TO THE EDITOR:
Rearrangements in cytokine receptor–like factor 2 (CRLF2r) represent a subset of B-cell acute lymphoblastic leukemia (B-ALL) that demonstrates poorer survival in children and adults.1,2 An increased incidence of acute lymphoblastic leukemia (ALL) with CRLF2r has been consistently reported in patients of Latinx ethnicity,1-3 likely contributing to poorer outcomes.3 Separately, Hispanic/Latinx populations exhibit high rates of obesity,4 a host factor independently associated with the risk for developing B-ALL5,6 and poorer survival.7,8 Obesity may promote leukemogenesis and impair disease response via multiple mechanisms, including hormones, adipokines, and direct interactions with adipocytes9-14 ; however, no studies to our knowledge have examined whether obese patients are at a higher risk of developing any specific genomic subtype of ALL. Indeed, despite the higher rates of obesity in the Hispanic/Latinx population, and the connection of obesity with B-ALL, prior studies of CRLF2r ALL and ethnicity have not explored the potentially confounding influence of high rates of obesity in the Hispanic/Latinx population. We hypothesized that obesity contributes to preferential development of CRLF2r in B-ALL, and that high rates of obesity in Hispanic/Latinx populations may explain some of the observed association between ethnicity and CRFL2r ALL.
To test this, we collected data from 3 consecutive prospective trials (2011-2020) examining body composition in patients with newly diagnosed ALL (supplemental Table 1, available on the Blood Web site).15,16 Fat mass (FM) and body fat (BF) percentage (BF%) were measured using whole-body dual-energy X-ray absorptiometry.15 Body mass index (BMI) percentile (BMI%) was classified using population norms (obese, BMI ≥95th percentile, BMI ≥30.0 for age ≥20 years). Based on preclinical data showing interactions between adipocytes and ALL, FM (adipocyte burden) was analyzed as the primary measure of obesity. Both BMI% and BF% were also analyzed, as BMI% is generally accessible, albeit imprecise, in ALL populations,15 and BF% is most closely associated with obese physiology.
Description of cohort
| Covariable . | Total cohort . | CRLF2r ALL . | Not CRLF2r ALL . | P* . | |||
|---|---|---|---|---|---|---|---|
| n . | % . | N . | % . | n . | % . | ||
| Total | 70 | 100 | 23 | 33 | 47 | 67 | |
| Sex | |||||||
| Female | 28 | 40 | 5 | 22 | 23 | 49 | .039 |
| Male | 42 | 60 | 18 | 78 | 24 | 51 | |
| Age at diagnosis, y | |||||||
| Mean (SD) | 15.2 (2.9) | 16.2 (2.0) | 14.8 (3.2) | .057 | |||
| <15 | 30 | 43 | 5 | 22 | 25 | 53 | .020 |
| ≥15, AYA | 40 | 57 | 18 | 78 | 22 | 47 | |
| Ethnicity | |||||||
| Not Hispanic/Latinx | 16 | 23 | 3 | 13 | 13 | 28 | .232 |
| Hispanic/Latinx | 54 | 77 | 20 | 87 | 34 | 72 | |
| Body composition, by DXA, mean (SD) | |||||||
| FM, kg | 23.8 (13.6) | 34.1 (13.2) | 18.9 (10.8) | <.0001 | |||
| BF, % | 31.8 (9.5) | 37.3 (8.0) | 29.1 (9.2) | .0007 | |||
| BMI, percentile† | |||||||
| Mean (SD) | 76.9 (29.7) | 91.3 (17.8) | 69.9 (31.9) | .004 | |||
| Not obese, <95th | 39 | 56 | 7 | 30 | 32 | 68 | .003 |
| Obese, ≥95th | 31 | 44 | 16 | 70 | 15 | 32 | |
| Covariable . | Total cohort . | CRLF2r ALL . | Not CRLF2r ALL . | P* . | |||
|---|---|---|---|---|---|---|---|
| n . | % . | N . | % . | n . | % . | ||
| Total | 70 | 100 | 23 | 33 | 47 | 67 | |
| Sex | |||||||
| Female | 28 | 40 | 5 | 22 | 23 | 49 | .039 |
| Male | 42 | 60 | 18 | 78 | 24 | 51 | |
| Age at diagnosis, y | |||||||
| Mean (SD) | 15.2 (2.9) | 16.2 (2.0) | 14.8 (3.2) | .057 | |||
| <15 | 30 | 43 | 5 | 22 | 25 | 53 | .020 |
| ≥15, AYA | 40 | 57 | 18 | 78 | 22 | 47 | |
| Ethnicity | |||||||
| Not Hispanic/Latinx | 16 | 23 | 3 | 13 | 13 | 28 | .232 |
| Hispanic/Latinx | 54 | 77 | 20 | 87 | 34 | 72 | |
| Body composition, by DXA, mean (SD) | |||||||
| FM, kg | 23.8 (13.6) | 34.1 (13.2) | 18.9 (10.8) | <.0001 | |||
| BF, % | 31.8 (9.5) | 37.3 (8.0) | 29.1 (9.2) | .0007 | |||
| BMI, percentile† | |||||||
| Mean (SD) | 76.9 (29.7) | 91.3 (17.8) | 69.9 (31.9) | .004 | |||
| Not obese, <95th | 39 | 56 | 7 | 30 | 32 | 68 | .003 |
| Obese, ≥95th | 31 | 44 | 16 | 70 | 15 | 32 | |
AYA, adolescent and young adult; DXA, whole body dual-energy X-ray absorptiometry; SD, standard deviation.
*Fisher exact test or Student t test significance: P < .05.
†Obesity defined as sex/age adjusted BMI percentile ≥95% according to Centers for Disease Control and Prevention pediatric norms.
To determine the presence of CRLF2r, all patients diagnosed since 2016 were evaluated with the following combination of techniques. Flow cytometry was used to detect CRLF2 expression. Fluorescent in situ hybridization (FISH) was performed using CRLF2 (Cytocell, Cambridge, United Kingdom) and IGH (Abbott Molecular Inc, Des Plaines, IL) breakapart probes; cases positive for IGH and CRLF2 rearrangements were classified as IGH-CRLF2. If FISH was positive for a loss of 5′ CRLF2 signal and negative for an IGH rearrangement, the case was classified as P2RY8-CRLF2. For these cases, chromosomal microarray confirmed a deletion in the Xp22.3/Yp11.3 pseudoautosomal region between the P2RY8 and CRLF2 genes. A proprietary platform17 for amplification-based next-generation sequencing of tumor DNA and RNA identified concurrent IKZF1, JAK, and interleukin 7 receptor mutations, which further supported the presence of a CRLF2r and directly detected the P2RY8-CRLF2 fusion. Prior to 2016, CRLF2r was identified on banked aspirate specimens using the same FISH approach. All pathologic diagnoses of CRLF2r were centrally reviewed. Following distributional analyses, locally weighted scatterplot smoothing (bandwidth, 0.8) was used to depict the smoothed average of the proportion of CRLF2r ALL cases by presenting FM. Linear and logistic regression models, adjusting for ethnicity and age, were constructed to, respectively, analyze the association of demographics with FM at diagnosis and FM with presence of CRLF2r. All analyses were 2-sided (P < .05) and calculated using R (www.r-project.org). All studies were approved by the institutional review board and informed consent was obtained from all participants.
Ninety-seven B-ALL patients ≥10 years old were included (supplemental Table 1).1 Of these, 27 of 97 (28%) were unevaluable due to technical difficulties performing FISH on the banked specimens. There were no significant differences in body composition or ethnicity for unevaluable cases nor between testing cohorts (supplemental Tables 2 and 3). Of those evaluable, 23 of 70 (33%) were diagnosed as CRLF2r ALL (16 of 23 IGH-CRLF2; 6 of 23 P2RY8-CRLF2; 1 of 23 CRLF2 to unknown). Ten (44%) of the 23 CRLF2 cases had concurrent IKZF1 deletions, 3 (13%) had JAK mutations, and 3 (13%) had interleukin 7 receptor mutations.
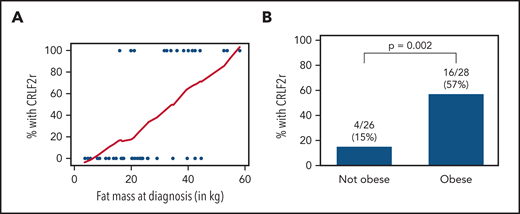
Patients with CRLF2r ALL presented with significantly higher FM, BF%, and BMI% (Table 1). Even within the subset of Hispanic/Latinx subjects, FM and obesity were associated with prevalence of CRLF2r (Figure 1). Obesity was similarly more prevalent in those with vs without CRLF2r (supplemental Figure 1). As expected, Hispanic/Latinx ethnicity was associated with a higher prevalence of obesity (supplemental Table 4) and was positively correlated with FM (β = 14.51; 95% confidence interval [95% CI], 7.12-21.90; P = .0002). In multivariable analysis adjusting for ethnicity, the odds of being CRLF2r increased by ∼13% for every kg of FM (odds ratio, 1.125; 95% CI, 1.056-1.198; P = .0003). As prevalence of CRLF2r is increased in adolescents and young adults,2,18 adjusting for age in addition to ethnicity yielded similar effects of obesity on CRLF2r risk (odds ratio, 1.119; 95% CI, 1.049-1.194; P = .0007).
Association of BF and obesity with CRLF2r ALL in Hispanic/Latinx patients. (A) In Hispanic/Latinx patients newly diagnosed with B-ALL, the proportion of those with CRLF2r vs non-CRLF2r increased with FM (kg) as measured by whole-body dual-energy X-ray absorptiometry. Blue dots represent each case (with/without CRLF2r); the red line represents the moving smoothed average for proportion of cases with CRLF2r by increasing FM (x-axis). (B) CRLF2r was similarly more prevalent in Hispanic/Latinx patients who were obese (BMI ≥95%) vs those who were nonobese (BMI <95%).
Association of BF and obesity with CRLF2r ALL in Hispanic/Latinx patients. (A) In Hispanic/Latinx patients newly diagnosed with B-ALL, the proportion of those with CRLF2r vs non-CRLF2r increased with FM (kg) as measured by whole-body dual-energy X-ray absorptiometry. Blue dots represent each case (with/without CRLF2r); the red line represents the moving smoothed average for proportion of cases with CRLF2r by increasing FM (x-axis). (B) CRLF2r was similarly more prevalent in Hispanic/Latinx patients who were obese (BMI ≥95%) vs those who were nonobese (BMI <95%).
We report here the new finding of an association between body fat and CRLF2r ALL in older children and adolescents. To our knowledge, this is the first description connecting obesity to a specific high-risk genomic variant in ALL. Increased prevalence of CRLF2r likely contributes to the poorer survival rates observed in obese patients with B-ALL.7,19 As obesity has recently been reported to increase the risk for developing B-ALL in Hispanic/Latinx populations,5 it is likely that obesity may also contribute to the association between Hispanic/Latinx ethnicity and CRLF2r ALL.1-3 How obesity predisposes to CRLF2r in this group remains unknown. A recent genome-wide association study found that GATA3 variants were associated with risk for developing CRLF2r ALL and that the penetrance of the risk-associated allele was higher in Hispanic/Latinx populations.20,21 Indeed, this same GATA3 polymorphism contributes to altered adipogenesis and diabetes risk,22,23 implicating a common susceptibility pathway. Alternatively, one might hypothesize that Hispanic/Latinx patients may be genetically susceptible to developing the CRLF2r variant of B-ALL, and obesity-induced signaling of phosphatidylinositol 3-kinase(PI3K)/AKT and mammalian target of rapamycin (mTOR) leukemia24 provides a “second hit” that promotes survival and propagation of the CRLF2r leukemic clone. Improved understanding of the interaction of genetic predisposition, ethnicity, and obesity with development of CRLF2r ALL may provide critical clues to preventing or treating this high-risk subtype of ALL.
This study has several strengths. Foremost, all patients underwent prospective and detailed measurement of BF, negating reliance on BMI as a surrogate and imprecise measure and allowing for quantification of the risk derived from increasing amounts of FM. Second, most patients received comprehensive testing for CRLF2r and concurrent mutations. Third, although our study was not powered to detect a direct association with Hispanic/Latinx, the rates of CRLF2r ALL found in aggregate and in the Hispanic/Latinx population are similar to that previously reported,1-3,18 further supporting the generalizability of these findings. These strengths were balanced by several innate limitations. Prior to 2016, CRLF2r was identified by FISH only; although FISH was 100% concordant with other testing, we cannot exclude the possibility, however unlikely, that flow cytometry or genomic analysis may have uncovered evidence of a cryptic CRFL2r. Additionally, as the trials only enrolled older children with ALL, we could not explore the impact from obesity at a younger age (ie, <10 years old). Finally, we acknowledge that to include precise measures of body composition, these findings were derived from a series of prospective trials with a relatively small sample size and validation is now required within larger cohorts.3,18 Nonetheless, these findings offer new insights into the complex relationship between ethnicity and CRLF2r. As a potentially modifiable risk factor, obesity offers a unique opportunity for prevention and/or early intervention in those with inherited susceptibility to a high-risk form of B-ALL.
Deidentified data are available upon reasonable request for 3 years from eorgel@chla.usc.edu.
The online version of this article contains a data supplement.
Acknowledgments
The authors thank Gerald Lu and Michael Lu from the Center of Personalized Medicine for their work on the CRFL2r testing. The authors thank the participants in the studies and their families, as well as the sponsors of this research (the Leukemia & Lymphoma Society, Gabrielle’s Angel Foundation for Cancer Research, and the National Institutes of Health, National Cancer Institute).
This work was supported by the Gabrielle’s Angel Foundation for Cancer Research and by Leukemia & Lymphoma Society grant LLS624911. This study was also supported, in part, by National Institutes of Health grants R01 CA201444 (S.D.M.), P30 CA16042 and P50 CA2110 (G.L.) from the National Cancer Institute, and UL1TR000124 (G.L.) from the National Center for Advancing Translational Sciences. This work was also supported, in part, by National Institutes of Health, National Center for Advancing Translational Sciences grants UL1TR001855 and UL1TR000130 via the Southern California Clinical and Translational Science Institute.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: E.O. was responsible for study conception; E.O. and S.D.M. designed the study; G.R. and M.J.O. were responsible for central review of hematopathology; E.O., S.D.M., J.K., and G.L. verified data; J.K. and G.L. analyzed data; and all authors interpreted data, revised the manuscript, and gave final approval of the manuscript.
Conflict-of-interest disclosure: E.O. was on a scientific advisory board for Jazz Pharmaceuticals outside of the scope of this work. The remaining authors declare no competing financial interests.
Correspondence: Etan Orgel, Cancer and Blood Disease Institute, Children’s Hospital Los Angeles, 4650 Sunset Blvd, MS#54, Los Angeles, CA 90027; e-mail: eorgel@chla.usc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal