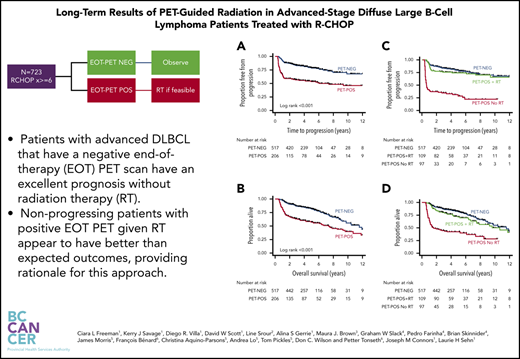
Key Points
Patients with advanced DLBCL who have a negative EOT PET scan have an excellent prognosis without RT.
PET-POS patients with nonprogressing disease given RT at EOT have better-than-expected outcomes, providing rationale for this approach.
Abstract
Consolidative radiation therapy (RT) for advanced-stage diffuse large B-cell lymphoma (DLBCL) remains controversial, with routine practice continuing to include RT in patients with initial bulky disease or residual masses. Positron emission tomography (PET)-computed tomography is a sensitive modality for detecting the presence of residual disease at the end of treatment (EOT). A PET-guided approach to selectively administering RT has been the policy in British Columbia since 2005. Patients with advanced-stage DLBCL diagnosed from 1 January 2005 to 1 March 2017 and treated with at least 6 cycles of R-CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisolone plus rituximab), who underwent EOT PET, were included in this analysis. Those with complete metabolic response (PET-negative [PET-NEG]) were observed; those with PET-positive (PET-POS) scans were offered consolidative RT, when feasible. Of the patient records reviewed, 723 were identified, with median follow-up of 4.3 years: 517 (72%) were PET-NEG; 206 (28%) were PET-POS. Time to progression (TTP) and overall survival (OS) at 3 years were 83% vs 56% and 87% vs 64%, in patients with PET-NEG and PET-POS scans, respectively. PET-POS patients with nonprogressing disease treated with consolidative RT (109 and 206; 53%) had outcomes approaching those of PET-NEG patients, with 3-year estimates of 76% and 80% for TTP and OS. PET-NEG patients who had bulky disease (≥10 cm) at diagnosis had outcomes indistinguishable from those without bulk, despite the omission of RT. These data suggest that patients with advanced-stage DLBCL who are PET-NEG at EOT and receive no RT have excellent outcomes. 18F-fluorodeoxyglucose-PET can reliably guide selective administration of consolidative RT, even in patients with initially bulky disease.
Introduction
Despite advances in the diagnosis and treatment of diffuse large B-cell lymphoma (DLBCL), outcomes remain variable, with durable remissions achieved in 60% to 70% of all patients treated with immunochemotherapy.1-5 Patients with advanced-stage disease are typically treated with 6 to 8 cycles of R-CHOP (rituximab with cyclophosphamide, doxorubicin, vincristine, and prednisolone), and consolidative radiation therapy (RT) is often considered for those presenting with bulky disease or residual masses after completion of immunochemotherapy.6,7 In addition, consolidative RT is often recommended for certain extranodal sites, such as craniofacial or isolated bone.7-11 Although RT is often administered in these settings, practices are variable and its use remains controversial.12-18
Positron emission tomography (PET) with 18F-fluorodeoxyglucose (FDG) is highly sensitive for the detection of DLBCL and is recommended for staging and remission assessment in international guidelines.19,20 Prospective studies have confirmed the prognostic utility of an end-of-treatment (EOT) PET scan to distinguish between patients with excellent clinical outcomes and those with higher likelihood of relapse, with 2-year progression-free survival (PFS) estimates for those who achieve a complete metabolic response at completion of R-CHOP of 72% to 86% compared with 24% to 64% for those with persistent sites of PET positivity.21-25 As a result, using an EOT PET scan to identify patients in need of consolidative RT is an attractive option, sparing low-risk patients from potentially unnecessary RT and selectively using radiation only in those at high risk. More evidence is needed in the era of contemporary R-CHOP and modern PET imaging to more accurately select patients for optimized therapy.15 No published prospective randomized trials have been performed in this patient population, where the decision to proceed to RT has been based on the findings of the EOT PET scan.
In British Columbia (BC), since 2005, 6 cycles of R-CHOP (up to 8 before the publication of the RICOVER study26 ), followed by an EOT-PET scan, have been recommended for patients with newly diagnosed, advanced-stage DLBCL. Initially, this recommendation was for those with residual sites of disease (≥2 cm) on EOT computed tomographic (CT) scans, but in practice, patients frequently underwent an outcome PET scan irrespective of the CT findings. Patients achieving a negative EOT PET (PET-NEG) were observed, regardless of initial bulk or site of disease, whereas those with residual FDG-accumulation (PET-POS), in the absence of overt disease progression, were referred for consolidative RT to residual FDG-avid areas, if feasible. We report on the 14-year experience of this risk-adapted RT approach in patients with advanced-stage DLBCL in BC.
Methods
This retrospective study was approved by the University of British Columbia-BC Cancer Research Ethics Board and was conducted in accordance with the Declaration of Helsinki. The BC Cancer Lymphoid Cancer Database was used to identify all consecutive patients with newly diagnosed DLBCL from 1 January 2005 to 1 March 2017. Diagnostic biopsies were reviewed by an expert BC Cancer hematopathologist and categorized according to the World Health Organization classification that was current at the time of diagnosis.27,28 Assessment for MYC and BCL2/BCL6 translocations by fluorescence in situ hybridization was not routinely performed in all patients before the more recent iteration of the classification and thus was not available for the whole cohort.
Patients were included for analysis if they were at least 18 years of age, had advanced-stage disease, had been treated with R-CHOP with curative intent, and had undergone an EOT PET scan. Advanced stage was defined as Ann Arbor stages III/IV or stages I/II with B symptoms and/or bulky disease (≥10 cm). Patients underwent 6 (or rarely, up to 8) cycles every 21 days. Patients who had disease progression during therapy were excluded. The BC Cancer Department of Functional Imaging database was cross-referenced to identify all patients who underwent an EOT PET. Patients without an initial pretreatment staging PET were included in this analysis. Patients were excluded if they were HIV positive; had evidence of an underlying indolent lymphoproliferative disorder, central nervous system (CNS) involvement at diagnosis, or primary-mediastinal B-cell lymphoma; were treated with <6 cycles of R-CHOP; or had RT or additional antineoplastic agents (apart from corticosteroids) during initial R-CHOP.
Patients were considered to have bulky disease if they had a documented site of involvement amounting to ≥10 cm in any of the longest diameters. Patients with documented cortical bone involvement at diagnosis were categorized as having skeletal involvement. Patients were deemed to have craniofacial involvement if 1 or more of the following extranodal sites was involved: periorbital area (excluding intraocular), nasopharynx, nasal or paranasal sinuses, salivary glands, or oropharynx.
Functional imaging and PET-guided algorithm
PET-CT scans were performed and reported centrally at the BC Cancer Vancouver Centre. It was recommended that PET scans be performed 4 to 6 weeks after completion of therapy. Between 1 July 2005 and 1 January 2014 PET scans were interpreted according to the International Harmonization Project (IHP) guidelines.29 This scale was subsequently replaced by the Deauville 5-point scale19 with Deauville X, 1 to 3, considered to be negative. As a result of the different thresholds for positivity between these 2 criteria, all FDG-PET scans performed locally before 2014 were reclassified, blinded to outcome, in accordance with the Deauville criteria (P.T.). We also performed a sensitivity analysis limited to those patients who had scans initially reported in accordance with Deauville only.
In the absence of overt progression, the local policy in BC is that patients who are PET-POS at EOT are immediately referred for RT, without confirmatory biopsy, provided the FDG-avid residual sites can be encompassed within a reasonable volume with acceptable anticipated toxicity. The dose administered ranged from 30 to 40 Gy and was delivered over 15 to 20 fractions. The treated area targeted the PET-POS residual mass plus an additional margin of 2 to 5 cm, which was individualized according to anatomic site.30,31
Statistical analysis
Descriptive statistics were used to summarize the baseline characteristics of the patients, and the χ2 test was used to evaluate differences between patients who were PET-NEG and PET-POS at EOT. The 2-sample Mann-Whitney U test was used to determine significance between the medians of standardized uptake values (SUVs) at EOT because of the nonnormally distributed data. Time to progression (TTP) was defined as time from date of diagnosis to disease progression, relapse, or death related to lymphoma or acute treatment toxicity. Observations were censored on the date the patient was last known to be alive or, for patients who died as a result of causes unrelated to lymphoma or treatment, the date of death. The Kaplan-Meier method was used to estimate overall survival (OS) and TTP, with the log-rank test for comparisons between groups.32,33 Cox proportional hazards regression models were used to estimate the hazard ratios (HRs) for potential prognostic factors influencing TTP.34 In the multivariate model, univariate factors with P < .1 were included, in addition to age. A value of P < .05 (2-sided) was considered significant. Statistical analyses were performed with STATA/IC, version 13 (StataCorp, College Station, TX).
Results
PET-NEG vs PET-POS at completion of therapy
A total of 723 patients meeting the eligibility criteria outlined above were included in the analysis (supplemental Figure 1, available on the Blood Web site). Their clinical characteristics are summarized in Table 1. The median age at diagnosis was 65 years (range, 18-89). A slight majority were male (57%), most had Ann Arbor stage III/IV disease (74%), 41% had an ECOG (European Cooperative Oncology Group) performance status (PS) of 2 to 4, and 285 (39%) had bulky disease (≥10 cm). The majority (94%) of patients received 6 cycles of R-CHOP, and 44 (6%) were treated with 7 or 8 cycles. At EOT, 517 (72%) had a PET-NEG scan and 206 (28%) had a PET-POS scan. Baseline characteristics were largely similar between both groups (Table 1), except that PET-POS patients were more likely to have elevated lactate dehydrogenase (LDH), B symptoms, low hemoglobin, bone marrow (BM) involvement, and bulky disease at presentation compared with those who became PET-NEG.
Comparison of baseline characteristics by EOT PET
| Characteristic . | All patients (n = 723), n (%) . | PET-NEG (n = 517), n (%) . | PET-POS (n = 206), n (%) . | P . |
|---|---|---|---|---|
| Age >60 years | 460 (64) | 333 (64) | 127 (62) | .49 |
| Male | 411 (57) | 289 (56) | 122 (59) | .42 |
| ECOG PS 2-4 | 299 (41) | 203 (41) | 96 (47) | .15 |
| Stage III/IV | 534 (74) | 389 (75) | 145 (70) | .18 |
| B symptoms | 320 (44) | 207 (40) | 113 (55) | <.001 |
| Marrow involvement | 77 (11) | 63 (12) | 14 (7) | .03 |
| Elevated LDH | 393 (54) | 257 (54) | 136 (69) | <.001 |
| Extranodal sites >1 | 221 (31) | 165 (44) | 56 (38) | .15 |
| Bulky site ≥10 cm | 285 (39) | 172 (33) | 113 (55) | <.001 |
| Skeletal involvement | 142 (20) | 103 (20) | 39 (19) | .76 |
| Craniofacial involvement | 41 (6) | 33 (6) | 8(4) | .19 |
| Hemoglobin <110 g/L | 191 (26) | 124 (26) | 67 (35) | .02 |
| IPI 3-5 | 377 (52) | 260 (55) | 117 (59) | .33 |
| Characteristic . | All patients (n = 723), n (%) . | PET-NEG (n = 517), n (%) . | PET-POS (n = 206), n (%) . | P . |
|---|---|---|---|---|
| Age >60 years | 460 (64) | 333 (64) | 127 (62) | .49 |
| Male | 411 (57) | 289 (56) | 122 (59) | .42 |
| ECOG PS 2-4 | 299 (41) | 203 (41) | 96 (47) | .15 |
| Stage III/IV | 534 (74) | 389 (75) | 145 (70) | .18 |
| B symptoms | 320 (44) | 207 (40) | 113 (55) | <.001 |
| Marrow involvement | 77 (11) | 63 (12) | 14 (7) | .03 |
| Elevated LDH | 393 (54) | 257 (54) | 136 (69) | <.001 |
| Extranodal sites >1 | 221 (31) | 165 (44) | 56 (38) | .15 |
| Bulky site ≥10 cm | 285 (39) | 172 (33) | 113 (55) | <.001 |
| Skeletal involvement | 142 (20) | 103 (20) | 39 (19) | .76 |
| Craniofacial involvement | 41 (6) | 33 (6) | 8(4) | .19 |
| Hemoglobin <110 g/L | 191 (26) | 124 (26) | 67 (35) | .02 |
| IPI 3-5 | 377 (52) | 260 (55) | 117 (59) | .33 |
Bold P values indicate statistical significance.
ECOG, European Cooperative Oncology Group; IPI, International Performance Index; LDH, lactate dehydrogenase.
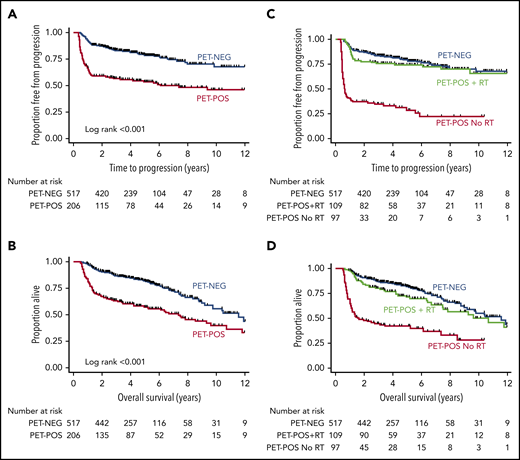
With a median duration of follow-up for living patients of 4.3 years (range, 0.9-14.2), estimates for 3-year TTP were 83% (95% confidence interval [CI], 80-86) for PET-NEG patients and 56% (95% CI, 49-63) for PET-POS patients (log-rank P < .001; Figure 1A). OS at 3 years was 87% (95% CI, 84-90) for PET-NEG patients and 64% (95% CI, 57-70) for PET-POS patients (log-rank P < .001; Figure 1B).
TTP and OS estimates for patients by EOT PET and RT status. Shown are TTP for PET-NEG vs PET-POS (A) and for PET-NEG vs PET-POS with RT and PET-POS without RT (C); OS for PET-NEG vs PET-POS (B) and for PET-NEG vs PET-POS with RT and PET-POS without RT (D).
TTP and OS estimates for patients by EOT PET and RT status. Shown are TTP for PET-NEG vs PET-POS (A) and for PET-NEG vs PET-POS with RT and PET-POS without RT (C); OS for PET-NEG vs PET-POS (B) and for PET-NEG vs PET-POS with RT and PET-POS without RT (D).
Outcomes for patients with bulky disease at presentation
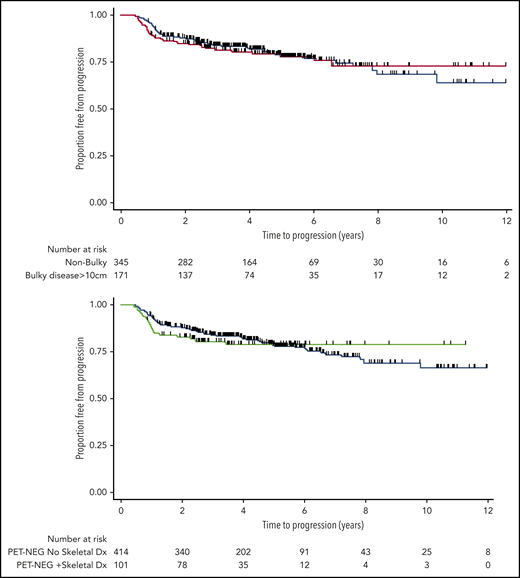
Of the 285 patients with sites of bulky disease (≥10 cm) at diagnosis, 172 (60%) became PET-NEG at EOT. Of those, only 1 was treated with RT at the discretion of the treating physician (excluded from PET-NEG group); the rest were observed. The estimates for 3-year TTP of patients with bulky disease who became PET-NEG was virtually identical with that of patients with nonbulky disease who also became PET-NEG: 82% (95% CI, 75-87) vs 84% (95% CI, 80-88), respectively (log-rank P = .92; Figure 2, top). There was no difference in these results when a cutoff of 7.5 cm was used to define disease bulk (results not shown). Equally, when these patients were subdivided according to stage, the presence of bulky disease at baseline still had no impact on the outcome of patients who had limited (log-rank P = .56) or advanced-stage disease, once complete metabolic response was observed on EOT PET (log-rank P = .98; supplemental Figure 2).
TTP estimates. TTP for PET-NEG patients with or without initial tumor bulk (≥10 cm at presentation) (top) and for PET-NEG patients with or without sites of skeletal involvement (bottom).
TTP estimates. TTP for PET-NEG patients with or without initial tumor bulk (≥10 cm at presentation) (top) and for PET-NEG patients with or without sites of skeletal involvement (bottom).
Outcomes for patients with skeletal lesions and craniofacial sites at presentation
Of the 142 patients with documented sites of skeletal involvement at diagnosis, 103 (73%) became PET-NEG and 39 (27%) remained PET-POS at EOT, proportions similar to those of patients who did not have skeletal involvement (P = .8). Of those, 2 were treated with consolidative RT to bony sites of original disease at the discretion of the treating physician (excluded from PET-NEG group). The presence of skeletal disease at diagnosis did not influence TTP in those patients who became PET-NEG (log-rank P = .67; Figure 2, bottom). A progression event was documented in only 20 of 103 (15%) patients who were PET-NEG at EOT: 8 patients relapsed in the CNS, 11 progressed with multiple sites of disease, and 1 patient later received RT on clinical suspicion arising from imaging changes at an original bony site of disease, without histological confirmation of relapse.
Forty-one patients had documented craniofacial sites of involvement at diagnosis, and of those, 33 (80%) patients became PET-NEG at EOT (supplemental Table 6). Of those, 1 patient received consolidative RT to the site of original disease at the discretion of the supervising physician (excluded from PET-NEG group). Compared with PET-NEG patients without craniofacial sites of involvement, there was no difference in TTP (log-rank P = .76, supplemental Figure 3). Six (18%) PET-NEG patients later relapsed; in 5 of 6 patients, the sites of progression were outside of the area that may have been included in an empirical radiation field.
Predictors of relapse in PET-NEG patients
In keeping with recommendations, 513 of 517 (99%) of the patients with PET-NEG EOT scans were observed. For the 517 patients achieving complete metabolic response at EOT, univariate analysis of baseline characteristics associated with risk of relapse included stage III/IV, elevated LDH, baseline anemia (hemoglobin <110 g/L), the presence of B symptoms, and documented BM involvement (supplemental Table 7). An IPI (International Performance Index) score of 3 to 5 was also associated with a higher risk of relapse. However, in multivariate analysis, only B symptoms and the presence of BM involvement remained significant (Table 2).
Multivariate analysis of baseline characteristics associated with time-to-progression events, restricted to those patients who became PET-NEG at EOT
| Characteristic . | HR . | 95% CI . | P . |
|---|---|---|---|
| Age >60 years | 0.9 | 0.5-1.4 | .5 |
| ECOG PS >1 | 0.9 | 0.5-1.5 | .6 |
| Extranodal sites >1 | 1.0 | 0.6-1.6 | .9 |
| Ann Arbor stage III/IV | 1.5 | 0.6-3.8 | .3 |
| Elevated LDH | 1.3 | 0.8-2.2 | .3 |
| Hb <110 g/L | 1.3 | 0.7-2.2 | .4 |
| B symptoms | 2.0 | 1.2-3.2 | .007 |
| Marrow involvement | 2.9 | 1.7-5.0 | <.0001 |
| Characteristic . | HR . | 95% CI . | P . |
|---|---|---|---|
| Age >60 years | 0.9 | 0.5-1.4 | .5 |
| ECOG PS >1 | 0.9 | 0.5-1.5 | .6 |
| Extranodal sites >1 | 1.0 | 0.6-1.6 | .9 |
| Ann Arbor stage III/IV | 1.5 | 0.6-3.8 | .3 |
| Elevated LDH | 1.3 | 0.8-2.2 | .3 |
| Hb <110 g/L | 1.3 | 0.7-2.2 | .4 |
| B symptoms | 2.0 | 1.2-3.2 | .007 |
| Marrow involvement | 2.9 | 1.7-5.0 | <.0001 |
Bold P values indicates statistical significance.
IPI, International Performance Index; LDH, lactate dehydrogenase.
PET-POS at completion of therapy and the use of consolidative RT
Of the 206 patients with PET-POS EOT scans, 109 (53%) received consolidative RT. The estimates for 3-year TTP were 76% (95% CI, 66-83) for PET-POS patients treated with RT, a result that was not statistically different when compared to 3-year TTP for patients who were PET-NEG at EOT (log-rank P = .3; Figure 1C). By comparison, 3-year TTP was 34% (95% CI, 25-44) for patients with PET-POS scans who were not treated with RT (log-rank P < .001; Figure 1C). The estimates for 3-year OS were 87% (95% CI, 84-90), 80% (95% CI, 71-87), and 44% (95% CI, 34-54) for PET-NEG, PET-POS treated with RT, and PET-POS not treated with RT, respectively (Figure 1D).
For a variety of reasons, the 97 patients with a PET-POS EOT scan did not undergo RT. One patient refused RT, and 4 patients had CNS relapse. Seven patients underwent surgical excision of a solitary site of PET positivity (splenectomy, n = 6; mastectomy, n = 1) with only 1 having DLBCL demonstrated in the resected specimen (spleen). The majority (59 of 97; 57%) had disease that could not be included in feasible RT fields, because of either location or extent, and were referred for salvage chemotherapy or palliation, as appropriate. In total, 15 patients ultimately underwent autologous stem cell transplant consolidation (ASCT). Of those, 7 were still alive at the most recent time of follow-up, in line with expectations from this approach. For the remaining PET-POS patients, the treating physician elected to perform further investigation or opted for surveillance.
Almost one-third of PET-POS patients who did not receive RT (29 of 97, 30%) did not relapse, despite having no further treatment of lymphoma. Within this cohort of 97, median SUVmax reported for the EOT PET differed significantly between progressors (n = 68) and nonprogressors (n = 29): 16.3 (range, 2.3-36) vs 4.5 (range, 1.7-18.1), respectively (P < .0001; supplemental Figure 4), albeit with notable overlap in the range of positivity.
Of the PET-POS patients who did receive consolidative RT (N = 109), there were a total of 30 relapses. Of those, 24 (80%) had progressive disease that developed outside of the radiated volume (n = 11 outside only, 13 both within and outside the radiated volume), 4 patients progressed only within the radiated volume, and for 2 patients, the sites of relapse were unknown.
Deauville-reported analysis
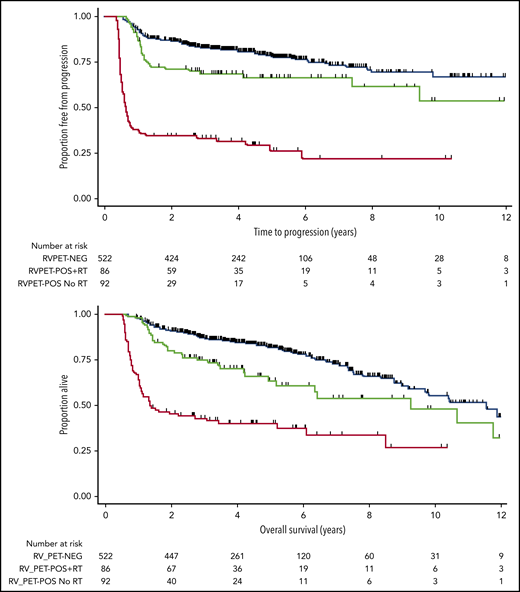
Given the differences in PET reporting criteria over time in our cohort, we reclassified all PET scans performed before 2014, blinded to outcome, in accordance with the Deauville criteria.19 As a result of the higher threshold for PET-POS (where a positive scan demonstrates an area of FDG uptake measuring greater than liver [Deauville],19 compared with the previous lower threshold of mediastinal blood pool [IHP]29 ), 545 patients were classified as PET-NEG and 178 patients as PET-POS at EOT. Patients with a PET-NEG scan (in accordance with Deauville thresholds) who received RT on the basis of their IHP-reported scan were not included in the survival estimates. Estimates of 3-year TTP were almost identical with the findings reported above: 83% (95% CI, 79-86) for patients who became PET-NEG, 69% (95% CI, 58-78) for those who were PET-POS treated with RT, and 33% (95% CI, 24-43) for those who were PET-POS and not treated with RT (Figure 3, top). OS at 3 years was 87% (95% CI, 84-90) for PET-NEG patients, 75% (95% CI, 64-83) for PET-POS patients treated with RT, and 52% (95% CI, 32-53) for those who were PET-POS and not treated with RT (Figure 3, bottom). We also confirmed that outcomes of patients achieving PET-NEG in accordance with the Deauville thresholds were identical with those of patients with bulky and nonbulky disease (81% vs 84%, respectively; log-rank P = .99; supplemental Figure 5).
TTP and OS estimates for patients by EOT PET and RT status when scans were reclassified in accordance with Deauville criteria. TTP (top); OS (bottom).
TTP and OS estimates for patients by EOT PET and RT status when scans were reclassified in accordance with Deauville criteria. TTP (top); OS (bottom).
In addition, we performed a separate subgroup analysis limited to the 345 (48%) patients who had their scans reported at the time of imaging strictly in accordance with the Deauville criteria.19 Although the median duration of follow-up was understandably shorter than that for the entire cohort (2.9 years; range, 0.9-5.8), outcomes were again similar to those for the group as a whole. The 3-year TTP was 81% (95% CI, 75-86) for patients who became PET-NEG, 71% (95% CI, 55-83) for those who were PET-POS treated with RT (supplemental Figure 6), and 41% (95% CI, 27-55) for those who were PET-POS and not treated with RT. OS at 3 years was 87% (95% CI, 82-91) for PET-NEG patients, 76% (95% CI, 60-86) for PET-POS patients treated with RT, and 51% (95% CI, 36-65) for those who were PET-POS and were not treated with RT.
Discussion
Although R-CHOP has become the standard of care for patients with advanced-stage DLBCL, the use of consolidative RT remains controversial, with guidelines endorsing its use in certain situations, mainly on the basis of multicenter retrospective analyses.4,7-9 As ∼97% of patients with DLBCL have lesions that exhibit FDG avidity, PET is a sensitive assessment tool that has been recommended as a key component of staging and posttreatment evaluation for more than a decade, with numerous studies demonstrating its strong predictive value at completion of therapy.24,25,35-39 However, no published prospective trials evaluate the use of EOT PET in advanced stage DLBCL, to guide the use of consolidative RT in the modern treatment era.
Since 2005, it has been standard practice in BC to tailor the use of consolidative RT based on EOT PET scan results, regardless of the presence of bulky disease or certain extranodal sites at presentation. This policy has been applied consistently across our universally treated cohort, with centralized PET imaging and reporting. This real-world experience with very mature follow-up is unique in its scope and should have practical value for clinicians, especially given the lack of prospective clinical evidence in this patient population.
We have demonstrated that patients who achieve a PET-NEG response at completion of therapy have excellent outcomes, with 83% free of relapse at 3 years. Similar results were reported in a subanalysis of the phase 3 GOYA trial, with a landmark analysis from EOT reporting an estimated 2.5-year PFS of 86.1% for those who became PET-NEG at treatment completion.24
Importantly, of the 285 patients in our analysis who had bulky disease, 172 (60%) had a PET-NEG scan, and all but 1 was observed without additional consolidative RT. This did not appear to compromise their outcome with virtually identical 3-year TTP compared with those without bulky disease (82% vs 84%, respectively).
Similar results were reported in an interim analysis of the OPTIMAL >60 trial of older patients with DLBCL, with a cutoff for bulky disease of ≥7.5 cm. The researchers concluded that a PET-guided approach could effectively spare 42% of patients the toxicity of radiation without affecting outcome.40
Previously published retrospective analyses, most performed in the absence of functional imaging for response assessment, have suggested benefit conferred by the use of routine consolidative RT in advanced DLBCL, in addition to its application to certain extranodal sites, in particular skeletal involvement.9,10,17,18,41-44 As a result, there are recommendations that continue to endorse RT in these patients.7,13,45 We acknowledge that there are centers that have changed their practice in the modern era and have already adopted a similar PET-guided algorithm, omitting RT in patients with PET-NEG scans at EOT. Our data provide the evidence that has been lacking to date in support of this practice, demonstrating the outcomes that can be expected by patients who achieve PET negativity at completion of therapy, including those with bulky, skeletal, or craniofacial sites of disease pretreatment, without the incorporation of additional consolidative RT.
We appreciate that in the absence of prospective data randomizing these patients to RT vs observation alone, we cannot exclude the possibility that there may be a proportion of patients who could benefit from the addition of RT. There is still room for improvement for those patients who experience relapse after R-CHOP, and the possibility that RT as an adjuvant strategy could improve outcomes for these patients is not disproven with these data. However, as there are no immediate plans to undertake such a randomized trial that we are aware of, this population-based analysis (with its limitations) can serve to inform treating physicians on the outcomes that may be anticipated by following a PET-guided approach.
For patients who are PET-POS at EOT, PFS estimates reported in the literature are highly variable at 2 to 3 years (range, 24% to 64%).21,23-25 However, those patients consistently have significantly worse outcomes than their PET-NEG counterparts. In contrast, the outcomes for patients in our cohort with PET-POS scans who underwent consolidative RT appeared to be considerably better than expected, suggesting that select patients benefit from this approach.
It is important to note that the PET-POS patients who proceeded to RT are a highly select cohort, with nonprogressive, radio-encompassable disease. In the absence of disease progression, patients in BC do not universally undergo biopsy, but proceed immediately to RT if amenable. This policy has been adopted because of the frequent challenges in obtaining reliable biopsies from sites of concern following immunochemotherapy, the high risk of false-negative biopsies, and the inevitable delay in starting RT. The decision to proceed to immediate RT in these patients is based on the statistical likelihood that these residual PET-POS sites harbor persistent disease.
In patients for whom RT is feasible, proceeding to consolidative radiation is believed to be a practical and well-tolerated alternative to proceeding immediately to salvage chemotherapy and consolidative ASCT and may eliminate the need for further therapy. This strategy has the potential to spare certain patients the toxicity associated with salvage regimens and ASCT. Our outcomes suggest benefit conferred by this algorithm when compared to historical reports, but we acknowledge that some patients treated with consolidative RT may not have harbored residual disease.
The outcomes observed in the heterogeneous group of patients who remained PET-POS at EOT and did not receive RT were, not surprisingly, poor. The majority of those patients were presumed to have refractory disease at completion of therapy and proceeded to immediate salvage chemotherapy or palliation. However, approximately one-third of those patients did not relapse, despite having no further treatment of lymphoma, consistent with reported estimates of false positivity.46 Interestingly, these patients tended to have lower SUVmax reported on EOT scans compared with those in whom the disease progressed, but there was considerable overlap in the range of values. These findings are similar to those reported in another study of patients with primary mediastinal B-cell lymphoma, with higher SUVmax values noted on EOT PET in treatment failures compared with patients with PET-POS scans who did not progress with surveillance and serial imaging.47 This observation certainly merits more detailed exploration, ideally with PET parameters that may be less subject to site and methodological variability than the SUVmax.48-51
Although our study was retrospective and not randomized, it has several unique strengths. It was large in scale, included mature follow-up, and represented real-world outcomes in a population treated with a comprehensively applied management algorithm. We appreciate that over the course of the reported period, PET technology has evolved. However, as PET scanning was limited to a single center, there was consistency in reporting among the small number of nuclear medicine physicians who reviewed all the images. Given the change in reporting criteria from IHP to Deauville during the course of this study, we retrospectively reclassified all scans in line with the Deauville criteria. Survival estimates across the 3 groups were almost identical. In addition, we performed a separate sensitivity analysis, limited only to scans reported by Deauville criteria at the time of imaging, which again demonstrated outcomes similar to those seen in the larger cohort.
Our analysis does have its limitations. Not all patients underwent a staging PET scan, which can make the reporting of scans at EOT more challenging. It is also possible that the few patients with apparent limited-stage disease who may have been upstaged by a baseline PET are not captured in our cohort,52 and therefore our results may be conservative in the estimation of true outcomes. Our analysis was restricted to those patients who successfully completed a full course of R-CHOP. Therefore, the value of PET scans in patients who received an abbreviated induction cannot be determined. In addition, PET scans were initially recommended only for patients with residual abnormalities on EOT CT, thus those achieving a complete radiological remission on CT scan were most likely underrepresented in our cohort.
This mature analysis effectively demonstrates that the use of a PET-guided approach to selectively administer consolidative RT in patients with advanced-stage DLBCL is feasible and appears to be associated with favorable outcomes. Using this approach, the most of our patients are not subjected to the potential toxicity and associated costs of consolidative RT, regardless of initial bulk or select extranodal sites of disease at presentation. The outcomes for nonprogressing patients who remain PET-POS and undergo consolidative RT appeared better than historical expectations. For this group of patients, our data suggest that benefit may be conferred by this management approach and could spare some patients the toxicity of proceeding immediately to salvage chemotherapy and ASCT.
Presented in abstract form at the 52nd annual meeting of the American Society of Hematology, Orlando, FL, 4 December 2010, and the International Conference on Machine Learning, Atlanta, GA, 16 June 2013, and updated at the 59th annual meeting of the American Society of Hematology, Atlanta, GA, 9 December 2017.
Original data are available upon request to Ciara L. Freeman (ciara.freeman@bccancer.bc.ca).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
No funding was received for this study.
Authorship
Contribution: G.W.S., P.F., and B.S. were involved in pathological central review; F.B., D.C.W., and P.T. reviewed the PET scans; J.M., C.A.-P., A.L., and T.P. were responsible for the delivery of RT; M.J.B. contributed to radiology review; C.L.F. performed the final analysis and wrote the paper; and all authors were involved in data acquisition, contributed to the manuscript, and approved the final submission.
Conflict-of-interest disclosure: C.L.F. has received honoraria from Seattle Genetics, Janssen, Amgen, Celgene, and AbbVie and research funding from Roche and Teva. D.W.S. is a named inventor and receives royalties on a patent licensed to NanoString Technologies; has received research funding from NanoString Technologies, Janssen, and Roche; and research funding, consultancy fees, and travel expenses from Celgene. J.M.C. has received research funding from Merck, Janssen, Bristol Myers-Squibb, Cephalon, Amgen, Bayer Healthcare, Genentech, Lilly, F. Hoffmann-La Roche, Roche Canada, and Amgen; is a named inventor and receives royalties on a patent licensed to NanoString Technologies; and has received research funding and honoraria from Seattle Genetics and Takeda. L.H.S. has consulted for and/or received honoraria from Roche/Genentech, AbbVie, Amgen, Apobiologix, Astra Zeneca, Acerta, Celgene, Gilead, Janssen, Kite, Karyopharm, Lundbeck, Merck, Morphosys, Seattle Genetics, Teva, Takeda, TG Therapeutics, and Verastem; and has received research funding from Roche/Genentech. A.S.G. has received research funding from Roche, Lundbeck, Janssen, and Accerta (all institutional funding) and honoraria from Roche and Janssen; has served on the AbbVie Advisory Board; and has received honoraria from Janssen, AbbVie, and Celgene. K.J.S. has consulted for and received honoraria from Seattle Genetics, BMS, Merck, AbbVie, Astra-Zeneca, and Servier; has served on the steering committee for Beigene; has received research funding from Roche/Genentech; and has received institutional clinical trial funding from BMS, Merck, Takeda, and Beigene. D.R.V. has received honoraria from Janssen, Roche, Lundbeck, Celgene, Seattle Genetics, AbbVie, AstraZeneca, Gilead, and NanoString Technologies. L.S. has received honoraria from Janssen, Roche, Servier, Seattle Genetics, Amgen, Novartis, and AbbvVie. T.P. has received honoraria from Tarsera, AbbVie, and Sanofi. The remaining authors declare no competing financial interests.
Correspondence: Ciara L. Freeman, British Columbia Cancer Agency, 600 West 10th Ave, Vancouver, BC V5Z 4E6, Canada; e-mail: ciara.freeman@bccancer.bc.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal