Abstract
Infections are a common cause of morbidity and mortality in patients with lymphoid cancer. Because cancer therapeutics, including new targeted therapies and immunotherapies, are evolving, clinicians need to be aware of additional risk factors and infections that may arise in patients treated with these agents. This article highlights fundamental issues in treating patients with lymphoid cancer, including risk factors for infection, screening for infectious diseases, and recommendations for antimicrobial prophylaxis in patients with lymphoid cancers. We present 4 scenarios of patients with lymphoid cancers who have various infections, and we describe a treatment approach based on a combination of evidence-based data and experience because objective data are limited regarding infections, especially with newer agents. The goal of this discussion is to provide a framework for institutions and health care providers to help them develop their own approach to preventing and treating infections in patients with lymphoid cancer.
Introduction
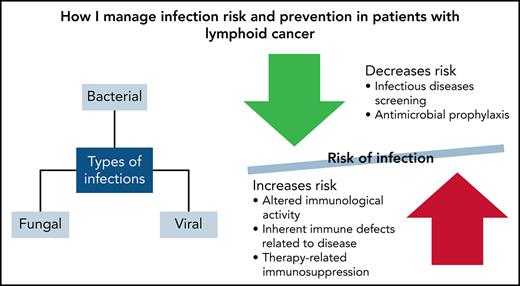
The World Health Organization classifies more than 100 types and subtypes of lymphoid, histiocytic, and dendritic neoplasms.1 The most common of these is lymphoma, which accounts for ∼4% of all cancers in the United States.2 Infections remain a common cause of morbidity and mortality because of altered immunologic activity and inherent immune defects related to the primary lymphoid cancer.3 Chemotherapy, immunologic therapies, and steroids can also cause therapy-related immunosuppression resulting in neutropenia, abnormal cell-mediated immunity, and immune defects.3
Table 1 displays the inherent immune defects associated with common lymphoid cancers that contribute to risk of infection.
Table 1. Infection risk associated with disease-related inherent immune defects
| Disease . | Disease-related inherent immune defects . |
|---|---|
| Chronic lymphocytic leukemia |
|
| Multiple myeloma |
|
| Hairy cell leukemia |
|
| Hodgkin lymphoma |
|
| Non-Hodgkin lymphoma |
|
| Disease . | Disease-related inherent immune defects . |
|---|---|
| Chronic lymphocytic leukemia |
|
| Multiple myeloma |
|
| Hairy cell leukemia |
|
| Hodgkin lymphoma |
|
| Non-Hodgkin lymphoma |
|
EBV, Epstein-Barr virus; NK, natural killer.
Treatment-induced neutropenia increases the risk of infections in patients with lymphoma and other cancers. The mortality rate with febrile neutropenia (FN) can be as high as 50% in the setting of severe sepsis or septic shock.4-6 The American Society of Clinical Oncology (ASCO) and the Infectious Diseases Society of America (IDSA) published guidelines in 2018 to help guide the use of antibiotic, antifungal, antiviral, and Pneumocystis jirovecii pneumonia (PJP) prophylaxis.7
Antibacterial and antifungal prophylaxis is recommended for patients who are at high risk of infection, such as those who are expected to have profound protracted neutropenia defined as <100 neutrophils per microliter for 7 days or other risk factors.7 Most centers will stop antibacterial prophylaxis when the neutropenia has resolved or, for patients who develop FN, when empiric antibiotics are started. Emerging data suggest that routine use of antimicrobial prophylaxis may not be required for patients with lymphoma.8,9 International guidelines recommend the use of prophylactic granulocyte colony-stimulating factor for mitigating chemotherapy-induced neutropenia when using a regimen associated with FN in >20% patients, when dose-dense or dose-intense chemotherapy strategies have survival benefits, or if reductions in dose intensity or density are known to be associated with a poor prognosis.10-13
Antiviral prophylaxis against herpesviruses is recommended in certain situations. Herpes simplex virus (HSV) prophylaxis is recommended for HSV-seropositive patients undergoing chemotherapy for acute leukemia.11 Patients who received allogeneic stem cell transplantation (allo-SCT) and developed graft-versus-host disease (GVHD) or received immunosuppressive treatment, including steroids, may also require HSV prophylaxis.11 Leukemic patients and SCT candidates and recipients should be informed about how varicella zoster virus (VZV) is transmitted and should be educated on how to avoid exposure.11 Family members, household contacts, and health care workers known to be VZV seronegative or children without a history of VZV infection should be given varicella vaccine.11 Seronegative individuals who may be in contact with the patient during transplantation should be vaccinated >4 weeks before conditioning starts.11
With the development of targeted therapy, key components involved in normal immune homeostasis or cell cycle control are blocked, which leads to impaired immune function and increased risk of infection.4 Targeted therapies may have an impact on both innate and adaptive immunity and may affect responses to acute infection and control of latent or chronic infections.5 Because more novel agents and immune-based therapies are being used to treat cancer, infectious complications have diversified, which has required us to broaden our differential to include fungal and viral infection in the absence of neutropenia.1 For example, 1 study of patients with lymphoid cancer who received ibrutinib during a 5-year period found serious infections in 43 of 378 patients with chronic lymphocytic leukemia (CLL) and mantle cell lymphoma.3 New guidelines from the National Comprehensive Cancer Network (NCCN) include evaluations of targeted therapies regarding risk of infection, and they propose that prophylaxis be considered.12Table 2 describes some of the new agents used to treat lymphoid malignancies, their associated infectious risks, and considerations for prophylaxis.
Table 2. Infection risk associated with therapy-related immunosuppression
| Therapy-related immunosuppression . | Infection risk . | Considerations . | |
|---|---|---|---|
| Chronic lymphocytic leukemia | CD52 target (eg, alemtuzumab) | Fungal, HSV, VZV, CMV, listeria, BK, PML, and TB |
|
| Purine-analog (eg, fludarabine and cladribine) | Fungal, PJP, HSV, and VZV |
| |
| Bruton-kinase inhibitors (eg, Ibrutinib and alcalabrutinib) | Fungal, PJP, and PML |
| |
| Phosphatidylinositol-3 kinase (PIK3) inhibitors (eg, copanlisib and idelasilib) | Fungal, PJP, CMV, and PML |
| |
| CD20 target (eg, rituximab, ofatumumab, and obinatuzumab) | HBV, HCV, VZV, PML, neutropenia, and Low IgG |
| |
| Multiple myeloma | Ubiquitin-proteasome pathway inhibitor (eg, bortezomib, ixazomib, and carfilzomib) | Pneumonia, influenza, and VZV |
|
| Immunomodulatory drugs (eg, thalidomide, lenalidomide, and pomalidomide) | No clear increased infection risk from drug |
| |
| CD38 target (eg, daratumumab) | Neutropenia, VZV, PJP, and low IgG |
| |
| SLAMF7, CD319 target (eg, elotuzumab) | VZV and PJP |
| |
| Hairy cell leukemia | Purine-analog (eg, fludarabine and cladribine) | Fungal, PJP, HSV, and VZV |
|
| CD20 target (eg, rituximab, ofatumumab, and obinatuzumab) | HSV, VZV, and PJP |
| |
| Moxetumomab pasudotox | HSV, VZV, and PJP |
| |
| Vemurafenib | No clear increased infection risk from drug |
| |
| Hodgkin lymphoma | CD30 target (eg, brentuximab) | HSV, CMV, PJP, PML, and neutropenia |
|
| mTOR inhibitors (eg, everolimus and sirolimus) | VZV, HBV, HCV, PJP, PML, and TB |
| |
| Checkpoint inhibitors (eg, nivolumab and pembrolizumab) | No clear increased infection risk from drug, but the drug leads to immune upregulation, which can necessitate steroids |
| |
| Non-Hodgkin lymphoma | CD52 target (eg, alemtuzumab) | Fungal, HSV, VZV, CMV, listeria, BK, PML, and TB |
|
| CD19 directed (eg, axicabtagene ciloleucel and tisagenlecleucel) | Bacterial, fungal, HSV, HBV, PJP, and low IgG |
| |
| CD20 target (eg, rituximab, ofatumumab, and obinatuzumab) | HBV, HCV, VZV, PML, neutropenia, and Low IgG |
| |
| Bruton-kinase inhibitors (eg, Ibrutinib and alcalabrutinib) | Fungal, PJP, and PML |
| |
| BCL-2 inhibitor (eg, venetoclax) | VZV and PJP |
| |
| Ubiquitin-proteasome pathway inhibitors (eg, bortezomib, ixazomib and carfilzomib) | Pneumonia, influenza, and VZV |
| |
| mTOR inhibitors (eg, everolimus and sirolimus) | VZV, HBV, HCV, PJP, PML, and TB |
| |
| PI3K inhibitors (eg, copanlisib and idelasilib) | Fungal, PJP, PML, and CMV |
| |
| Checkpoint inhibitors (eg, nivolumab and pembrolizumab) | No clear increased infection risk from drug, but the drug leads to immune upregulation, which can necessitate steroids |
| |
| ACV, acyclovir; BK, BK virus; CMV, cytomegalovirus; HBV, hepatitis B virus; IgG, immunoglobulin G; PML, progressive multifocal leukoencephalopathy; TB, tuberculosis.*Paucity of prospective data to inform institutional practices.†Per NCCN guidelines. |
| Therapy-related immunosuppression . | Infection risk . | Considerations . | |
|---|---|---|---|
| Chronic lymphocytic leukemia | CD52 target (eg, alemtuzumab) | Fungal, HSV, VZV, CMV, listeria, BK, PML, and TB |
|
| Purine-analog (eg, fludarabine and cladribine) | Fungal, PJP, HSV, and VZV |
| |
| Bruton-kinase inhibitors (eg, Ibrutinib and alcalabrutinib) | Fungal, PJP, and PML |
| |
| Phosphatidylinositol-3 kinase (PIK3) inhibitors (eg, copanlisib and idelasilib) | Fungal, PJP, CMV, and PML |
| |
| CD20 target (eg, rituximab, ofatumumab, and obinatuzumab) | HBV, HCV, VZV, PML, neutropenia, and Low IgG |
| |
| Multiple myeloma | Ubiquitin-proteasome pathway inhibitor (eg, bortezomib, ixazomib, and carfilzomib) | Pneumonia, influenza, and VZV |
|
| Immunomodulatory drugs (eg, thalidomide, lenalidomide, and pomalidomide) | No clear increased infection risk from drug |
| |
| CD38 target (eg, daratumumab) | Neutropenia, VZV, PJP, and low IgG |
| |
| SLAMF7, CD319 target (eg, elotuzumab) | VZV and PJP |
| |
| Hairy cell leukemia | Purine-analog (eg, fludarabine and cladribine) | Fungal, PJP, HSV, and VZV |
|
| CD20 target (eg, rituximab, ofatumumab, and obinatuzumab) | HSV, VZV, and PJP |
| |
| Moxetumomab pasudotox | HSV, VZV, and PJP |
| |
| Vemurafenib | No clear increased infection risk from drug |
| |
| Hodgkin lymphoma | CD30 target (eg, brentuximab) | HSV, CMV, PJP, PML, and neutropenia |
|
| mTOR inhibitors (eg, everolimus and sirolimus) | VZV, HBV, HCV, PJP, PML, and TB |
| |
| Checkpoint inhibitors (eg, nivolumab and pembrolizumab) | No clear increased infection risk from drug, but the drug leads to immune upregulation, which can necessitate steroids |
| |
| Non-Hodgkin lymphoma | CD52 target (eg, alemtuzumab) | Fungal, HSV, VZV, CMV, listeria, BK, PML, and TB |
|
| CD19 directed (eg, axicabtagene ciloleucel and tisagenlecleucel) | Bacterial, fungal, HSV, HBV, PJP, and low IgG |
| |
| CD20 target (eg, rituximab, ofatumumab, and obinatuzumab) | HBV, HCV, VZV, PML, neutropenia, and Low IgG |
| |
| Bruton-kinase inhibitors (eg, Ibrutinib and alcalabrutinib) | Fungal, PJP, and PML |
| |
| BCL-2 inhibitor (eg, venetoclax) | VZV and PJP |
| |
| Ubiquitin-proteasome pathway inhibitors (eg, bortezomib, ixazomib and carfilzomib) | Pneumonia, influenza, and VZV |
| |
| mTOR inhibitors (eg, everolimus and sirolimus) | VZV, HBV, HCV, PJP, PML, and TB |
| |
| PI3K inhibitors (eg, copanlisib and idelasilib) | Fungal, PJP, PML, and CMV |
| |
| Checkpoint inhibitors (eg, nivolumab and pembrolizumab) | No clear increased infection risk from drug, but the drug leads to immune upregulation, which can necessitate steroids |
| |
| ACV, acyclovir; BK, BK virus; CMV, cytomegalovirus; HBV, hepatitis B virus; IgG, immunoglobulin G; PML, progressive multifocal leukoencephalopathy; TB, tuberculosis.*Paucity of prospective data to inform institutional practices.†Per NCCN guidelines. |
ACV, acyclovir; CMV, cytomegalovirus; HBV, hepatitis B virus; HSV, herpes simplex virus; IgG, immunoglobulin G; PJP, Pneumocystis jirovecii pneumonia; PML, progressive multifocal leukoencephalopathy; TB, tuberculosis; VZV, varicella-zoster virus.
Current strategies to prevent or treat infection
Case 1: Clostridioidesdifficile and CMV infection
A 21-year-old woman presented with abdominal pain and was found to have free air by abdominal radiograph. At surgery, a gastric perforation was repaired; pathology revealed classic Hodgkin disease. She was treated with brentuximab vedotin, doxorubicin, vinblastine, and dacarbazine; 1 course was complicated by FN (which was treated with cefepime) and diarrhea. She was diagnosed with Clostridioides difficile infection (CDI) and was treated with oral vancomycin. Because of persistent gastrointestinal (GI) symptoms, CMV polymerase chain reaction (PCR) was performed, and she was found to have CMV viremia, with a PCR level of 1200 IU/mL. She received treatment with ganciclovir and was transitioned to valganciclovir once the diarrhea resolved.
CMV reactivation is common among patients with lymphoid malignancies, including those who receive therapy with brentuximab vedotin, and occurs most frequently between 3 and 6 weeks after initiation of therapy when T-cell counts reach a nadir.13-16 The most relevant risk factors seem to be advanced disease, poor performance status, CD34+ selected autografts, total body irradiation, and treatment with alemtuzumab, fludarabine, bortezomib, rituximab, or high-dose steroids.17 Routine CMV screening is not necessary in most patients with lymphoma. However, it is important to consider CMV infection and perform testing in patients with risk factors and unexplained fever, cytopenias, lung infiltrates, or GI symptoms.
Preemptive therapy or treatment of asymptomatic CMV viremia is not generally recommended in patients with lymphoid malignancies, with the exception of high‐risk patients such as those with hematologic malignancies who are undergoing allo‐SCT, high‐risk patients undergoing autologous SCT (auto-SCT), patients with acute or chronic GVHD, those who require higher doses of steroids and other immunosuppressive drugs, and those treated with alemtuzumab.18-20 For patients with viremia who have no evidence of end organ disease, preemptive therapy is warranted: at least 2 weeks of induction therapy or treatment until CMV viral load by PCR is below a specific lower limit of quantitation followed by an additional 2 weeks of maintenance therapy. To prevent recurrent reactivation of CMV, routine surveillance using PCR or antigen-based methods once per week during therapy and for at least 2 months after completion of treatment is recommended.
IV ganciclovir or oral valganciclovir are the drugs of choice for treating CMV infections. The oral formulation of valganciclovir is preferred, which may prevent or reduce hospital stays and minimize the infectious and vascular complications associated with IV therapy.21 However, if there is concern for GI or other end organ disease, IV ganciclovir is preferred. IV foscarnet is an option in patients with severe cytopenias, despite its less-than-optimal safety profile and the limited efficacy data for treating CMV infection in settings other than allo-SCT.19 For tissue invasive CMV disease, at least 3 weeks of induction therapy or treatment until CMV viral load is below the lower limit of quantitation is indicated. Optimal duration of maintenance therapy depends upon the organ involved and the degree of ongoing immunosuppression.20-23
Several agents have been studied for CMV prophylaxis. IV ganciclovir and oral valganciclovir have been tested in several randomized trials, all showing a reduction in the risk of CMV disease compared with placebo but not improved survival.24-27 Ganciclovir given at engraftment causes prolonged neutropenia, which leads to more invasive bacterial and fungal infections. Foscarnet prophylaxis is associated with dose-dependent renal toxicity and electrolyte abnormalities.28-30
Letermovir (AIC-246), a CMV terminase inhibitor, is another highly selective anti-CMV agent.31 Letermovir is not myelotoxic or nephrotoxic and does not require dose adjustments for renal or mild to moderate hepatic dysfunction. It is available in oral and IV formulations.32 In a phase 3 trial with patients who were CMV seropositive after allo-SCT, prophylaxis with letermovir decreased the rate of CMV infection.33,34 All-cause mortality was decreased by week 24 after allo-SCT, but statistical significance was lost by week 48.33,34 Randomized studies of letermovir outside the allo-SCT setting have not yet been published. CMV resistance to letermovir has emerged in both experimental and clinical settings.18 The low genetic barrier for letermovir resistance and the risk of breakthrough infections indicates that physicians should be cautioned against using it during infections associated with high levels of viral replication.18
Maribavir is currently being investigated as a prophylactic drug for CMV. A randomized, placebo-controlled dose-ranging phase 2 study in SCT recipients showed significantly lower risk of CMV infection and borderline reduction of CMV disease compared with placebo with some GI toxicity but no myelotoxicity.35-37 The medication has in vitro activity against ganciclovir- or cidofovir-resistant CMV, and small case series suggest a possible clinical benefit at higher doses.38 However, along with another novel antiviral agent (brincidofovir) and a DNA vaccine (ASP113), maribavir failed to improve the CMV-related outcomes in phase 3 prophylaxis trials.32,39,40 Maribavir interacts with other drugs, for example, with inhibitors of the cytochrome P450 3A4 (CYP3A4) system.41,42
CDI is a leading cause of infectious complications in allo-SCT recipients. In a study of lymphoma patients who had been discharged, CDI was present in 2.13% of those with lymphoma and 0.8% of those without lymphoma (P < .001).43 The significant predictors were infection, SCT, GVHD, race, chemotherapy, GI surgery, and Charlson Comorbidity Index score.43 CDI in lymphoma was associated with worse hospital outcomes, including increased mortality, increased length of stay, mean total hospital charges, rate of intubation, and rate of total parenteral nutrition.43
Clostridioides difficile is spread from person to person through the fecal-oral route.44,45 The incidence of Clostridioides difficile can be decreased by limiting the use of antibiotics through antibiotic stewardship, strict adherence to infection prevention measures, including the use of gloves, gowns, and hand hygiene, and environmental cleaning and disinfection.44,46
For an initial episode of CDI, vancomycin (125 mg orally 4 times per day) or fidaxomicin (200 mg twice per day) for 10 days is recommended.46 First recurrence is generally treated with vancomycin with a taper or fidaxomicin. For multiple recurrences, fecal microbiota transplantation is recommended.46-49 International guidelines have endorsed fecal microbiota transplantation in treating recurrent CDI after the publication of clinical trial data showing the superiority of this procedure compared with antibiotic treatment.50,51
There is currently no standardized, approved prophylaxis for Clostridioides difficile, but recent studies have evaluated prophylactic oral vancomycin to prevent CDI in those who received an allo-SCT.52 In 1 study, oral vancomycin was found to be highly effective in preventing CDI in allo-SCT recipients without increasing the risk of GVHD or disease relapse.52 However, more data is needed before routine use is implemented because the impact on long-term outcomes in malignancy has not been assessed, and the optimal regimen has not been defined.53,54
Bezlotoxumab is a human immunoglobulin G1 (IgG1) monoclonal antibody that binds to Clostridioides difficile toxin B and neutralizes it to prevent its toxic effects. In the MODIFY I and MODIFY II trials, participants received antibiotic treatment for primary or recurrent Clostridioides difficile infection. Use of bezlotoxumab was associated with a decrease in recurrent infection compared with placebo and with a safety profile similar to that of placebo.55 In the MODIFY I and II post hoc analysis of patients with cancer, the rate of recurrent CDI in participants treated with bezlotoxumab was lower than in participants treated with placebo.56
Probiotic supplementation has been promoted for numerous health conditions, but its safety in immunosuppressed patients is not known. In 1 study, bloodstream infections within 1 year of SCT57 were evaluated, and organisms frequently incorporated into available over-the-counter probiotics were not common causes of bacteremia.57 However, data are limited that support the use of probiotics in treatment or prophylaxis for Clostridioides difficile infections.
Case 2: fungal infection and prophylaxis
A 65-year-old man with CLL who was receiving ibrutinib was admitted and was intubated for fever, altered mental status, and respiratory failure. A computerized tomographic examination of the chest showed a left lower lobe infiltrate. A lumbar puncture revealed an opening pressure of 180 mm H2O, glucose 3 mg/dL, protein 77 mg/dL, and white blood cell count of 198/μL. A cerebrospinal fluid cryptococcal antigen (CRAG) was 1:128 and the serum CRAG was 1:1024. He was given liposomal amphotericin B and flucytosine induction therapy for 2 weeks. Cryptococcus neoformans was grown from blood samples. After induction, lumbar puncture was repeated and it showed a cerebrospinal fluid CRAG of 1:64 and serum CRAG of 1:128. Consolidation therapy was started with fluconazole 800 mg once per day for 8 weeks followed by 400 mg per day for 1 year, and ibrutinib dose was adjusted to 280 mg/d. The patient had serum CRAG studies every 3 months; after 9 months, the patient’s serum CRAG became negative.
Invasive fungal infections (IFIs) are a cause of morbidity and mortality in patients with lymphoproliferative disorders.58 The SEIFEM-2004 study investigated the incidence of IFIs in patients with chronic lymphoproliferative disorders in 18 Italian hematology units with a cohort of 11 802 patients with hematologic malignancies.59 There were 538 proven or probable IFIs (4.6%); 41% of those occurred in patients with chronic leukemia, lymphoma, or multiple myeloma. More than half (346 of 538) of the infections were caused by molds, in most cases Aspergillus.59 Overall mortality rates were 2% and a rate of 39% was attributable to IFIs59; the highest IFI-attributable mortality rates were associated with zygomycosis (64%) followed by fusariosis (53%), aspergillosis (42%), and candidemia (33%).59 Recent data from the prospective Italian Hema e-Chart registry reported 147 episodes of fungal infections, among which 9.5% were associated with chronic lymphoproliferative diseases.60
With the introduction of targeted therapies such as ibrutinib come the challenges of assessing the risk of developing an IFI and managing the drug–drug interactions between antifungals and the targeted therapy.61,62 The risk associated with developing an IFI in patients with lymphoid cancers has historically been associated with treatment-mediated risk factors, such as neutropenia as a result of using chemotherapy and corticosteroids. Using Candida prophylaxis (eg, fluconazole) is recommended for patients who have prolonged neutropenia and using extended-spectrum azoles (eg, posaconazole) is recommended for heavily pretreated patients to prevent mold infections. According to the ASCO, IDSA, and NCCN Clinical Practice Guidelines, antifungal prophylaxis is recommended for patients who are at high risk of infection and for certain targeted therapies7, but careful vigilance or consideration is required. Coadministration of ibrutinib with a moderate CYP3A4 inhibitor requires close monitoring and adjustment of the ibrutinib dose.
Invasive candidiasis (IC) is the most common nosocomial mycosis.63 Recently, non-albicans species such as Candida tropicalis, Candida parapsilosis, Candida krusei, and Candida lusitaniae, have emerged as important pathogens,63,64 and they have various sensitivities to antifungal medications. IC represents 25% to 30% of IFIs among patients with hematologic cancer, but the incidence of IC has decreased because azole prophylaxis has become common practice.59,65-67 Risk factors for Candida infections include mucosal damage from chemotherapy, increased use of broad-spectrum antibiotics, prolonged neutropenia, use of corticosteroids, and the presence of a central venous catheter.63
The use of corticosteroids and neutropenia put patients who have received a bone marrow transplantation and those with leukemia or lymphoma at high risk for developing invasive aspergillosis.59,63,66-68Aspergillus fumigatus and Aspergillus flavus are the most common organisms that cause invasive infections.59,67-72 To help the patient recover, decreasing immunosuppression, if possible, and antifungal therapy are recommended. Surgical excision can be considered for patients for whom medical treatment has failed and who have localized Aspergillus lesions.68,73
Cryptococcus is different from other fungi, and having neutropenia does not seem to put patients at high risk for this type of infection.68,74-78 Risk factors include cellular immunodeficiencies as in patients with AIDS or lymphomas or patients who have received bone marrow transplantations or corticosteroids.77 Fever and headache are common symptoms in patients with meningitis.78
Cryptococcus gattii is an important fungal pathogen that is endemic in British Columbia, Canada, and in the United States Pacific Northwest. Infection manifests most often as meningoencephalitis and/or pulmonary disease.74,75 Compared with the more common Cryptococcus neoformans, Cryptococcus gattii frequently causes infection in people who are immunocompetent, although it has been hypothesized that some patients may have subclinical defects in immunity.76,79,80 For central nervous system and disseminated disease due to Cryptococcus gattii, induction, consolidation, and suppressive treatment are the same as that for Cryptococcus neoformans.77
Case 3: HBV and antiviral prophylaxis
A 53-year-old woman with mantle cell lymphoma was treated with cyclophosphamide, vincristine, doxorubicin, and dexamethasone (hyper-CVAD) chemotherapy. Her treatment course was complicated by FN and increases in the results of liver function tests on day 10 of cycle 2B (alanine aminotransferase [ALT], 435 U/L, aspartate aminotransferase [AST], 149 U/L). She reported a remote history of liver inflammation and was unsure of her vaccine history. The patient was found to be positive for hepatitis B core antibody (HBcAb), negative for hepatitis B surface antibody (HBsAb), and nonreactive for hepatitis B surface antigen (HBsAg). Her hepatitis B (Hep B) DNA was 1 813 597 IU/mL. She was seen by hepatology clinicians and started on tenofovir with a diagnosis of reactivation of HBV. Within 2 months, liver function tests normalized. After 3 months of treatment, Hep B DNA was undetectable.
Reactivation of HBV can be severe and even fatal, but reactivation is preventable. In patients with a malignancy, HBV reactivation is most commonly reported in those receiving cancer chemotherapy, especially rituximab-containing therapy for those with hematologic malignancies and those receiving SCT.81-83 Several of the newer agents (eg, brentuximab, ofatumumab, obinutuzumab, axicabtagene, brexucabtagene, and tisagenlecleucel) are associated with HBV reactivation.12,17 It is critical to assess patients who have spent significant time in areas where HBV is endemic or who have risk factors for blood-borne exposure because they could be at risk for HBV infection.
Universal screening for HBV is recommended at a minimum for patients receiving anti-CD20 therapies or SCT7,84 but it should (along with screening for hepatitis C virus [HCV] and HIV), be considered for all patients before they undergo chemotherapy or other immunosuppressive therapies. If HBV screening is pursued, patients should also be tested for HBcAb, HBsAb, and HBsAg.7,84,85 The ASCO provisional clinical opinion clinicians recommend starting antiviral therapy for patients who are positive for HBcAb or HBsAg before starting or simultaneously given with cancer therapy. The group also recommends that patients who are positive for HBcAb or negative for HBsAg should be monitored for reactivation by assessing Hep B DNA and ALT levels every 1 to 3 months; they also recommend starting antivirals if reactivation occurs.85 Clinicians can initiate antivirals for patients who are positive for HBcAb or negative for HBsAg in anticipation of starting cancer therapies associated with a high risk of reactivation, or they can monitor HBV DNA and ALT levels and initiate on-demand antivirals.85 Patients who are positive for both HBcAb and HBsAb likely have an even lower risk of reactivation86 and, depending on other risk factors, clinicians may choose the monitoring and on-demand antiviral approach. The Canadian Association for the Study of the Liver and the Association of Medical Microbiology and Infectious Disease Canada recommend that all high-risk individuals be screened for HBV infection87 and all high-risk individuals who are negative for HBcAb, HBsAb, or HBsAg should receive the HBV vaccine, and their response to the vaccine should be assessed.87 Consultation with experts in infectious diseases or hepatology should be strongly considered.
Ziakas et al88 found that anti-HBV prophylaxis can improve survival rates by 2.4% in patients who are positive for HBsAg and are receiving chemotherapy for lymphoma. Small randomized controlled trials and prospective cohort studies suggest that HBV reactivation rates can be reduced to near zero with the use of prophylactic antiviral medication.82,83,89 There is some uncertainty regarding the optimal duration of antiviral prophylaxis; however, the ASCO panel consensus recommends continuing treatment for 6 to 12 months after the conclusion of chemotherapy, with the preferred agents for HBV prophylaxis being entecavir or tenofovir.85
Case 4: Pneumocystis jirovecii and respiratory viral infections
A 61-year-old man with relapsed CLL and small lymphocytic lymphoma was started on ofatumumab and oral prednisone for hypercalcemia 4 months before admission. One month before admission, he was started on ibrutinib, and 1 day later, he developed fevers that continued for weeks with progressive shortness of breath, pleuritic chest pain, cough, night sweats, and weight loss. He received a course of levofloxacin and showed no improvement.
He was admitted to an outside hospital and was treated for pneumonia with ceftriaxone and azithromycin and still showed no improvement. He was then transferred to our hospital where a computed tomography chest scan showed diffuse pneumonia, and a bronchoscopy revealed both influenza and PJP by cytopathology and PCR. He also had severe hypogammaglobulinemia and profound CD4 lymphopenia. He was treated with oseltamivir for 5 days and received a 21-day course of trimethoprim-sulfamethoxazole (TMP/SMX) followed by prophylaxis as well as intravenous immunoglobulin once per month.
Prophylaxis against PJP is indicated in recipients of autologous hematopoietic SCT (auto-HSCT) or allo-SCT; patients with acute lymphoblastic leukemia (ALL); patients receiving purine analog therapy (eg, fludarabine, cladribine) and other T-cell–depleting agents, CD30 antibodies, PI3K inhibitors, concomitant temozolomide and radiotherapy; and in patients with neoplastic diseases receiving intensive corticosteroid treatment (eg, the equivalent of ≥20 mg of prednisone once per day for ≥4 weeks).26,90,91 Fludarabine plus prednisone results in a uniform depression of CD4+ cells that may persist for several months after completion of therapy.26,90-92, Prophylaxis should be continued until the patient has recovered from lymphocytopenia.93
TMP/SMX prophylaxis is highly effective in preventing PJP.94 TMP/SMX also has the advantage of being active against other infectious complications (eg, common bacterial infections, listeriosis, nocardiosis, toxoplasmosis) that may affect patients with severe T-cell depletion or impairment.95 Dapsone or atovaquone given once per day and aerosolized or IV pentamidine given once per month are thought to be effective alternatives to TMP/SMX, although some data suggest that these agents may be inferior when used prophylactically in recipients of allo-SCT.96-99 A common practice among institutions is continuing PJP prophylaxis for 6 months to a year or until immunosuppressive therapy is completed (Table 3).
Table 3. PJP prophylaxis
| Agent . | Dose . | Route . | Schedule . | Special notes . |
|---|---|---|---|---|
| Preferred | ||||
| TMP/SMX | 80/400 mg (SS)160/800 mg (DS) | Oral | If use SS give dailyIf use DS give 3 × week | Renally dose if renal dysfunctionMonitor for neutropenia and transaminase elevations |
| Alternatives | ||||
| Dapsone | 100 mg | Oral | Daily | Should not be given to patients with glucose-6-phosphate dehydrogenase deficiency |
| Atovaquone | 1500 mg | Oral | Daily | Take with fatty meal |
| Pentamidine | 300 mg | Inhaled/intravenous | Monthly |
| Agent . | Dose . | Route . | Schedule . | Special notes . |
|---|---|---|---|---|
| Preferred | ||||
| TMP/SMX | 80/400 mg (SS)160/800 mg (DS) | Oral | If use SS give dailyIf use DS give 3 × week | Renally dose if renal dysfunctionMonitor for neutropenia and transaminase elevations |
| Alternatives | ||||
| Dapsone | 100 mg | Oral | Daily | Should not be given to patients with glucose-6-phosphate dehydrogenase deficiency |
| Atovaquone | 1500 mg | Oral | Daily | Take with fatty meal |
| Pentamidine | 300 mg | Inhaled/intravenous | Monthly |
DS, double strength; SS, single strength.
Influenza and other respiratory viral infections cause significant morbidity and mortality in patients with cancer.100-102 Annual vaccination against influenza with the inactivated influenza virus is recommended for all individuals at increased risk because of immunosuppression, as well as their household contacts.103 Trivalent inactivated influenza vaccine is the formulation most commonly administered.104 The live-attenuated intranasal influenza vaccine (FluMist) should be avoided by patients who have suppressed immune systems and by their household contacts.103,105,106 Preliminary data have shown that the high-dose influenza vaccine is safe for patients with cancer and may show more immunogenicity than standard-dose influenza vaccine.107-109 However, there is currently not enough data to recommend the high-dose vaccine over the standard-dose influenza vaccine. When feasible, vaccines should be administered before planned immunosuppressive chemotherapy, preferably more than 2 weeks before receiving chemotherapy or between chemotherapy cycles, if possible.110
Other viral respiratory tract infections, including respiratory syncytial virus, parainfluenza, adenovirus, human metapneumovirus, and others have been shown to cause significant morbidity in patients with hematologic malignancy. Not surprisingly, recent data have suggested that patients with cancer may be more vulnerable to severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) sequelae, with higher fatality rates, particularly in those with hematologic malignancies, than in patients who do not have cancer.111 Detailed discussion of SARS-CoV-2 is beyond the scope of this review, but numerous ongoing studies are addressing the impact, optimal treatments, and vaccination strategies in this patient population. Because there is a scarcity of approved effective treatments and vaccines for many non-influenza respiratory viral infections, a focus on prevention through strict adherence to infection control guidance is paramount.
Conclusions
Preventing, accurately diagnosing, and treating infections helps decrease the morbidity and mortality in patients with lymphoma. The immune defects caused by the disease process itself, as well as therapy-induced adverse effects increase the risk of infection in these patients. Because more novel agents are now being developed and used, we must consider a broader infectious differential to include not only bacterial etiologies but also viral and fungal causes, even in the absence of neutropenia. Because of the demands of prophylaxis and treatment of various infections in this population, it is also important to be stewards of antimicrobials to avoid the emergence of multidrug-resistant organisms.
Acknowledgments
The authors thank Divya Koura and Justine Ross for reviewing the manuscript.
Authorship
Contribution: N.L. and R.A.T. contributed equally to the original design, draft, and revisions of this manuscript; and both authors reviewed and approved final submission.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Randy A. Taplitz, Division of Infectious Diseases, Department of Medicine, City of Hope National Medical Center, City of Hope, 1500 East Duarte Rd, Duarte, CA 91010; e-mail: rtaplitz@coh.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal