Abstract
Thrombotic complications in hematologic malignancies have important clinical implications. In this meta-analysis we sought to obtain accurate estimates of the thrombotic risk in lymphoma patients. Articles were searched in electronic databases and references. Eighteen articles were identified (29 cohorts, 18 018 patients and 1149 events). Pooled incidence rates (IRs) were calculated by the use of a method based on the exact maximum likelihood binomial distribution. The global IR of thrombosis was 6.4% (95% confidence interval [CI] 6.0%-6.8%). The global IRs of venous or arterial events were 5.3% (95% CI, 5.0%-5.7%) and 1.1% (95% CI, 0.9%-1.2%), respectively. The IR of thrombosis observed in subjects with non-Hodgkin lymphoma (NHL) was 6.5% (95% CI, 6.1%-6.9%), significantly greater than that observed for patients with Hodgkin lymphoma (4.7%; 95% CI, 3.9%-5.6%). Within NHL, patients with high-grade disease had a greater risk of events (IR 8.3%; 95% CI, 7.0%-9.9%) than low-grade disease (IR 6.3%; 95% CI, 4.5%-8.9%). This meta-analysis shows that the IR of thrombosis in lymphoma patients is quite high, especially in those with NHL at an advanced stage of the disease. These results may help better defining lymphoma populations at high thrombotic risk, to whom prophylactic approaches could be preferentially applied.
Introduction
The link between cancer and thrombosis is well recognized,1-3 and the occurrence of thrombotic complications in cancer patients has important implications, including need for chronic anticoagulation with the associated risk of bleeding, possible delays in delivering chemotherapy, a high risk of recurrent thrombosis, along with a decreased quality of life.
However, because antithrombotic prophylaxis is associated with increased hemorrhagic risk and implies additional treatment costs, it is important to determine which population could receive a true benefit from it. The search for thrombosis risk factors in cancer patients has been the aim of several clinical studies. Although patients with solid tumors initially were thought to experience the greatest rate of thrombotic complications, the authors of recent investigations4-8 suggest that thrombotic risk in subjects with hematologic malignancies also may be considerably high. Specifically, incidence rates (IRs) of thrombosis in subjects with lymphoma vary between 3% and 13% when systemic forms of the disease are studied and reach up to 60% in primary brain locations.9
Despite these findings, no clinical subpopulation of patients with lymphoma has been identified so far as being at greater risk for thrombosis, and no clear indications have been provided regarding the need and the conditions required to prevent thrombotic events in these patients. The aim of the present study was to quantitatively combine and meta-analyze the available data on thrombosis occurrence in patients with lymphoma and to obtain accurate estimates of the thrombotic risk. Several subgroup analyses were conducted in an attempt to identify lymphoma subpopulations at greater risk for thrombotic complications.
Methods
Selection of articles
Articles were searched in the PubMed, MEDLINE, Embase, and Cochrane databases by use of the following keywords: lymphoma, cancer and tumors combined with thrombosis, “thrombotic events,” hypercoagulability, and coagulation. Subsequently, we searched for cross-references. Studies on pediatric populations were excluded. In addition to studies specifically designed to evaluate the occurrence of thrombosis in lymphoma patients, articles including all malignancies that separated results by type of cancer were included, as well as articles reporting laboratory findings on hypercoagulability associated with clinical events reporting. The articles were independently reviewed by 2 authors (V.C. and S.M.). Disagreements were resolved through discussion, and consensus was achieved in the selection of articles for analysis. Only articles written in English published after 1985 were included.
The end point used for the analysis was the occurrence of “symptomatic” thrombotic events, defined as “symptomatic thrombotic event,” or “symptomatic thrombosis,” or “symptomatic venous thrombosis,” or merely “thrombosis/thrombotic event,” followed by the description of clinical symptoms. All events had to be confirmed by objective methods. Studies in which the definition of events was unclear or unspecific were excluded.
Extracted data were as follows: site of thrombotic event; whether it occurred before, during, or after therapy; characteristics of the study population (mean age, lymphoma subtype and location, stage of disease, risk stratification, and use of central venous catheters [CVCs]); and treatment protocol when available. When studies described events for the different subtypes of lymphoma, patients experiencing these distinct subtypes were considered as belonging to different “populations.”
From a total of 70 articles retrieved, 47 were excluded for one of the following reasons: review articles (n = 13),9-21 no data about incidence of clinical thrombotic events (n = 13),22-34 no distinction of type of cancer (n = 9),35-43 duplicated data (n = 3),44-46 case reports (n = 7),47-53 and case-control studies of patients with a first event of deep vein thrombosis (DVT) and subsequent cancer diagnosis (n = 2).54,55 In addition, studies in which the authors evaluated the incidence of thrombosis after bone marrow transplantation (n = 3)56-58 were not considered in the present analysis.
The remaining 23 articles were divided as summarized in Table 1.59-79 Thirty-six cohorts were identified, which included 3 331 489 patients and 84 165 events. The great majority of the patients were included in record linkage studies of epidemiologic registries and/or disease/hospitalization databases. In this group, the IR of thrombotic events was significantly lower than that observed in retrospective or prospective studies specifically designed to study thrombotic complications in cancer patients (Table 1). Because recall of events in this type of studies could be rather erratic, and many cases may not be included into the databases, this group was excluded from further analysis. The final selection comprised 18 studies (8 prospective and 10 retrospective), with 29 independent cohorts of patients, including 18 018 patients and 1149 events. Approval was obtained from the Catholic University at Campobasso Institutional Review Board for these studies. The study was conducted in accordance with the Declaration of Helsinki.
Classification of 23 selected studies according to their methodologic design
| Class of study . | No. of studies . | Cohorts . | Patients . | Events . | IR for thrombosis, % (95% CI) . | Homogeneity test, P . |
|---|---|---|---|---|---|---|
| Retrospective6,59-67 | 10 | 17 | 16 861 | 1069 | 6.3 (6.0-6.7) | < .001 |
| Prospective68-75 | 8 | 12 | 1157 | 80 | 6.9 (5.5-8.6) | .034 |
| Registries*4,76-79 | 5 | 7 | 3 313 471 | 83 016 | 2.5 (2.4-2.5) | < .001 |
| Class of study . | No. of studies . | Cohorts . | Patients . | Events . | IR for thrombosis, % (95% CI) . | Homogeneity test, P . |
|---|---|---|---|---|---|---|
| Retrospective6,59-67 | 10 | 17 | 16 861 | 1069 | 6.3 (6.0-6.7) | < .001 |
| Prospective68-75 | 8 | 12 | 1157 | 80 | 6.9 (5.5-8.6) | .034 |
| Registries*4,76-79 | 5 | 7 | 3 313 471 | 83 016 | 2.5 (2.4-2.5) | < .001 |
95% CI indicates 95% confidence interval; and IR, incidence rate.
Studies determined on the basis of record linkage of epidemiologic registries and/or disease/hospitalization databases.
Subgroup analyses
Subgroups were analyzed, taking into account type of events, time of occurrence, type of lymphoma, stage of the disease, compression, thrombophilia, use of central catheter, and treatments. However, a separate analysis could only be performed for type of events, time of event occurrence, type of lymphoma, and stage of the disease.
Five studies included patients with different cancers, either hematologic or solid, with subsequent description of thrombotic events by type of malignancy. Five studies included “lymphoma” patients; 3 of them discriminated events according to the type of lymphoma. In 3 studies the authors evaluated only non-Hodgkin lymphoma (NHL) and 1 only Hodgkin lymphoma (HL) patients. More specifically, 3 studies included only patients with high-grade NHL (hgNHL) and one only central nervous system lymphoma (CNSL).
A particular effort was made to categorize lymphomas in the most accurate way. However, although the authors of most studies distinguished between NHL and HL, few of them differentiated between hgNHL and low-grade NHL (lgNHL), and none separated events by different subtypes of HL. Therefore, different IRs of thrombosis were calculated for the following populations: (1) lymphoma, from studies in which different types of lymphoma were not discriminated; (2) NHL, from studies in which different subtypes of NHL were not distinguished; (3) lgNHL; (4) hgNHL; (5) HL, and (6) CNSL. From studies in which the authors separated subjects according to the Ann Arbor staging system, different IRs of thrombosis were calculated for different stages of the disease.
Statistical analysis
Pooled IRs were calculated by the use of an exact method, as proposed in Martin and Austin.80 This approach used exact maximum likelihood binomial distribution for calculated pooled rates and 95% confidence intervals (95% CIs); 0.5 was not added to numbers of events in studies with 0 events because the method accounts for sparseness of individual studies. Homogeneity across studies was tested by use of the Breslow-Day test. The method provides stratum-specific estimates and tests of differences across subgroups.
Results
Global meta-analysis
Of 1149 events, 959 (83.5%) were venous and 190 (16.5%) were arterial thrombosis; 16.5% were pulmonary embolism events (Table 2). In two-thirds of the cases, the site of thrombosis was not described (Table 2). Arterial thromboses were mainly myocardial infarctions (Table 2).
Sites of thrombosis
| Site of thrombosis, n = 1149 . | No. of events (%) . |
|---|---|
| Venous thrombosis | 959 (83.5) |
| Pulmonary embolism | 121 (10.5) |
| Deep vein thrombosis without pulmonary embolism | 769 (66.9) |
| Deep vein thrombosis and pulmonary embolism | 69 (6.0) |
| Among patients with deep vein thrombosis, n = 838 | |
| Without description of site | 772 |
| Portal-mesenteric | 3 |
| CNS | 3 |
| Lower limbs | 39 |
| Upper limbs | 21 |
| Among upper limbs: catheter-related thrombosis | 7 |
| Arterial thrombosis | 190 (16.5) |
| Acute myocardial infarction | 106 (9.2) |
| Stroke | 65 (5.7) |
| Not described | 19 (1.6) |
| Site of thrombosis, n = 1149 . | No. of events (%) . |
|---|---|
| Venous thrombosis | 959 (83.5) |
| Pulmonary embolism | 121 (10.5) |
| Deep vein thrombosis without pulmonary embolism | 769 (66.9) |
| Deep vein thrombosis and pulmonary embolism | 69 (6.0) |
| Among patients with deep vein thrombosis, n = 838 | |
| Without description of site | 772 |
| Portal-mesenteric | 3 |
| CNS | 3 |
| Lower limbs | 39 |
| Upper limbs | 21 |
| Among upper limbs: catheter-related thrombosis | 7 |
| Arterial thrombosis | 190 (16.5) |
| Acute myocardial infarction | 106 (9.2) |
| Stroke | 65 (5.7) |
| Not described | 19 (1.6) |
CNS indicates central nervous system.
When considering all the studies (18 studies, 29 independent cohorts, 18 018 patients and 1149 thrombotic events), the global IR of symptomatic thrombosis was 6.4% (95% CI, 6.0%-6.8%; homogeneity test: P < .001; Table 3). The global IRs of venous and arterial events were 5.3% (95% CI, 5.0%-5.7%) and 1.1% (95% CI, 0.9%-1.2%), respectively.
IRs of thrombosis for different groups of studies according to the overall population included
| . | No. studies . | No. cohorts . | No. patients . | No. events . | IR, % (95% CI) . |
|---|---|---|---|---|---|
| Lymphoma patients* | 5 | 8 | 15 397 | 922 | 6.0 (5.6-6.4) |
| Lymphoma patients† | 5 | 12 | 1222 | 99 | 8.1 (6.6-9.9) |
| Only non-Hodgkin lymphoma (NHL)‡ | 3 | 4 | 189 | 22 | 11.6 (7.6-17.6) |
| Only high-grade NHL | 3 | 3 | 991 | 71 | 7.2 (5.7-9.0) |
| Only Hodgkin lymphoma | 1 | 1 | 177 | 10 | 5.6 (3.0-10.5) |
| Only central nervous system lymphoma | 1 | 1 | 42 | 25 | 59.5 (40.2-88.1) |
| Total | 18 | 29 | 18 018 | 1149 | 6.4 (6.0-6.8) |
| . | No. studies . | No. cohorts . | No. patients . | No. events . | IR, % (95% CI) . |
|---|---|---|---|---|---|
| Lymphoma patients* | 5 | 8 | 15 397 | 922 | 6.0 (5.6-6.4) |
| Lymphoma patients† | 5 | 12 | 1222 | 99 | 8.1 (6.6-9.9) |
| Only non-Hodgkin lymphoma (NHL)‡ | 3 | 4 | 189 | 22 | 11.6 (7.6-17.6) |
| Only high-grade NHL | 3 | 3 | 991 | 71 | 7.2 (5.7-9.0) |
| Only Hodgkin lymphoma | 1 | 1 | 177 | 10 | 5.6 (3.0-10.5) |
| Only central nervous system lymphoma | 1 | 1 | 42 | 25 | 59.5 (40.2-88.1) |
| Total | 18 | 29 | 18 018 | 1149 | 6.4 (6.0-6.8) |
P < .001 for difference among groups of studies.
Studies in which the authors included patients with different cancers, with subsequent description of thrombotic events by type of malignancy, including lymphoma. Three of these differentiate NHL from HL, whereas 2 do not discriminate type of lymphoma. Numbers of patients and events refer to lymphoma cases.
Studies which included only lymphoma patients. Three studies differentiate NHL from HL and discriminated subtypes of NHL, whereas 2 do not discriminate type of lymphoma.
One of the studies discriminates low-grade NHL from high-grade NHL.
Time of event occurrence
Fourteen studies (17 660 patients; 1134 thrombotic events) described the time when the thrombotic event occurred in relation to diagnosis and treatments. Ninety-five percent of events occurred during treatment, whereas 3.8% took place at presentation of the disease, before initiating chemotherapy, and only 1.2% after completion of the treatment.
Type of lymphoma
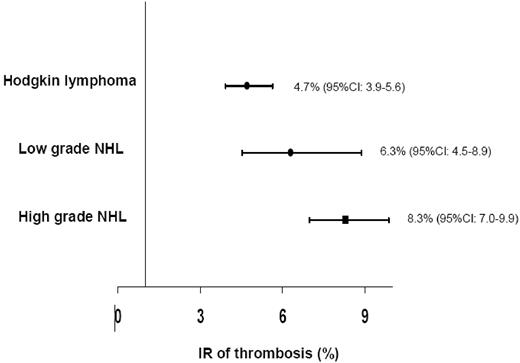
The IR of thrombosis observed in subjects with NHL (15 cohorts; 997 events in 15 410 patients) was 6.5% (95% CI, 6.1%-6.9%), significantly greater (P < .001) than that observed for HL patients (7 cohorts; 2505 patients; 118 events; IR 4.7%; 95% CI, 3.9%-5.6%). Within NHL, for 5 cohorts it was not possible to distinguish the grading of the disease (13 332 patients; 835 events; IR 6.3%; 95% CI, 5.9%-6.7%); NHL patients with high-grade disease had the most elevated risk of events (7 cohorts; 1542 patients; 128 events; IR 8.3%; 95% CI, 7.0%-9.9%) compared with low-grade disease (3 cohorts; 536 patients; 34 events; IR 6.3%; 95% CI, 4.5%-8.9%; Figure 1). Although the number of patients with CNSL was low, the incidence of thrombosis in this population was extremely high (2 cohorts; 54 patients; 26 events; IR 48.1%; 95% CI, 32.8%-70.7%).
Stage of disease
Only 3 studies (894 patients) described the rate of thrombotic events according to the stage of the disease. A trend, although not statistically significant, was observed to proportionally increase the IRs with the stage of the disease (Table 4).
IRs of thrombosis according to stage of disease, using the Ann Arbor staging system
| Stage . | No. patients . | No. events . | IR, % (95% CI) . |
|---|---|---|---|
| I | 80 | 4 | 5.0 (1.98-13.3) |
| II | 271 | 21 | 7.7 (5.0-11.9) |
| III | 160 | 11 | 6.9 (3.8-12.4) |
| IV | 382 | 44 | 11.5 (8.6-15.5) |
| Stage . | No. patients . | No. events . | IR, % (95% CI) . |
|---|---|---|---|
| I | 80 | 4 | 5.0 (1.98-13.3) |
| II | 271 | 21 | 7.7 (5.0-11.9) |
| III | 160 | 11 | 6.9 (3.8-12.4) |
| IV | 382 | 44 | 11.5 (8.6-15.5) |
95% CI indicates 95% confidence interval; and IR, incidence rate.
P = .14 for differences according to stage of disease.
Discussion
Frequency of thrombosis in lymphoma patients
The occurrence of venous thrombosis in association with malignancy has been described long ago, but only recently the clinical relevance of this phenomenon has been fully appreciated, both in relation to cancer itself and, particularly, in association with cancer therapies, such as surgery and chemohormone therapy.1-3 Thrombosis may be an indicator of an occult malignancy, and in fact, recurrent “idiopathic” vein thrombosis must prompt the search for a possible associated malignant condition.1,81
In the present study, the global IR of thrombosis in lymphoma patients was found to be more than 6%, most of the events occurring during treatment of the disease. Because in the majority of the studies, patients were followed-up from diagnosis to an extent of 1 to 3 years (treatment period being usually of 6-12 months), this pooled IR is to be considered particularly high. However, because 95% of thromboses occurred during treatment, it is impossible to distinguish between thrombogenic effects of lymphoma itself or its treatment. Nevertheless, the high rate of thrombotic events in lymphoma patients stands as a problem in their clinical management, irrespective of the cause, either cancer or the treatments.
The estimated annual incidence of thrombosis in cancer population is indeed approximately 0.5%, compared with 0.1% in the general population, thrombosis representing the second-leading cause of death in cancer patients.81,82 The occurrence of a thrombotic event once a malignant disease has been diagnosed and a therapeutic regimen has been started represents a significant clinical obstacle, not only related to the increased morbidity and mortality associated to the event itself but also because of the detrimental effect an interruption or modification in therapy secondary to the event may exert on the primary disease.
In a prospective observational study of outpatients with cancer starting chemotherapy, venous and arterial thrombosis accounted for 9% of deaths,22 whereas cancer patients with thrombosis had an independent 2-fold increase in mortality compared with cancer patients without this complication,54,78 even in the absence of metastatic disease.6 Although solid tumors were initially thought to be associated with the greatest rate of thrombotic complications, the authors of recent investigations4-6 suggest that thrombotic risk in subjects with hematologic malignancies too may be considerably high. Studies evaluating thrombosis in lymphoma patients report incidences that vary between 3% and 13% for systemic forms of lymphoma, reaching up to 60% in primary brain locations of the disease.9
Thrombotic risk in lymphoma patients was slightly lower than those observed for ovarian, CNS, and pancreas cancer, 3 tumors well known for their prothrombotic characteristics76 Recently, a large population-based case-control study (Multiple Environmental and Genetic Assessment [MEGA] of risk factors for venous thrombosis) showed that patients with lymphoma had the greatest risk of venous thrombosis (odds ratio [OR] 10.2, 95% CI, 1.4-76.9), followed by lung and gastrointestinal cancer patients.30
Among more than 65 000 neutropenic cancer patients with more than 5000 thrombotic events, patients with lymphoma and leukemia accounted for one third of venous and nearly one half of arterial events6 ; recently, the same research group included lymphoma within the high-risk group for developing thrombosis, along with lung, gynecologic, and genitourinary cancers in a predictive model for cancer-associated thrombosis.83
In addition, the authors of studies evaluating the occurrence of cancer after a primary thrombotic event found a high incidence of lymphoma.55,84 In a review of more than 500,000 cancer patients with unprovoked thrombosis during the year preceding the diagnosis of cancer, the incidence in NHL was found to be 2.7 times greater than expected, greater than those found for other solid tumors such as lung and colon cancer.5
Subtype of lymphoma
In subgroup analyses, different pooled IRs of thrombotic events were obtained for different subtypes of lymphoma. Compared with all types of NHL, HL patients had a statistically lower incidence of thrombosis. Between NHL subtypes, those with hgNHL exceeded lgNHL in the rate of thrombotic events.
Thrombotic risk in CNSL was found to be extremely high in one single study including only CNSL patients (IR 59%).60 In a study including different types of lymphomas, the authors observed 1 thrombotic event in 12 patients with CNSL (IR 8.3%), similar to that found in other hgNHLs.64 Almost all events occurred during the early period of intensive therapy, which consisted of high-dose methotrexate and corticosteroids.
Ottinger and coworkers73 investigated risk factors for thrombosis in different subtypes of hgNHL, finding only the presence of mediastinal clear cell subtype as an independent risk factor for thrombosis. However, in these patients, lymphoma compression of veins by bulky mediastinal disease was documented as the dominant cause of thrombosis. Because the aforementioned study was the only one that accurately classified NHL, further estimation of different IRs according to subtypes of hgNHL and lgNHL was not possible.
Advanced stage of disease
A proportionally increased IR of thrombosis with stage of the disease was confirmed by our meta-analysis. Experimental models suggest that activation of the coagulation cascade confers growth stimulation to the tumor.85 Therefore, the presence of thrombosis could indicate aggressive tumor biology and, therefore, a worse short-term prognosis.
Thrombosis in general was previously associated with an advanced cancer stage and a 3-fold lower survival at 1 year54 ; in addition, Ann Arbor stage IV was an independent risk factor for thrombosis in a study on hgNHL patients.73 Moreover, a study on HL patients reported that most of the thrombotic events occurred in subjects with advanced stage according to Ann Arbor classification systems and the presence of B symptoms.63 In both studies, patients who developed thrombosis had a shorter survival, principally when the events occurred during the treatment.
Time of occurrence
Ninety-five percent of thromboses occurred during lymphoma treatment. Although it was difficult to analyze the time elapsed from diagnosis to the event because few researchers provided this information, those describing the time of thrombosis reported that most events occurred early in the course of the disease. Similarly, when considering all cancers, the risk of venous thrombosis was highest in the first few months after the diagnosis of malignancy (OR 53.5) and, as time progressed, the risk of a thrombotic event decreased.30
Type of thrombosis
The observation that in this study more than 16% of cases (approximately 1 of 6) were arterial thrombosis prompts, some reevaluation of the rather exclusive association of malignant disorders with deep vein thrombosis and pulmonary embolism.
Compression
The presence of compression of venous vessels was indicated as a risk factor in several studies; however, the characteristics of the available data did not allow us to include them in this meta-analysis. Compression of veins by local growth of lymphoma was identified as the cause in 50% of cases, including all 12 patients who developed thrombosis before treatment and one third of patients with thrombosis during chemotherapy73 and was correlated to clinical events in 11% of cases with advanced-stage disease.64
Congenital thrombophilia
No study evaluated the effect of congenital thrombophilia specifically in lymphoma patients. A 12-fold increase in the risk of thrombosis in an overall cancer population with factor V Leiden mutation, compared with a 5-fold increase in cancer patients without the mutation, was found in the MEGA study.30 Similar results were indirectly calculated for the prothrombin 20210A mutation.30
Regarding antiphospholipid antibodies (APA), in a retrospective study of 120 cancer patients, lymphoma was found to be the most common malignancy associated with APA.86 However, the thrombogenicity of APA in lymphoma patients is still controversial, with contradictory findings in different studies.
In one study, with a prevalence of APA of 27% in lymphoma patients, the rate of thrombosis was significantly greater in patients with than without APA (5.1% vs 0.75% patients/year) APA. These 2 groups were similar with respect to relapse and death rate, but the presence of APA identified patients at high risk to develop thrombotic complications.70 On the contrary, Genvresse et al68 did not observe any increase of thrombosis rate in lymphoma patients with APA. The number and characteristics of patients were similar in both studies. A meta-analysis that included these 2 studies only failed to show any significant result (data not shown).
Use of central venous catheter
The contribution of CVCs to thrombosis in lymphoma patients could not be thoroughly investigated in this meta-analysis because the authors of only a few studies reported the proportion of patients bearing CVCs, and less than one half of venous thrombosis sites were recorded. Available data show that 7 of 21 upper-limb DVTs were catheter related (< 1% of total DVT events). The rate of symptomatic catheter-related thrombosis in patients with hematologic malignancies has been reported to range between 11% and 45% in studies of populations with CVCs,74,75 with greater rates when asymptomatic events were included.58 These findings suggest CVCs to be an important local risk factor for site-specific venous thrombosis.
Effect of treatment
Almost all patients included in this analysis were treated according to current chemotherapy protocols used in each treating center, with or without radiotherapy according to type and stage of lymphoma. Because data regarding therapy were heterogeneous across studies, and not all authors reported name and dosage of the drugs used, an evaluation of the effect of different treatment interventions in the risk of thrombosis was not possible.
Because the use of anticoagulation in cancer patients may contribute to an increased risk of bleeding, medical oncologists rarely prescribe thromboprophylaxis to their patients. However, because thrombosis is a frequent event in the cancer population and it is associated with several detrimental effects, the possibility of stratifying the risk would allow an appropriate use of VTE prophylaxis in patients at high risk.
At this moment, guidelines recommend antithrombotic prophylaxis in hospitalized patients and those undergoing surgery; in outclinic patients, prophylactic anticoagulation is recommended only for those receiving thalidomide or lenalidomide.87
A predictive model for chemotherapy-associated thrombosis in outpatients was proposed.83 Five predictive variables were identified in a stage-adjusted multivariate model: site of cancer, platelet count 350 000/mm3 or greater, hemoglobin less than 10 g/dL and/or use of erythropoiesis-stimulating agents, leukocyte count greater than 11 000/mm3, and body mass index 35 kg/m2 or greater. Interestingly, lymphomas were part of the high-risk group together with lung, gynecologic, and genitourinary cancers, with an independent OR for thrombosis of 1.5 (0.9-2.7).83
Conclusions
In conclusion, our meta-analysis shows that the IR of thrombosis in lymphoma patients is quite high, especially in those with hgNHL at an advanced stage of the disease (IR 10.3%; 95% CI 7.3%-14.5%). Although not analyzed here, the presence of vein compression, as well as the use of CVCs could be additional risk factors for thrombosis occurrence.
These results may help better defining lymphoma populations at high thrombotic risk, in whom prophylactic approaches presently lacking could be preferentially developed. However, confirmation of these findings, along with further studies of prothrombotic risk factors in lymphoma are needed in the setting of well-designed prospective clinical studies.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to Professor Jozef Vermylen, Catholic University, for his critical review of the manuscript.
This study was partially supported by an unrestricted grant from Glaxo-Smith Kline, and by a grant of MIUR–Programma Triennale di Ricerca, Decreto no. 1588 del 19/11/2004.
Authorship
Contribution: V.C. performed research, analyzed data, and wrote the paper; A.D. designed research and analyzed data; S.M. performed research; M.A.L. critically reviewed the manuscript; G.d.G. designed research and critically reviewed the manuscript; S.S. critically reviewed the manuscript; L.I. designed research and wrote the paper; and M.B.D. designed research and critically reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Licia Iacoviello, MD, PhD, Laboratory of Genetic and Environmental Epidemiology, “RE ARTU” Research Laboratories, Centre for High Technology Research and Education in Biomedical Sciences, Catholic University, Largo Gemelli 1, 86100 Campobasso, Italy; e-mail: licia.iacoviello@rm.unicatt.it.