) therapy. Her medical history is pertinent for class I obesity, impaired fasting plasma glucose, dyslipidemia, hyperuricemia, and systemic hypertension. Her current medications include pravastatin 20 mg/day, allopurinol 200 mg/day, and metoprolol 75 mg twice a day. The latter was started also in view of a history of sinus tachycardia and reduced ejection fraction of 35% to 40%. Her current left ventricular ejection fraction (LVEF) is 55%, and global longitudinal strain (GLS) is −18%. Ventricular dimension, wall thickness, atrial sizes, and valves are normal; right ventricular (RV) systolic pressure is 30 mm Hg. The patient has no cardiopulmonary symptoms.
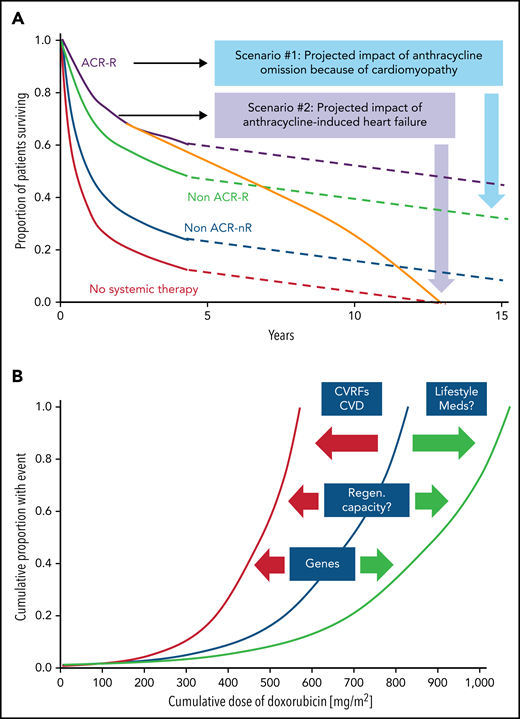
DLBCL is a potentially curable disease; 10-year overall survival is more than 40% to 65% with first-line therapy.8-11 Although often not of primary concern, cardiac comorbidities and complications can significantly influence choice of therapies and long-term outcomes. These aspects are illustrated in Figure 1A for anthracyclines, which are an integral part of R-CHOP therapy, the current standard of care for patients with DLBCL. Other elements of R-CHOP therapy with cardiotoxicity risk include rituximab, which can cause hypertension, hypotension, angina, cardiomyopathy, and ventricular arrhythmias.5,6 Furthermore, cyclophosphamide has been associated with vascular events and hemorrhagic myopericarditis at higher doses used in stem cell transplantation, which can be fatal.8 Beyond the acute risks, recent data from the Dutch Childhood Oncology Group indicate a long-term risk for heart failure after (higher-dose) cyclophosphamide treatment in childhood cancer survivors.9 Similar data for patients, and especially adult patients with lymphoid malignancies, are not available; in this patient population, anthracycline cardiomyopathy remains the most common long-term cardiovascular concern.
Impact of cardiomyopathy and the correlation of cumulative anthacycline doseage. (A) Illustration of the impact of cardiomyopathy (decline in cardiac function) and/or heart failure before, during, or after anthracycline-based therapy in older patients with diffuse large B-cell lymphoma. ACR, anthracycline-containing regimen; R, rituxumab (modified from Tien et al,87 and based on data from Ammar et al.88 (B) Outline of the correlation of cumulative anthracycline dose (doxorubicin equivalent) and risk of heart failure and the impact of modulating factors such as cardiovascular risk factors (CVRFs), CVDs, and genetic predisposition, as well as lifestyle factors such as exercise/fitness and adjunctive/concomitant medications and therapies which shift the curve to the left (ie, increasing the likelihood of heart failure at a lower-dose spectrum) or to the right (ie, lower risk of heart failure at similar or even higher dose range).
Impact of cardiomyopathy and the correlation of cumulative anthacycline doseage. (A) Illustration of the impact of cardiomyopathy (decline in cardiac function) and/or heart failure before, during, or after anthracycline-based therapy in older patients with diffuse large B-cell lymphoma. ACR, anthracycline-containing regimen; R, rituxumab (modified from Tien et al,87 and based on data from Ammar et al.88 (B) Outline of the correlation of cumulative anthracycline dose (doxorubicin equivalent) and risk of heart failure and the impact of modulating factors such as cardiovascular risk factors (CVRFs), CVDs, and genetic predisposition, as well as lifestyle factors such as exercise/fitness and adjunctive/concomitant medications and therapies which shift the curve to the left (ie, increasing the likelihood of heart failure at a lower-dose spectrum) or to the right (ie, lower risk of heart failure at similar or even higher dose range).
Defining a patient’s risk of cardiotoxicity with anthracycline therapy is inherently difficult and cannot be expressed in a fixed percentage (which could then be weighed against the projected therapeutic benefit in a shared decision-making approach). Total cumulative dose is a key determinant, and the dose–cardiotoxicity relationship is deemed to be exponential with a deflection point at 250 to 300 mg/m2 (Figure 1B). The cardiotoxicity risk increases substantially after this deflection point, although considerable variability is seen, especially in cardiac function decline. Cardiovascular (CV) risk factors, especially hypertension, CVDs, and age <15 or ≥65 years, shift the dose–cardiotoxicity curve to the left (higher risk at lower doses).10 Single nucleotide polymorphisms can have similar effects,11 and most of these are related to drug metabolism, although 1 study also reported a titin mutation.12 Titin gene mutations are 1 of the most frequent causes of dilated cardiomyopathy, found in approximately 25% of familial cases of idiopathic dilated cardiomyopathy. An alternative expression of the constellation of factors influencing the risk of cardiac function decline and disease manifestation is that of “multiple hits.” This is a key concept in cardio-oncology and underlies various risk models.13,14
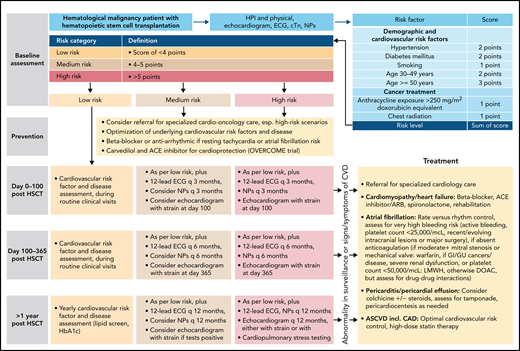
The Mayo Cardiotoxicity Score was the first model outlining the concept of combining therapy-related risk factors (graded by the strength of evidence for the association of the cancer drug with cardiotoxicity) and patient-related risk factors (additional points for each CVD or CV risk factor element) to stratify cardiotoxicity risk levels before cancer therapy.10 The joint Heart Failure Association (HFA)–International Cardio-Oncology Society (IC-OS) model expanded on this concept and devises cardiovascular toxicity risk levels: low, medium, high, and very high risk (Figure 2) (corresponding to a quantitative [likelihood] level of risk of <2%, 2% to 9%, 10% to 19%, and ≥20%, respectively).15 The American Society of Clinical Oncology practice guideline devises a binary risk model: either low or high, based on the combination of exposure to anthracycline, and/or radiation therapy to the chest, trastuzumab, or CVDs or multiple CV risk factors (Table 2).16 The National Comprehensive Cancer Network Guidelines consider patients exposed to a higher cumulative dose of anthracyclines (≥250 mg/m2) or with 1 or more heart failure risk factors as at risk (Table 2).16 The European Society of Medical Oncology consensus defines at-risk patients similar to the American Society of Clinical Oncology and National Comprehensive Cancer Network Guidelines but with some nuances and includes abnormal cardiac biomarkers, cardiac troponin, and natriuretic peptides (NPs) to define risk (supplemental Table 1, available on the Blood Web site). Assessment of these biomarkers is recommended at baseline and during follow-up in patients at risk of cardiotoxicity.
Outline of a risk-based management approach to anthracycline cardiomyopathy. For more on the outline, see Lyon et al,15 Pudil et al,17 and Öztürk et al.19 cTn, cardiac troponin, ECG, electrocardiogram, HTN, hypertension, NPs, natriuretic peptides.
Recommendations for cardiotoxicity risk identification and management of patients with hematological malignancies
| . | Recommendation . |
|---|---|
| Definition of at risk or high risk (risk increases with number of risk factors present) | Planned, ongoing, or completed cancer therapy • High dose anthracycline therapy (≥250 mg/m2 doxorubicin equivalent) • Chest radiation therapy (treatment doses ≥30 Gy) • Combination of anthracycline and chest radiation therapy Cardiovascular risk factors, especially • Age ≥65 • Hypertension • Diabetes • Smoking Cardiovascular diseases, especially • Reduced or borderline cardiac function (ejection fraction ≤55%) • Heart failure • Coronary artery disease • Prior cardiovascular events such as myocardial infarction and stroke |
| Prevention | • Healthy lifestyle, including physical exercise, optimized weight, blood pressure, lipid, and glucose control • Control of cardiovascular risk factors (preferred use of beta-blockers such as carvedilol and nebivolol, and angiotensin converting enzyme inhibitors/angiotensin receptor blockers for management of hypertension, statins for hyperlipidemia) • Optimal management of cardiovascular diseases (preferred use of beta-blockers such as carvedilol and nebivolol, and angiotensin converting enzyme inhibitors/angiotensin receptor blockers, statins as indicated for cardiomyopathy, heart failure, and coronary artery disease) • Consider non-anthracycline-based regimens, use of dexrazoxane, or liposomal doxorubicin if doxorubicin lifetime dose >300 mg/m2 or underlying cardiomyopathy/ heart failure (history) |
| Surveillance | • Baseline assessment of cardiac function (preferred echocardiogram with strain imaging), ECG, and cardiac biomarkers (cardiac troponin [cTn] and natriuretic peptides [NPs]), especially in high-risk patients, ideally in all undergoing potentially cardiotoxic therapy (as a reference) • cTn and/or NPs every 3-6 wk or before each cycle depending on therapy and risk scenario • Reassessment of cardiac function (ideally echocardiogram with strain) after a cumulative dose of 250 mg/m2 doxorubicin equivalent and every 100 mg/m2 thereafter and end of therapy • Echocardiogram and cardiac biomarkers at 6-12 mo, 2 y and possibly periodically thereafter following completion of anthracycline therapy |
| Treatment | New abnormal cardiac biomarkers or significant change from baseline (significant delta for cTn, 100% increase for NPs): • Cardiology (preferably cardio-oncology) consultation • Consider echocardiogram (with strain), other tests as needed • Consider beta-blockers such as carvedilol and nebivolol, and angiotensin converting enzyme inhibitors/angiotensin receptor blockers, if not already prescribed • Anticancer therapy may be continued, if only mild elevation and without significant change in cardiac function or other acute cardiac pathology, recognizing that a decline in cardiac function may not become evident until after cancer therapy New abnormal global longitudinal strain or significant change from baseline (12% to 15% relative change or 3% to 5% absolute change): • Cardiology, preferably cardio-oncology consultation • Consider beta-blockers such as carvedilol and nebivolol, and angiotensin converting enzyme inhibitors/angiotensin receptor blockers, if not already prescribed • Anticancer therapy may be continued if no significant change in cardiac function or other acute cardiac pathology, recognizing that a decline in cardiac function may not become evident until after cancer therapy Asymptomatic decrease in LVEF by >10% to 40% to 49%: • Cardiology, preferably cardio-oncology consultation • Initiation of beta-blockers such as carvedilol and nebivolol, and angiotensin converting enzyme inhibitors/angiotensin receptor blockers, if not already prescribed • Intensification of cardiac surveillance with echocardiogram before each or every other cycle, cTn and NPs after each anthracycline dose, recognizing that a decline in cardiac function may not become evident until after cancer therapy • If further anthracycline-based therapy is planned, risk-benefit discussion and consideration for non-anthracycline-based regimens, use of dexrazoxane, or liposomal doxorubicin Symptomatic heart failure or LVEF <40%: • Cardiology, preferably cardio-oncology consultation • Cardiac function re-assessment and other tests as needed • Initiation and optimization of guideline-directed therapy • Withdrawal of therapy only after a period of stabilization and no active risk factors, and no further anticancer therapy • Hold anti-cancer therapy until careful risk-benefit discussion, seek alternatives to cardiotoxic medications, close surveillance (every cycle) if proceeding, recognizing that a decline in cardiac function may not become evident until after cancer therapy • At least annual review indefinitely Chest radiation therapy exposure: • Evaluation for heart failure, coronary artery, valvular heart, and chronic pericardial disease, starting at 5 y post-treatment and then at least every 3-5 y thereafter |
| . | Recommendation . |
|---|---|
| Definition of at risk or high risk (risk increases with number of risk factors present) | Planned, ongoing, or completed cancer therapy • High dose anthracycline therapy (≥250 mg/m2 doxorubicin equivalent) • Chest radiation therapy (treatment doses ≥30 Gy) • Combination of anthracycline and chest radiation therapy Cardiovascular risk factors, especially • Age ≥65 • Hypertension • Diabetes • Smoking Cardiovascular diseases, especially • Reduced or borderline cardiac function (ejection fraction ≤55%) • Heart failure • Coronary artery disease • Prior cardiovascular events such as myocardial infarction and stroke |
| Prevention | • Healthy lifestyle, including physical exercise, optimized weight, blood pressure, lipid, and glucose control • Control of cardiovascular risk factors (preferred use of beta-blockers such as carvedilol and nebivolol, and angiotensin converting enzyme inhibitors/angiotensin receptor blockers for management of hypertension, statins for hyperlipidemia) • Optimal management of cardiovascular diseases (preferred use of beta-blockers such as carvedilol and nebivolol, and angiotensin converting enzyme inhibitors/angiotensin receptor blockers, statins as indicated for cardiomyopathy, heart failure, and coronary artery disease) • Consider non-anthracycline-based regimens, use of dexrazoxane, or liposomal doxorubicin if doxorubicin lifetime dose >300 mg/m2 or underlying cardiomyopathy/ heart failure (history) |
| Surveillance | • Baseline assessment of cardiac function (preferred echocardiogram with strain imaging), ECG, and cardiac biomarkers (cardiac troponin [cTn] and natriuretic peptides [NPs]), especially in high-risk patients, ideally in all undergoing potentially cardiotoxic therapy (as a reference) • cTn and/or NPs every 3-6 wk or before each cycle depending on therapy and risk scenario • Reassessment of cardiac function (ideally echocardiogram with strain) after a cumulative dose of 250 mg/m2 doxorubicin equivalent and every 100 mg/m2 thereafter and end of therapy • Echocardiogram and cardiac biomarkers at 6-12 mo, 2 y and possibly periodically thereafter following completion of anthracycline therapy |
| Treatment | New abnormal cardiac biomarkers or significant change from baseline (significant delta for cTn, 100% increase for NPs): • Cardiology (preferably cardio-oncology) consultation • Consider echocardiogram (with strain), other tests as needed • Consider beta-blockers such as carvedilol and nebivolol, and angiotensin converting enzyme inhibitors/angiotensin receptor blockers, if not already prescribed • Anticancer therapy may be continued, if only mild elevation and without significant change in cardiac function or other acute cardiac pathology, recognizing that a decline in cardiac function may not become evident until after cancer therapy New abnormal global longitudinal strain or significant change from baseline (12% to 15% relative change or 3% to 5% absolute change): • Cardiology, preferably cardio-oncology consultation • Consider beta-blockers such as carvedilol and nebivolol, and angiotensin converting enzyme inhibitors/angiotensin receptor blockers, if not already prescribed • Anticancer therapy may be continued if no significant change in cardiac function or other acute cardiac pathology, recognizing that a decline in cardiac function may not become evident until after cancer therapy Asymptomatic decrease in LVEF by >10% to 40% to 49%: • Cardiology, preferably cardio-oncology consultation • Initiation of beta-blockers such as carvedilol and nebivolol, and angiotensin converting enzyme inhibitors/angiotensin receptor blockers, if not already prescribed • Intensification of cardiac surveillance with echocardiogram before each or every other cycle, cTn and NPs after each anthracycline dose, recognizing that a decline in cardiac function may not become evident until after cancer therapy • If further anthracycline-based therapy is planned, risk-benefit discussion and consideration for non-anthracycline-based regimens, use of dexrazoxane, or liposomal doxorubicin Symptomatic heart failure or LVEF <40%: • Cardiology, preferably cardio-oncology consultation • Cardiac function re-assessment and other tests as needed • Initiation and optimization of guideline-directed therapy • Withdrawal of therapy only after a period of stabilization and no active risk factors, and no further anticancer therapy • Hold anti-cancer therapy until careful risk-benefit discussion, seek alternatives to cardiotoxic medications, close surveillance (every cycle) if proceeding, recognizing that a decline in cardiac function may not become evident until after cancer therapy • At least annual review indefinitely Chest radiation therapy exposure: • Evaluation for heart failure, coronary artery, valvular heart, and chronic pericardial disease, starting at 5 y post-treatment and then at least every 3-5 y thereafter |
Integrative approach based on recommendations by American and European cancer societies (see supplemental Table 1).
Translating risk stratification into action in terms of prevention and monitoring of cardiotoxicity was also first outlined in the Mayo Cardiotoxicity Score and then further developed by the HFA of the European Society of Cardiology. According to the HFA model, different levels of cardiac imaging and cardiac biomarker–based surveillance strategies are aligned with different levels of estimated cardiotoxicity risk (Figure 2).10,17,18 Importantly, the illustrated model is for anthracycline cardiotoxicity, and other models must be used for nonanthracycline cardiotoxicity. General models encompassing multiple drugs into 1 score remain challenging given the rapidly changing therapeutic landscape.19 With oncolytics accounting for nearly 30% of new drug approvals, any general model must be continuously reviewed for adaptation to new therapies. Likewise, any cancer agent–specific models need to be refined as new information becomes available.
A number of small- and moderate-sized studies have identified medications that mitigate anthracycline cardiotoxicity (Table 3).10 In patients with hematologic malignancies, carvedilol, a combined α- and β1- and β2-blocker with antioxidant properties, is the only beta-blocker proven to be efficacious for primary prevention of anthracycline cardiomyopathy. The benefit of angiotensin converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) seems to be a class effect (Table 3). Drug–drug interactions are important, and for the third class of cardioprotective agents, statins, pravastatin or rosuvastatin are often chosen because of the lowest potential of interactions with antifungals and other medications regularly used in patients with cancer. Dexrazoxane is a cardioprotective agent that may be considered to prevent cardiotoxicity in patients planned to receive high-dose anthracyclines (eg, doxorubicin ≥250 mg/m2 or epirubicin ≥600 mg/m2).16,20,21 Utilization is generally in unique circumstances (existing cardiomyopathy, prior exposure to anthracyclines, radiation, etc.) and taken in concert with the treating hematologist in situations with curative intent and limited alternative options. Liposomal doxorubicin is another alternative, and tailored approaches have been used in patients with lymphoma.22 Whether anthracyclines other than doxorubicin have a lower risk has not conclusively been demonstrated. Dose reduction, omission, or substitution of doxorubicin, for example, with etoposide, are common alternative approaches.23 Anthracycline exposure is a lifetime measure of all anthracyclines and must be assessed routinely, particularly with ongoing therapy and relapsed disease. Each anthracycline potency must be taken into consideration, and total anthracycline exposure can be evaluated in doxorubicin equivalents to best estimate long-term risk.
Adjunctive pharmacologic strategies for the prevention of chemotherapy-induced cardiotoxicity
| Study . | Year . | Cohort (n) . | Follow-up time . | Cardiotoxic chemotherapy . | Radiation therapy . | Preventive therapy . | Cardiotoxicity definition . | Event rate with vs without previous therapy . |
|---|---|---|---|---|---|---|---|---|
| Beta-blocker | ||||||||
| Kalay et al78 | 2006 | Breast cancer (68%), lymphoma (18%) | 6 mo | Anthracyclines: doxorubicin 520 mg/m2 or epirubicin 780 mg/m2 | 0% | Carvedilol 12.5 mg per day, started before CT and continued for 6 mo | LVEF baseline to 6 mo | Carvedilol: 70.5% to 69.7% Control: 68.9% to 52.3%* |
| Georgakopoulos et al79 | 2010 | HL and NHL (n = 125) | 12 mo | ABVD R-CHOP | 21% | Metoprolol 25-50 mg/BID or Enalapril 2.5-10 mg/BID started with CT and continued | LVEF baseline to 12 mo | Metoprolol: 65.7% to 63.3% Enalapril: 65.2% to 63.9% Control: 67.6% to 66.6% |
| Jhorawat et al80 | 2014 | NHL (n = 44), HL (n = 9), ALL (n = 1) | 6 mo | Doxorubicin 428 and 395 mg/m2 in control and intervention gorup | NA | Carvedilol 12.5 mg per day, started before CT and continued for 6 mo | LVEF baseline to 6 mo Incidence of LVEF decrease >10% from baseline | Carvedilol: 63.2% to 63.9% Control: 67.6% to 60.8%* Carvedilol vs placebo: 14.3% vs 40.9%* |
| Abuosa et al81 | 2018 | Breast (n = 72), NHL (n = 95), other (n = 46) | 6 mo | Doxorubicin 252-282 mg/m2 | NA | Carvedilol 6.25 mg, 12.5 mg, or 25 mg per day, started prophylactically and continued for 6 mo | LVEF baseline to 6 mo Incidence of LVEF decrease to 50% | 62% to 58.2% w/placebo, 61.4% to 60% w/6.25 mg carvedilol, 60% to 58% w/12.5 mg carvedilol, 60.4% to 59.2% w/25 mg carvedilol Carvedilol 12.5 mg vs control 1% vs 11%* |
| ACE inhibitor/ARB | ||||||||
| Nakamae et al82 | 2005 | NHL (n = 40) | Day 3 after initiation | CHOP | 0% | Valsartan 80 mg/d started and continued with CT | LVEDD BNP QTc | 45 vs 49 mm* 30 vs 80 pg/dL* 420 vs 435 ms* |
| Cardinale et al83 | 2006 | HDC (n = 114, 60% NHL and breast cancer) + cTnI > ULN within 3 d of any cycle | 12 mo | Various, cumulative doxorubicin equivalent dose 335 mg/m2 | 11% | Enalapril 2-20 mg per day, started after cTnI elevation and continued in f/u | LVEF decline >10% to <50% HF Arrhythmias | 0% vs 43%* 0% vs 24%* 2% vs 17%* |
| Dessi et al84 | 2011 | Various (n = 49, breast cancer 37%) | 12 mo | Epirubicin 400 mg/m2 | 0% | Telmisartan 40 mg/d, 1 wk before, 6 mo after CT | Strain rate | 1.75 vs 1.5* |
| Beta-blocker ACE inhibitor combination | ||||||||
| Bosch et al. (OVERCOME trial)85 | 2013 | Acute leukemia (n = 36) or HSCT (n = 54) | 6 mo | Anthracyclines (40% before, 40% during, cumulative 265 mg/m2) | 18% | Carvedilol (6.25- 25 mg BID) and Enalapril (2.5-10 mg BID), started 24 h before CT and continued in f/u | LVEF by TTE LVEF by CMR | −0.17 vs −3.28* 0.36 vs −3.04 |
| Statin | ||||||||
| Acar et al86 | 2011 | Various (n = 40) NHL, 60%) | 6 mo | Anthracyclines: doxorubicin 256 mg/m2, idarubicin 297 mg/m2 | NA | Atorvastatin 40 mg per day, started before and continued for 6 mo after CT | LVEF LVEDD LVESD | 1.3 vs −7.9* −0.15 vs 2.0* −1.35 vs 2.1* |
| Study . | Year . | Cohort (n) . | Follow-up time . | Cardiotoxic chemotherapy . | Radiation therapy . | Preventive therapy . | Cardiotoxicity definition . | Event rate with vs without previous therapy . |
|---|---|---|---|---|---|---|---|---|
| Beta-blocker | ||||||||
| Kalay et al78 | 2006 | Breast cancer (68%), lymphoma (18%) | 6 mo | Anthracyclines: doxorubicin 520 mg/m2 or epirubicin 780 mg/m2 | 0% | Carvedilol 12.5 mg per day, started before CT and continued for 6 mo | LVEF baseline to 6 mo | Carvedilol: 70.5% to 69.7% Control: 68.9% to 52.3%* |
| Georgakopoulos et al79 | 2010 | HL and NHL (n = 125) | 12 mo | ABVD R-CHOP | 21% | Metoprolol 25-50 mg/BID or Enalapril 2.5-10 mg/BID started with CT and continued | LVEF baseline to 12 mo | Metoprolol: 65.7% to 63.3% Enalapril: 65.2% to 63.9% Control: 67.6% to 66.6% |
| Jhorawat et al80 | 2014 | NHL (n = 44), HL (n = 9), ALL (n = 1) | 6 mo | Doxorubicin 428 and 395 mg/m2 in control and intervention gorup | NA | Carvedilol 12.5 mg per day, started before CT and continued for 6 mo | LVEF baseline to 6 mo Incidence of LVEF decrease >10% from baseline | Carvedilol: 63.2% to 63.9% Control: 67.6% to 60.8%* Carvedilol vs placebo: 14.3% vs 40.9%* |
| Abuosa et al81 | 2018 | Breast (n = 72), NHL (n = 95), other (n = 46) | 6 mo | Doxorubicin 252-282 mg/m2 | NA | Carvedilol 6.25 mg, 12.5 mg, or 25 mg per day, started prophylactically and continued for 6 mo | LVEF baseline to 6 mo Incidence of LVEF decrease to 50% | 62% to 58.2% w/placebo, 61.4% to 60% w/6.25 mg carvedilol, 60% to 58% w/12.5 mg carvedilol, 60.4% to 59.2% w/25 mg carvedilol Carvedilol 12.5 mg vs control 1% vs 11%* |
| ACE inhibitor/ARB | ||||||||
| Nakamae et al82 | 2005 | NHL (n = 40) | Day 3 after initiation | CHOP | 0% | Valsartan 80 mg/d started and continued with CT | LVEDD BNP QTc | 45 vs 49 mm* 30 vs 80 pg/dL* 420 vs 435 ms* |
| Cardinale et al83 | 2006 | HDC (n = 114, 60% NHL and breast cancer) + cTnI > ULN within 3 d of any cycle | 12 mo | Various, cumulative doxorubicin equivalent dose 335 mg/m2 | 11% | Enalapril 2-20 mg per day, started after cTnI elevation and continued in f/u | LVEF decline >10% to <50% HF Arrhythmias | 0% vs 43%* 0% vs 24%* 2% vs 17%* |
| Dessi et al84 | 2011 | Various (n = 49, breast cancer 37%) | 12 mo | Epirubicin 400 mg/m2 | 0% | Telmisartan 40 mg/d, 1 wk before, 6 mo after CT | Strain rate | 1.75 vs 1.5* |
| Beta-blocker ACE inhibitor combination | ||||||||
| Bosch et al. (OVERCOME trial)85 | 2013 | Acute leukemia (n = 36) or HSCT (n = 54) | 6 mo | Anthracyclines (40% before, 40% during, cumulative 265 mg/m2) | 18% | Carvedilol (6.25- 25 mg BID) and Enalapril (2.5-10 mg BID), started 24 h before CT and continued in f/u | LVEF by TTE LVEF by CMR | −0.17 vs −3.28* 0.36 vs −3.04 |
| Statin | ||||||||
| Acar et al86 | 2011 | Various (n = 40) NHL, 60%) | 6 mo | Anthracyclines: doxorubicin 256 mg/m2, idarubicin 297 mg/m2 | NA | Atorvastatin 40 mg per day, started before and continued for 6 mo after CT | LVEF LVEDD LVESD | 1.3 vs −7.9* −0.15 vs 2.0* −1.35 vs 2.1* |
ABVD, Adriamycin (doxorubicin), bleomycin, vinblastine, and dacarbazine; ALL, acute lymphocytic leukemia; BB, beta-blocker; BID, twice a day; CMR, cardiac magnetic resonance imaging; CT, chemotherapy; cTnI, cardiac troponin I; F/u, follow-up; FS, fractional shortening; HF, heart failure; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; LVESWS, left ventricular end-systolic wall stress; NS, nonsignificant; NT-pro-BNP, N-terminal-probrain natriuretic peptide; QTc, corrected QT interval; TTE, transthoracic echocardiogram; ULN, upper limit of normal.
P < .05.
Back to our patient: case 1
Based on the HFSA-IC-OS model, the patient’s history of cardiomyopathy sufficed to consider her as being at very high risk for a decline in cardiac function with R-CHOP therapy, and she was started on lisinopril and carvedilol. However, she did not tolerate carvedilol, and heart rate control was inadequate. For this reason, metoprolol was reinstituted, and pravastatin was continued. An echocardiogram after 3 of 6 planned cycles showed a drop in her LVEF from 55% to 60% to 35% to 40%, and she developed symptoms of dyspnea on exertion and mild lower extremity edema shortly thereafter. Etoposide was substituted for doxorubicin in the remaining cycles. Repeat staging demonstrated complete remission, and her LVEF improved slowly after 1 year, to 50%, with up-titration of lisinopril and the addition of spironolactone. She has had no recurrence of heart failure symptoms. The patient is alive and has remained free of malignancy 10 years after treatment.
The patient showed a drop in LVEF midway through standard R-CHOP therapy. This may raise concerns for acute anthracycline cardiotoxicity, which has been deemed to be an uncommon (<5%), poorly predictable, toxic myocarditis. It can manifest in an acute reduction of cardiac function and/or atrial and ventricular arrhythmias (usually within 1 week of therapy) with subsequent recovery.5 The role of cardioprotective agents such as carvedilol and ACE inhibitor/ARBs are not as defined and neither is the correlation with chronic anthracycline cardiomyopathy and long-term outcomes. This being said, seminal studies using nuclear multigated acquisition scans indicated that the LVEF response after a cumulative dose of 200 mg/m2 identifies patients with lymphoma at high risk and in need of closer cardiac monitoring (a decline in LVEF of >4% from baseline after 200 mg/m2 has 90% sensitivity for a final decline of >10% to an LVEF <50% after 500 mg/m2).24 These historic data have provided support for surveillance algorithms during anthracycline therapy and reassessment of cardiac function even during CHOP therapy in very high-risk patients as in our case example.25 More modern data with serial echocardiograms indicate that in patients who do develop anthracycline cardiotoxicity a decline in LVEF already emerges over the course/by the end of chemotherapy and becomes fully evident within 3-6 months thereafter.26 Furthermore, as seen in our case, recovery from anthracycline cardiomyopathy is slow and often only partial with the institution of standard heart failure medications.26
In conclusion, case 1 points out the value of considering the end from the beginning (ie, the cardiotoxic risks of cancer therapies even before they are started). Although not robustly validated yet, cardiotoxicity risk stratification models help conceptually to direct preventive strategies and surveillance approaches. Practice guidelines recommend a cardiac function assessment 6 to 12 months after completion of anthracycline-based therapy in high-risk patients such as those undergoing R-CHOP therapy.16,27 Even beyond this time period, patients exposed to anthracyclines remain at risk of progression along the American Heart Association heart failure (HF) stages: stage A, at risk; stage B, asymptomatic cardiac dysfunction; stage C, symptomatic HF; stage D, refractory HF.28 This needs to be integrated into survivorship plans, recognized by all care providers during follow-up, and conveyed to the patient as their own advocate.
Case 2
A now 50-year-old woman was diagnosed with DLBCL 5 years ago and underwent 6 cycles of R-CHOP therapy but relapsed after 2 years. She received 2 more cycles of rituximab-ifosfamide, carboplatin, and etoposide therapy, followed by an autologous HSCT with myeloablative carmustine (BCNU), etoposide, cytarabine, and melphalan conditioning. She had 1 documented episode of atrial fibrillation at the time of BCNU (carmustine), etoposide, cytarabine, and melphalan conditioning. With another relapse after 2 years, she underwent lymphodepleting chemotherapy with fludarabine and cyclophosphamide and now presents for consideration of chimeric antigen receptor (CAR) T-cell therapy with axicabtagene ciloleucel. Dyspnea on exertion and palpitations have persisted since her HSCT.
Atrial fibrillation is a recognized challenge in patients with lymphoid malignancy, especially in the acute active treatment period.29 Thromboembolism and heart failure are tangible risks, and patients with cancer are conceivably more prone to these complications because of procoagulant states and the use of additional cardiotoxic treatments. In the setting of HSCT and CAR T-cell therapy, excessive tachycardia, hypotension, bleeding predisposition, and drug–drug interactions can complicate the care considerably. Prevention of atrial fibrillation is henceforth very appealing, especially as the risk period in HSCT and CAR T-cell therapy is relatively defined and not infinite. It typically emerges during conditioning in HSCT, with melphalan as a recognized stressor (reported incidence of atrial fibrillation, 11%).29 However, atrial fibrillation can also be prompted by pericarditis or other factors in patients undergoing HSCT. Cytokine release is the main trigger for atrial fibrillation with CAR T-cell therapy. Efforts to prevent atrial fibrillation in these settings therefore need to cover the different scenarios of vulnerability. It has been our practice to start patients at high risk for atrial fibrillation with HSCT or CAR T-cell therapy on prophylactic antiarrhythmic therapy. We identify those with a prior episode of atrial fibrillation as being at high risk.29 Other indicators of a predisposition to atrial fibrillation include left atrial enlargement, diastolic dysfunction, and mitral valve regurgitation, all of which can be captured on a pretherapy echocardiogram.29 Long-standing hypertension with or without chronic kidney disease is another important risk factor.29 In general, predisposing factors for atrial fibrillation-related stroke (thromboembolism) risk are captured in the CHA2DS2-VASc (congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, stroke or transient ischemic attack [TIA], vascular disease, age 65-74 years, sex category) score. However, the utility of this risk score for atrial fibrillation risk stratification in patients undergoing CAR T-cell therapy or HSCT has not been determined. In terms of prophylactic antiarrhythmic therapy, the easiest and most efficacious to use for these short-term applications is amiodarone. Concerns for drug–drug interactions and side effects must be considered. This being said, amiodarone toxicities are commonly a function of dose and duration of therapy. No adverse effects and a high degree of efficacy have been demonstrated in patients undergoing cardiac surgery in the Prophylactic Oral Amiodarone for the Prevention of Arrhythmias That Begin Early After Revascularization, Valve Replacement, or Repair (PAPABEAR) trial (10 mg/kg started 6 days prior and continued through 6 days after surgery).30 Although this protocol can be considered in the HSCT and CAR T-cell population, further prospective data are needed to provide guidance for the management of these patients.
Such primary prevention efforts are not taken in patients at long-term risk such as those with CLL on ibrutinib.31 Patients with CLL have a 2 times higher risk of atrial fibrillation, further increased by a factor of 3 to 4 in patients on ibrutinib (average incidence, 8%; range, 3% to 16%).31-35 The Mayo Clinic risk prediction model performs well to predict atrial fibrillation risk in patients with CLL in general and also on ibrutinib.31,36 Age carries 2 or 3 points (65-74 or >74 years), male sex is 1 point, valvular heart disease is 2 points, and hypertension is 1 point.31,36 Based on the sum score (0 to 1, 2-3, 4, or >4), 4 risk categories are assigned, with a doubling of the risk of atrial fibrillation with each category step-up (none, twofold, fourfold, and eightfold increase in risk, respectively). In general, however, patients who develop atrial fibrillation on ibrutinib have either a history of or a predisposition to atrial fibrillation.32,37 Patients at high risk may be advised to be treated with second-generation Bruton tyrosine kinase inhibitors.
The principles of therapy in patients with hematology malignancy who develop atrial fibrillation are the same as in the general population: lenient rate control, rhythm control as needed for symptom and complication management, and anticoagulation. Indeed, the need for anticoagulation is a major complicating factor in the setting of thrombocytopenia and bleeding predisposition.38 Drug–drug interactions, liver, and kidney dysfunction can further amplify the bleeding risk. Judicious use of anticoagulants is therefore advised. In the active acute treatment phases (eg, with HSCT and CAR T-cell therapy), heparin is usually the anticoagulation therapy of choice. For long-term therapy needs, warfarin and direct oral anticoagulants (DOACs) are the main options, the latter becoming increasingly preferred even in patients with cancer given lower bleeding risks at similar if not better therapeutic efficacy. Fluctuations in international normalized ratio levels with warfarin are very problematic and are to be expected in patients with cancer on multiple cycles of chemotherapy with alternating drug–drug interactions and oral uptake patterns because of induction of nausea and other gastrointestinal side effects. Warfarin use is discouraged in patients on ibrutinib because of severe bleeding events, including intracranial hemorrhage in early clinical trials.39 Of note, ibrutinib exerts a unique antiplatelet effect (inhibition of von Willebrand factor, fibrinogen, and collagen-mediated platelet activation), leading to an increased bleeding risk when combined with other antiplatelet agents or anticoagulants.39,40 Furthermore, ibrutinib has the potential to increase the serum levels of all DOACs, especially dabigatran and edoxaban.41,42 Drug–drug interactions and more complicated (and costly) acute reversal scenarios are the main concerns with DOACs. Cardiology referral is recommended when anticoagulation questions arise and especially for decisions on rate vs rhythm control of atrial fibrillation (including cardioversion decisions). Rate control with either a beta-blocker or a calcium channel blocker can usually be started while awaiting the consultation unless there is hemodynamic instability or concerns for intolerance for other reasons. With several rate and rhythm controlling agents available, the cardiovascular comorbidity and drug–drug interaction profile will dictate management decisions. For instance, amiodarone is the drug of choice in patients with heart failure, although particular care needs to be taken, for instance, in patients on ibrutinib. Ibrutinib can increase the plasma levels of amiodarone, carvedilol, digoxin, diltiazem, and verapamil.43 Conversely, amiodarone, diltiazem, and verapamil can increase the plasma levels of ibrutinib several fold.43 Atenolol and metoprolol are therefore preferred first-line agents for rate control in patients with atrial fibrillation on ibrutinib (the latter if there are renal function concerns).43
Back to our patient: case 2
The patient did well with CAR T-cell therapy without atrial fibrillation events on prophylactic amiodarone therapy. Hypertension continued to be well controlled (blood pressure during office visits <130/80 mm Hg). She continued, however, to experience dyspnea on exertion. An exercise oxygen consumption echocardiographic stress 1 year after CAR T-cell therapy and 3 years after HSCT showed mild generalized LV hypokinesis and an LVEF of 50% at rest (57% before CAR T-cell therapy) but increasing to 55% with stress without regional wall motion abnormalities. Her exercise capacity was confirmed to be severely limited to 4.7 metabolic equivalents and a peak VO2 of 14.1 mL/min per kilogram at near maximal cardiometabolic effort (peak respiratory exchange ratio 1.1), corresponding to 51% of predicted functional aerobic capacity. The patient was started on spironolactone and referred for cardio-oncology rehabilitation, covering not only physical activity but lifestyle and cardiovascular risk factor awareness and control in general.
As previously outlined by Bhatia et al44 in this series, patients after HSCT have a twofold increased risk of developing hypertension, diabetes, and hyperlipidemia, an at least twofold increased risk of developing CVDs, especially arterial disease and HF, and a two- to fourfold increased cardiovascular mortality.44-47 Indeed, cardiovascular disease is 1 of the leading causes of nonrelapse mortality after HSCT. Attention to signs and symptoms of CVD, especially coronary artery disease (CAD) and HF (eg, chest pain, dyspnea, reduction in exercise tolerance, fluid retention/edema/weight gain, inability to lay supine), as well as CV risk factors such as hypertension, diabetes, and hyperlipidemia should therefore be given during survivorship. Aspired goals for CV risk factors include a blood pressure of <130/80 mm Hg, ideally <120/80 mm Hg, hemoglobin A1c level <6.5%, and low-density lipoprotein level <100 mg/dL. The impact of CV risk factors cannot be overemphasized; not only do they increase the risk of CAD and ischemic cardiomyopathy but also of nonischemic cardiomyopathy in interaction with anthracyclines.48-50 Pre-HSCT ejection fraction <50%, conditioning with high-dose cyclophosphamide, and total body radiation are risk factors for HF after HSCT, as well as the total lifetime dose exposure to anthracyclines. Doxorubicin equivalent exposure of 250 mg/m2 or more increases the HF risk 10-fold; in combination with diabetes, this risk is increased 27-fold and in combination with hypertension is 35-fold. Most importantly, HF development after HSCT carries a 50% 5-year mortality risk.51,52 The goal is henceforth to identify and change any trajectory in this direction early and effectively. Although the current standard resting echocardiograms do not fulfill this goal, exercise testing can unmask abnormalities much earlier. In particular, oxygen consumption exercise testing with echocardiography allows for the most comprehensive noninvasive evaluation of functionally relevant CAD, cardiomyopathy, pulmonary capacity, and general fitness/conditioning.
Peak VO2 on oxygen consumption studies reflects peak exercise capacity (ie, the maximum capacity of the pulmonary and cardiovascular system to take up and deliver oxygen to exercising skeletal muscle groups and their extraction of oxygen from the blood). It is determined by pulmonary gas exchange (ventilation and diffusion capacity), cardiovascular performance (cardiac output and vascular function), and skeletal muscle metabolism. Peak VO2 is 1 of the best predictors for cardiovascular and overall mortality in adults.53 Importantly, a reduction in peak VO2 is not uncommon in patients with lymphoma who have undergone HSCT. Approximately 20% and 30% of these patients will have a reduction in peak VO2 by 20% and <16 mL/min per kilogram, respectively, on average 10 years after HSCT.54-56 The impairment is more pronounced after allogenic versus autologous HSCT and with myoablative versus reduced-intensity conditioning; peak VO2 is lowest in those with graft-versus-host disease.54 On the contrary, a high level of physical activity seems to prevent the progressive decline in functional aerobic capacity, which is seen with increasing cumulative doses of doxorubicin otherwise.54 Smoking and impaired diffusion capacity are additional factors associated with reduced exercise capacity.56 As illustrated in the example of our patient, resting LVEF is not a good measure of functional aerobic capacity; on the contrary, GLS shows a significant, although still only moderate, correlation (r = −0.67, P < .01).54 GLS is a measure of deformation of the myocardium during the cardiac cycle, expressing the change in length of a segment as a proportion (percentage) to baseline (longitudinal shortening). It is a more sensitive and more reproducible measure of cardiac (systolic) function than ejection fraction and generally precedes and predicts a decline in ejection fraction in anthracycline-treated patients.
Accordingly, echocardiography in combination with either GLS or an oxygen consumption exercise study should be the preferred testing modality after HSCT. Patients with lymphoid malignancy and cardiac surveillance abnormalities, concerns, or uncertainties should be referred for a cardio-oncology consultation. This includes those with a normal LVEF, bearing in mind that its definition remains a matter of debate. Although 50% has been the traditional cutoff for LVEF, the American Society of Echocardiography document on multimodality imaging of patients with cancer defines an LVEF value of 53% as the lower limit.57 Invasive hemodynamic studies are at times required to further address concerns for diagnoses such as HF with preserved ejection fraction. The role for beta-blocker and ACE inhibitor/ARB therapy is not as defined in these patients. Spironolactone is the only medication that has shown some therapeutic promise in clinical trials and most recently sodium glucose co-transporter-2 inhibitors.58-60 Last, but not least, especially in symptomatic patients with a reduced functional capacity such as the 1 in the case presentation, referral to a cardio-oncology rehabilitation program (as well as exercise efforts and weight reduction) is important.53,61
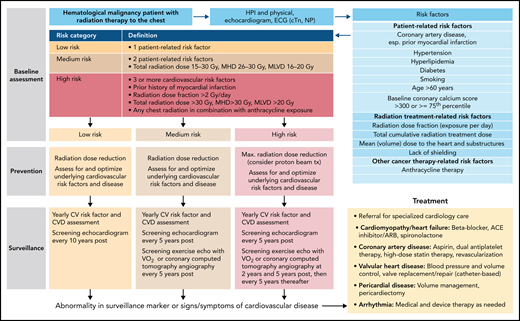
In summary, case 2 illustrates the cardiovascular toxicities challenges seen in patients undergoing HSCT, early and late.44,46 A risk stratification model for late effects is available, and a management outline is proposed in Figure 3.62 Control of risk factors is extremely important to prevent both arterial diseases and HF in these patients.51 Changes can be subtle, not seen on resting evaluation, but are unmasked when the cardiovascular system is challenged.63
Outline of a risk-based management approach to HSCT. Outline based on Armenian et al.62 ASCVD, atherosclerotic cardiovascular disease; cTn, cardiac troponin; ECG, electrocardiogram; GI, gastrointestinal; GU, genitourinary; HTN, hypertension; LMWH, low molecular weight heparin.
Outline of a risk-based management approach to HSCT. Outline based on Armenian et al.62 ASCVD, atherosclerotic cardiovascular disease; cTn, cardiac troponin; ECG, electrocardiogram; GI, gastrointestinal; GU, genitourinary; HTN, hypertension; LMWH, low molecular weight heparin.
Case 3
A now 56-year-old woman underwent radiation therapy to the chest and chemotherapy (mechlorethamine, vincristine, procarbazine, and prednisone/Adriamycin (doxorubicin), bleomycin, vinblastine, and dacarbazine) for Hodgkin lymphoma in 1986, at age 25. Twenty-six years later, in 2012, she underwent mechanical aortic valve replacement and coronary bypass graft surgery. Thereafter she has been maintained on warfarin, metoprolol 50 mg twice a day, atorvastatin 20 mg/day for hyperlipidemia, and sitagliptin-metformin 50 to 1000 mg twice a day for type 2 diabetes mellitus. Dyspnea on exertion has been a gradually progressive symptom over the last 5 years. She now presents to the emergency room with sudden onset of lightheadedness and a feeling of general unwellness. Shortly after arrival, she loses consciousness. Cardiac monitoring demonstrates a heart rate of 20 to 30 beats per minute in the setting of a new complete heart block. The patient is successfully resuscitated and admitted to the cardiac care unit with transcutaneous pacing in place.
An increased risk of a broad spectrum of CVDs can be seen over decades after mediastinal/chest radiation (eg, for lymphoma) with life-threatening implications. In this patient population, CV mortality is 7 times higher than expected, and CVDs remain a leading cause of long-term mortality next to secondary malignancies.64 The overall frequency and timing of clinically evident CVD may vary, to some degree, by disease entity and age at exposure. CAD and valvular heart disease (VHD) show a higher overall incidence burden, and time-to-event curves are steeper in older individuals (ie, shorted latency to events). This is even more so the case when additional CV risk factors are present, which accelerate disease development. Our patient had hyperlipidemia and diabetes, 2 very potent risk factors for CAD disease. Their control is paramount and should be checked at least annually during survivorship visits in the hematology clinic and/or by the patient’s primary care provider. Other controllable risk factors include hypertension and smoking. An inquiry for lifestyle habits should be made and vital signs should be taken during each follow-up visit. The CV risk factors goals are as outlined for case 2.
Importantly, the risk of arterial events in patients with lymphoid malignancies is likely dynamic and not static. The risk of acute arterial thromboembolic events shows an early peak around the time of diagnosis of NHL and then to gradually decrease over 2 years.65 However, a long-term perspective needs to be taken, and the risk of arterial events can be expected to increase again after the early malignancy period, eg because of exposure to radiation therapy. A decade has been the conventional standard for latency of events; however, some patients may experience cardiovascular events much sooner. In fact, the risk of clinical CAD emerges right after chest radiation therapy and continuous to rise thereafter in patients with lymphoma who were 35 years and older at the time of diagnosis and treatment. The American Society of Echocardiography algorithm indicates that a functional stress test can be performed 5 to 10 years after exposure in high-risk patients (ie after anterior or left sided chest radiation with 1 or more additional risk factors: high [>30 Gy total or >2 Gy/day] radiation exposure, lack of shielding, tumor in or next to the heart, concomitant chemotherapy, CV risk factors, or preexisting CVD, or age <50) and every 5 years thereafter. The Society for Cardiovascular Angiography and Interventions (SCAI) algorithm expands on this with an additional stress test at 2 years after radiation therapy in patients with CV risk factors including age > 60 years, known CAD, or additional vasotoxic drug exposure (eg, cyclophosphamide). Furthermore, the SCAI algorithm recommends an exercise echocardiogram with an oxygen consumption study. For VHD, an initial evaluation 5 years after chest radiation therapy suffices with repeats every 5 years. It is expected to see more left-sided valve disease, more aortic than mitral valve disease, and more aortic regurgitation than aortic stenosis with longer follow-up.
Patients are often not aware of or are not volunteering signs and symptoms of cardiopulmonary disease, providing an educational opportunity at each follow-up visit.66 Standardized questionnaire-based assessments have revealed a higher-than-expected disease burden in lymphoma survivors after chest radiation therapy, mainly (exertional) fatigue and dyspnea on exertion. Abnormalities in the electrocardiogram (ECG) are not uncommon but commonly not assessed or overlooked. Key abnormalities include a higher resting heart rate, which can be indicative of autonomic dysfunction (vs other etiologies such as thyroid dysfunction, anemia, or hypoxemia). Abnormal heart rate recovery after exercise (defined as ≤12 beats/min if active cool-down or ≤18 beats/min if passive recovery), as another reflection of autonomic dysfunction, is a potent predictor of an increased mortality risk. Other changes on the ECG include conduction abnormalities such as bundle branch block and atrioventricular block. Patients with a more than simple block pattern (eg, bifascular block or greater than first-degree atrioventricular block) should be considered at increased risk. A 12-lead ECG is therefore a very useful, low-cost test to add on during routine annual follow-up visits. Exercise testing and oxygen consumption testing, as described before, can be easily added to any planned echocardiographic follow-ups and can provide important additional information. Finally, noting calcification on the aorto-mitral curtain on echocardiographic surveillance can indicate increased risk for degeneration of the atrioventricular nodal area and risk of complete heart block. In fact, calcifications of cardiovascular structures, and the progression thereof, on routine restaging or surveillance computed tomography–positron emission tomography scans are readily available information for risk determination easily integrated.
Back to our patient: case 3
Echocardiographic evaluation showed calcification of the aorto-mitral curtain and a left ventricular ejection fraction in the range of 25% to 30%, a decline from 55% 7 months prior. Coronary angiography showed a 60% left main stenosis and 80% ostial right coronary stenosis with a patent left internal mammary graft to the left anterior descending artery and a patent sequential venous bypass to the first obtuse marginal branch and the distal right coronary artery. The hemodynamic catheterization revealed severely elevated right atrial and ventricular pressures and pulmonary artery and pulmonary wedge pressures. Additional hemodynamic assessments were indicative of restriction but not constriction. Cardiac output was normal at a heart rate of 120 beats/min on norepinephrine. The patient underwent dual chamber pacemaker implantation, was started on heart failure medications, and was listed for heart transplantation. Her cardiac function did not improve, and her pacemaker was upgraded to a cardiac resynchronization therapy-defibrillator device. Unfortunately, she experienced progressive HF and died before receiving a heart transplant.
HF is the “final common pathway” in the presentation of radiation-induced heart disease. Systolic dysfunction per se is less common and usually indicative of additional injury such as anthracycline exposure or the consequences of other radiation-induced disease processes such as CAD and VHD. VHD severe enough to lead to HF is usually very evident on echocardiography. CAD may reflect in regional wall motion abnormalities, but usually an invasive coronary angiography is needed to fully define its extent.67 As illustrated in this case, a cardiac catheterization is very helpful in defining the hemodynamics. The classical finding of radiation cardiomyopathy is that of restriction, which needs to be differentiated from constriction (which can also develop after radiation) as the management implications are different.68 Indeed, pericardiectomy can be life-changing in patients with chronic constrictive pericarditis but will be neutral at best in others. Even when indicated, the timing of such intervention is very important (not too soon and not too late). Evaluation for constriction vs restriction should start early during routine echocardiograms, which become the 1-stop evaluation tool for patients after radiation therapy. Societal recommendations are that evaluation for the various forms of radiation-induced heart disease should start 5 years after completion of chest radiation and should continue every 5 years therafter.68,69
Patients with abnormalities on screening or concerns should be referred to a cardiologist. The management of HF in patients with lymphoid malignancy follows general societal guidelines, with beta-blocker, ACE inhibitor/ARB, mineralocorticoid antagonist, and neprilysin inhibitor as therapies with proven mortality benefit. Diuretics are used for volume management. Furthermore, in select patients with a reduced heart function (≤35% LVEF), a wide QRS (mainly left bundle branch block), and functional class II to IV dyspnea on exertion, cardiac resynchronization therapy should also be considered.70 Cardiac resynchronization therapy is also indicated in selected patients with LVEF between 35% and 50% who are anticipated to require frequent ventricular pacing or who have a QRS ≥ 150 ms with left bundle branch block and refractory HF. Cancer survivors derive the same benefit as patients without cancer from implantable cardioverter defibrillators for either primary or secondary prevention.71
Mechanical circulatory support (eg, left ventricular assist device [LVAD]) and heart transplantation should be considered early for patients expected to have a poor prognosis. This includes those with radiation-induced heart disease because therapeutic options are limited, other than addressing what can be done interventionally (including stenting, coronary artery bypass, valve replacement, pericardiectomy, or device therapy). Outcomes of cancer survivors with severe progressive cardiotoxicity with LVAD and heart transplantation support the use of these options.72-74 With LVAD therapy, patients with anthracycline cardiomyopathy more frequently show signs of and require support for RV failure.74 In these patients and in those after chest radiation, pulmonary pressure and RV function should be evaluated carefully. LVAD can serve as a bridge to transplantation and on occasion to recovery, or be pursued as destination therapy.75 Current guidelines exclude patients who are within 5 years of either a solid tumor or a hematologic malignancy from advanced mechanical circulatory support therapies. Beyond this time frame, studies support outcomes of pediatric and adult survivors of cancer comparable to patients without cancer. Relapse and secondary malignancy with immune suppression have been the main concerns with heart transplantation.73
Taken together, case 3 outlines the broad and fatal spectrum of radiation-induced heart disease, and the best treatment is primary prevention.76 Over the last decades, the risk has been reduced with the use of involved field and involved node radiation therapy, as well as proton beam therapy. The management approach (Figure 4) includes a baseline assessment, primary preventive efforts, and structured cardiac surveillance.77 Early recognition and timely intervention are important. The relentless character of radiation-induced heart disease, however, can culminate in considerations of advanced HF therapies and heart transplantation as a last resort.
Outline of a risk-based management approach to radiation-induced heart disease. See Iliescu et al68 and Araujo-Gutierrez et al.72 cTn, cardiac troponin; ECG, electrocardiogram; MHD, mean heart dose; MLVD, mean left ventricular dose.
Conclusions
Patients with lymphoid malignancies are at risk of toxicities from cancer therapies. Herein we focused on the treatment modalities of greatest concern for cardiovascular toxicities, not only acutely but even more so long term. This chronicity aspect calls for interaction across the various disciplines so that these patients would be optimally treated throughout their cancer continuum, including and especially in the survivorship stage. Recognition of risk starts before the initiation of therapy and continues thereafter. Acute complications and acute decompensations of chronic cardiovascular comorbidities are of concern during the active treatment phase of lymphoid malignancies. The greatest challenge, however, remains cost-effective long-term surveillance and interventions. Studies, including randomized controlled trials, are needed to build the knowledge base in this area. The cardiovascular care of patients with lymphoid malignancies will remain important in the years to come and calls for close interaction between cardiologists and hematologists and even general internist and primary care providers.
Acknowledgments
This work was supported by the National Institutes of Health, National Cancer Institute (grant CA233610), and the Miami Heart Foundation.
Authorship
Contribution: J.H. conceptualized and wrote the manuscript; and K.B.M. and T.M.H. provided critical revisions of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Joerg Herrmann, Department of Cardiovascular Diseases, Mayo Clinic, 200 First St SW, Rochester, MN 55902; e-mail: herrmann.joerg@mayo.edu.
The online version of this article contains a data supplement.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal