Abstract
Plasminogen is an abundant plasma protein that exists in various zymogenic forms. Plasmin, the proteolytically active form of plasminogen, is known for its essential role in fibrinolysis. To date, therapeutic targeting of the fibrinolytic system has been for 2 purposes: to promote plasmin generation for thromboembolic conditions or to stop plasmin to reduce bleeding. However, plasmin and plasminogen serve other important functions, some of which are unrelated to fibrin removal. Indeed, for >40 years, the antifibrinolytic agent tranexamic acid has been administered for its serendipitously discovered skin-whitening properties. Plasmin also plays an important role in the removal of misfolded/aggregated proteins and can trigger other enzymatic cascades, including complement. In addition, plasminogen, via binding to one of its dozen cell surface receptors, can modulate cell behavior and further influence immune and inflammatory processes. Plasminogen administration itself has been reported to improve thrombolysis and to accelerate wound repair. Although many of these more recent findings have been derived from in vitro or animal studies, the use of antifibrinolytic agents to reduce bleeding in humans has revealed additional clinically relevant consequences, particularly in relation to reducing infection risk that is independent of its hemostatic effects. The finding that many viruses harness the host plasminogen to aid infectivity has suggested that antifibrinolytic agents may have antiviral benefits. Here, we review the broadening role of the plasminogen-activating system in physiology and pathophysiology and how manipulation of this system may be harnessed for benefits unrelated to its conventional application in thrombosis and hemostasis.
Introduction
The fibrinolytic system has long been recognized as the cornerstone for the removal of fibrin deposits and blood clots. However, overactivation of this system can result in severe bleeding. Current therapeutic modulation of fibrinolysis has been devoted to boosting this to aid in the removal of occlusive thrombi or to slow it down to reduce bleeding. Plasminogen, via its proteolytic form, plasmin, underpins the entire fibrinolytic process. Plasminogen is also one of the most abundant plasma proteins associated with hemostasis, yet it is rarely considered a diagnostic tool in clinical management. Perhaps the clinical imperative to justify this was lacking, possibly as a result of the perception that anomalies on the coagulation side of hemostasis have been considered as being more relevant, and sufficient interpretation could be gleaned from changes in levels of D-dimer or fibrinogen. However, the “yin and yang” of hemostasis places fibrinolysis on an equal footing with coagulation, because what is formed needs to be removed. This has also stymied the development of testing systems in pathology laboratories that enable accurate and rapid evaluation of the in vivo status or capacity of the fibrinolytic system in individuals with bleeding or clotting disorders. Adding further fuel to this is the recently discovered importance of plasminogen in other processes that have little or nothing to do with fibrin removal. Although these noncanonical roles of the “fibrinolytic” system have been surmised for some time, indeed even decades, what has changed recently is the extent to which plasminogen, and, in fact, the entire plasminogen-activation process impacts on numerous “nonfibrinolytic” processes. Indeed, a number of these plasminogen-dependent changes have clinically relevant consequences.
Here, we review the role of plasmin(ogen) in immunity, infection and inflammation, and wound healing, among others, and how in vivo modulation of plasmin generation has impacted on these processes in humans. A greater appreciation and more detailed investigation into plasmin(ogen)’s varied noncanonical properties will aid in further fundamental advances and widen the potential therapeutic application of this zymogen.
Fibrinolysis: the early days
The term “fibrinolysis” was coined in 1893 by the French physiologist Albert Dastre, who described the spontaneous time-dependent loss of fibrin in clotted blood.1 The protease responsible for this fibrinolytic process remained a mystery for the best part of half a century. However, a key advance, and indeed the unraveling of the entire process, started with the discovery of a fibrinolytic component produced by certain strains of β-hemolytic streptococci2 and Staphylococcus3 (streptokinase and staphylokinase, respectively). Neither streptokinase nor staphylokinase displayed fibrinolytic activity on its own; they were capable of generating potent activity only when combined with human plasma,4,5 hence revealing that these bacterial-derived entities needed to activate a plasma-derived zymogen.
The nomenclature in the early days was aligned toward fibrin(olysis) for obvious reasons; the unknown zymogen was initially referred to as “profibrinolysin,” and the active moiety was known as “fibrinolysin.”6 However, in 1945 Christensen and MacLeod7 revealed that the fibrinolytic substance was not fibrin specific, because it could cleave other substrates, including casein and gelatin. Therefore, these investigators abandoned this original nomenclature and imposed the new term “plasminogen” to define the “plasma zymogen” and “plasmin” as the active fibrinolytic protease.7,8
Biochemistry
Plasminogen is a 90-kDa glycoprotein that circulates in blood at a concentration ∼180 μg/mL (2 μM) with a plasma half-life ∼2 days. Although the protein concentration falls within a twofold range in healthy individuals, the extent of plasminogen activation (measured after ex vivo activation) occurs over an eightfold range and decreases with age.9 However, individuals in the lower range do not seem to be at risk for thrombosis10 although it remains to be determined whether this becomes clinically relevant in combination with other thrombophilic conditions.
Plasminogen also exists in 2 forms: glu-plasminogen (mature form) that predominates in plasma and lys-plasminogen, which is transiently formed after removal of the first 77 aa by plasmin. Lys-plasminogen is often reported as being more readily cleaved into plasmin than is glu-plasminogen; however, earlier reports cast doubt on this11 but showed that lys-plasminogen was more readily inactivated by antiplasmin.12 Adding further complexity to this is the fact that plasminogen possesses 2 glycoforms (I and II).13 Hence, mammalian plasma contains 4 versions of plasminogen and plasmin, each with likely various biological roles that are yet to be fully elucidated. Numerous genetic variants of plasminogen have also been reported.14
Physiologically, plasminogen activation essentially occurs on a needs-only basis. This feature is biochemically economical, sparing plasminogen from premature activation by tissue-type plasminogen activator (t-PA) or urokinase-type plasminogen activator (u-PA), yet restricting plasmin formation to the substrate intended. This targeted activation is due to its unique structure that maintains the zymogenic glu-plasminogen molecule in a closed activation-resistant form that, nonetheless, allows it to bind to its intended substrate before being activated into plasmin.15
Plasmin formation needs to be tightly controlled to ensure that fibrin (cross-linked by factor XIII) is removed appropriately and in a timely manner. The regulation of t-PA and u-PA activity is controlled by plasminogen activator inhibitor 1 (PAI-1) and PAI-216 , although PAI-1 is more relevant in plasma. The most important direct plasmin inhibitor is alpha2-antiplasmin, although plasmin can be further inhibited by alpha2-macroglobulin17 and CI-esterase inhibitor.18 Additional levels of regulation are provided by thrombin activatable fibrinolysis inhibitor (TAFI),19 a carboxypeptidase that removes exposed lysine residues that is important for plasminogen-fibrin interactions. These regulatory molecules are vitally important in controlling plasmin generation; hence, changes in levels of ≥1 of these components can dramatically alter plasmin formation (Figure 1).
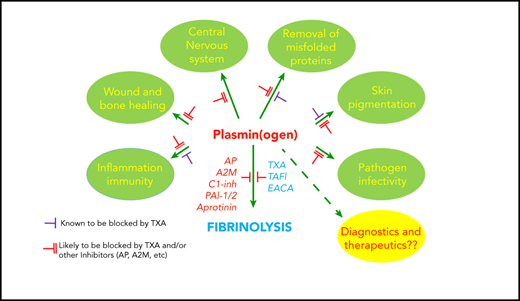
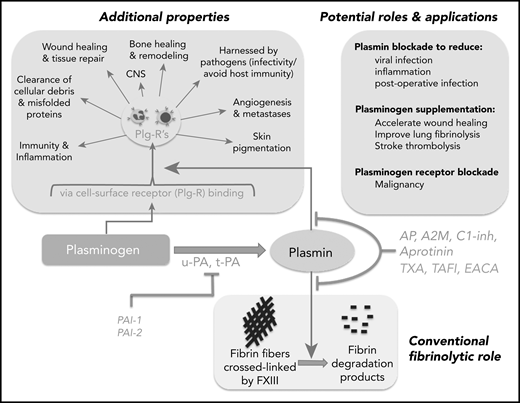
Schematic representation of the broad reach of plasminogen and the plasminogen activating system. The conventional function of this system has been in the removal of fibrin deposits and blood clots. Fibrin fibers formed following activation of coagulation are crossed linked by factor XIII (FXIII) and subsequently removed by plasmin. Activation of plasminogen by t-PA or u-PA can be inhibited by PAI-1 or PAI-2. Plasmin generation or activity can also be regulated by endogenous inhibitors: α2-antiplasmin (AP), TAFI, α2-macroglobulin (A2M), CI-inhibitor (C1-inh), or therapeutically by tranexamic acid (TXA), ε amino caproic acid (EACA; also a lysine analog), or aprotinin. Direct plasmin inhibitors are shown in red (AP, A2M, C1-inh, aprotinin), whereas those that inhibit plasmin generation (TAFI, TXA, EACA) are shown in blue. Plasminogen influences numerous other “additional” processes, some occurring via binding to plasminogen receptors (Plg-Rs) located on most leukocytes, permitting localized plasmin generation. Recent evidence has suggested that blocking plasmin formation might reduce viral infection and inflammation and improve immune function. On the other hand, plasminogen supplementation could be considered to improve wound healing, to more effectively remove parenchymal fibrin deposits, and to improve stroke thrombolysis. Plasminogen receptor blockade has also been proposed as a novel approach for malignant conditions.
Schematic representation of the broad reach of plasminogen and the plasminogen activating system. The conventional function of this system has been in the removal of fibrin deposits and blood clots. Fibrin fibers formed following activation of coagulation are crossed linked by factor XIII (FXIII) and subsequently removed by plasmin. Activation of plasminogen by t-PA or u-PA can be inhibited by PAI-1 or PAI-2. Plasmin generation or activity can also be regulated by endogenous inhibitors: α2-antiplasmin (AP), TAFI, α2-macroglobulin (A2M), CI-inhibitor (C1-inh), or therapeutically by tranexamic acid (TXA), ε amino caproic acid (EACA; also a lysine analog), or aprotinin. Direct plasmin inhibitors are shown in red (AP, A2M, C1-inh, aprotinin), whereas those that inhibit plasmin generation (TAFI, TXA, EACA) are shown in blue. Plasminogen influences numerous other “additional” processes, some occurring via binding to plasminogen receptors (Plg-Rs) located on most leukocytes, permitting localized plasmin generation. Recent evidence has suggested that blocking plasmin formation might reduce viral infection and inflammation and improve immune function. On the other hand, plasminogen supplementation could be considered to improve wound healing, to more effectively remove parenchymal fibrin deposits, and to improve stroke thrombolysis. Plasminogen receptor blockade has also been proposed as a novel approach for malignant conditions.
Most of the unraveling of substrate-dependent plasminogen activation was accomplished by investigating the interaction between plasminogen and fibrin, its most renowned substrate. Put simply, the substrate largely initiates the activation process: fibrin, once formed, exposes lysine residues on its surface that provide docking sites for critical “lysine binding sites” (LBSs) that are located within 4 of the 5 kringle domains of the plasminogen molecule. Even in its closed conformation, the LBS in kringle 1 of plasminogen is still exposed, allowing plasminogen to dock onto lysine residues on the fibrin surface.15,20 This initiates unfolding of the molecule, enabling the LBSs in the other domains to engage with other exposed lysine residues on fibrin. Hence, the fibrin surface provides the means for plasminogen to be released from its closed conformation, exposing its cleavage site to t-PA and u-PA to generate the proteolytically active moiety, plasmin. Therefore, fibrin is an essential cofactor for the activation of plasminogen to plasmin, essentially orchestrating its own demise after having served its purpose.21
There is little doubt regarding the physiological importance of targeted plasmin generation in the removal of fibrin. It has been well argued that fibrin is also the most important substrate for plasmin,22 but it is also true that plasmin has many other targets; indeed, plasminogen deficiency in mice results in fibrin accumulation, as well as in the reduced clearance of other nonfibrin substrates.23,24
Plasminogen activation outside of fibrinolysis: critical nonfibrin cofactors and substrates
Although the structural basis for the binding between fibrin and plasminogen was uncovered, this same process also facilitates the binding of plasminogen to many “nonfibrin” targets. O’Mullane and Baker25 reported that nonviable cells displayed ∼100-fold more lysine-dependent cell surface plasminogen binding and activation compared with their viable cell counterparts. The investigators referred to these plasminogen interacting sites as “plasminogen receptors” (Plg-Rs),25 but this implied that a physically intact receptor survived despite the cells being dead. Samson et al subsequently revealed that plasminogen was actually binding in a lysine-dependent manner to misfolded/necrotic proteins formed following cell death,24,26 resulting in their proteolytic removal.
The inclusion of misfolded proteins into the repertoire of lysine-dependent substrates for plasminogen further extends to proteins displaying an amyloid-like structure. This is not surprising because fibrin and misfolded proteins have a tendency to aggregate into fibrils and to display amyloid-like features.27 Indeed, Alzheimer amyloid β-peptide (αβ) analogs were reported to increase the stimulatory potential of t-PA to activate plasminogen28 ; this stimulation further increases when αβ-peptides form aggregated fibrillar structures similar to those found in amyloid deposits. Although plasminogen-deficient (Plg−/−) mice do not display any perturbation in amyloid levels under normal conditions,29 increasing plasminogen activation enhances αβ clearance.30
When considering this common mechanism, “fibrinolysis” per se may be better viewed as a general means to remove unwanted proteins from the body in a more general sense, with fibrin, although important, being only one of these substrates.
Plasminogen receptors
With fibrin removal being the primary focus of the fibrinolytic system at the very outset, and this being an enzymatic process occurring on the fibrin surface, there was little motivation to consider the possibility that plasminogen activation occurs on the cell surface, let alone performs any other cell-based function. This all changed in 1985 when Miles and Plow31 described a plasminogen receptor on the surface of platelets and subsequently on transformed cells.32 It has become apparent that ≥12 distinct plasminogen receptors exist,33 the most recent being Plg-RKT, which is gaining particular interest.34 Many of these receptors (and indeed misfolded proteins and fibrin) contain C-terminal lysine residues that are essential for plasminogen binding via its kringle domains.33 A number of the downstream consequences of plasminogen receptor–dependent cell modulation have now come to light.35,36
A puzzling question is why so many plasminogen receptors have evolved in the first place and why is there an evolutionary need for a given cell to harbor multiple plasminogen receptors simultaneously.37,38 Although, at first glance, this may be an example of extraordinary functional redundancy,37 it more likely reflects the fact that our knowledge is far from complete and that more research is required to understand the biological importance of such a diverse array of receptors. It also needs to be kept in mind that receptor specificity can be challenging, given the low affinity of plasminogen for lysine residues.
It had been reported that endothelial cells could bind several million plasminogen molecules per cell,39 and this binding capacity can increase threefold to 20-fold in response to various stimuli.37 Hence, the ability of a cell to capture plasminogen can be enormous. This extraordinary ability may provide an essential transportation means to deliver plasminogen to extravascular sites undergoing tissue/wound repair or remodeling.40 One recent publication reported a critical role for the plasminogen receptor Plg-RKT in cutaneous wound healing41 ; this particular receptor may explain how plasminogen levels in tissue increase approximately fivefold during the repair process.42
Nonfibrinolytic functions of plasminogen activation
Although hemostatic abnormalities were observed in Plg−/− mice,43 these mice also suffered from growth retardation, delayed wound healing, and reduced overall survival.43 This also revealed the broader relevance of fibrin, because these features were lost in mice deficient in both plasminogen and fibrinogen.22 This led to the suggestion that the only essential physiological role of plasminogen was, in fact, fibrinolysis. However, a series of more targeted investigations yielded information placing plasminogen as a key modulator of numerous processes, notably inflammation and immunity, and in the central nervous system (CNS) where fibrin was not necessarily the only driving culprit or the only target for plasmin.
Indeed, a primordial ancestor of plasminogen first appeared in protochordates based on studies of extant species.44 This molecule, cloned from the amphioxus Branchiostoma belcheri, can actually generate plasmin,45 but even more intriguing is that this lower-order species possesses a hemolymph that is unable to clot.46 Clearly, plasmin(ogen) exists in these species for purposes other than fibrin removal that remain enigmatic. The “noncanonical” findings for plasminogen reported in more recent times may, in fact, reflect some of these primitive functions.
Plasmin(ogen) in inflammation and immunity: fibrin and nonfibrin dependency
For decades, it has been known that fibrin deposition occurs in a number of acute and chronic inflammatory conditions47,48 and that the resolution of inflammation would require its timely removal.49 Fibrin itself was also revealed long ago as an immune stimulant,50 whereas fibrin fragments can promote51 or protect52 against inflammation. Most of these original observations and predictions have been confirmed and have extended into other areas, including neuroinflammation, where fibrin deposition is critical in the pathogenesis of some CNS disorders, including multiple sclerosis and Alzheimer disease.53,54
It is now evident that plasmin(ogen) intersects with the inflammatory system at a multitude of levels.55 Although fibrin deposition may be the initiating event in some examples, plasmin(ogen) can also interact directly, via its receptors, with numerous immune cells56 ; in doing so, it activates several proinflammatory pathways resulting in cytokine production.57 Plasminogen can further influence the innate immune response by activating complement58 and by promoting dendritic cell and macrophage phagocytosis.59,60
In contrast to its proinflammatory properties, plasmin also exhibits several anti-inflammatory and immunosuppressive responses. Plasmin(ogen) regulates key aspects of macrophage reprogramming, neutrophil apoptosis, and efferocytosis.61 For example, plasmin-treated dendritic cells fail to undergo maturation following phagocytosis, exhibit reduced migration to lymph nodes, and stimulate a 17-fold increase in transforming growth factor-β, which has immunosuppressive properties. These cells also have a reduced ability to induce allogeneic lymphocyte proliferation.59 These properties of plasmin are important in maintaining tissue homeostasis that may be important to prevent self-reactivity/autoimmunity.59,60
Wound and bone repair
Plg−/− mice exhibit significant delays to wound healing.36 Although plasmin-dependent removal of fibrin is essential for skin closure and healing,22 plasminogen now appears to be involved in the initiation, resolution, and proliferative phases of the inflammatory process during wound healing. Delayed formation of granulation tissue also suggests that the lack of plasminogen impairs fibroblast function.40 This was revealed in models of tympanic membrane perforations that fail to heal completely in Plg−/− mice.62,63
In acute burn wound models, Shen and colleagues showed that systemic administration of plasminogen accelerated wound healing, and improved the healing of chronic wounds, in a mouse model of diabetes.42 They proposed plasminogen as a novel therapeutic strategy for the management of chronic wounds. The benefit of exogenous plasminogen in the setting of diabetes may be further explained by the fact that plasmin(ogen) from diabetic patients has impaired fibrinolytic activity64 as a consequence of glycation impairing fibrin-dependent plasmin generation and activity.64,65 Although the animal studies are consistent with a beneficial effect of plasminogen during wound repair, additional research is needed to determine whether plasminogen supplementation will improve wound healing in diabetic and nondiabetic humans.
Plasmin(ogen)’s role in the induction and resolution of inflammation is further seen in radiodermatitis, a severe side effect of radiotherapy; however, in this example, its role is different from that seen in other models of skin wound healing. Fallah and colleagues66 showed that plasminogen levels increased in the irradiated area, leading to severe skin damage. Plg−/− mice were largely protected from radiodermatitis, which is in stark contrast to acute cutaneous injury models in which healing was delayed in Plg−/− mice. Furthermore, inhibition of plasmin generation with tranexamic acid (TXA) reduced radiodermatitis in wild-type mice and completely prevented pathology in heterozygous Plg+/− mice.66 However, a different scenario is seen when plasminogen administration is delayed after radiation injury. Daily administration commencing 10 days after injury significantly improved healing rates67 and was shown to alter the expression of numerous genes associated with inflammation and radiation injury. So why is plasminogen beneficial in the early phase of skin closure in models of cutaneous wound repair but deleterious in the early phase of radiation injury, yet protective later? This may come down to the timing of the inflammatory process in these vastly different models of skin injury and perhaps the differential recruitment of plasminogen receptors41 ; however, this remains speculative. Nonetheless, plasminogen is protective in all examples, depending on the timing of administration.
The role of plasminogen in the promotion of healing also extends to bone repair. Plg−/− mice exhibit delayed bone repair following femoral fracture.68 Furthermore, failure to clear fibrin from the fracture site severely impaired fracture vascularization and bone union, with resultant heterotopic ossification and dystrophic calcification.68,69
Plasminogen and infection by pathogens
As mentioned at the outset, the first indication of the existence of a naturally occurring profibrinolytic protease came from bacterial strains in the 1930s. It is now appreciated that plasmin can be harnessed by numerous bacteria, as well as fungi, helminths, parasites, and viruses to suppress the host immune response and evade local immune attack.70 These organisms have seemingly coevolved with humans and other mammals to harness host plasminogen for their defense and survival.71,72 More than 40 binding proteins have been reported in commensal and pathogenic bacterial species that target plasmin(ogen).70 Interestingly, Yersinia pestis, the causative pathogen of the plague that killed one third of the European population in the 14th century, expresses a plasmid gene (pla) that encodes a surface plasminogen activator that increases the virulence of Y pestis in the host.73
In 1973, Lazarowitz et al74 reported that plasmin was a major protease involved in the proteolytic cleavage of the hemagglutinin polypeptide of the influenza virus. More recently, plasminogen was shown to contribute to the lung inflammation and pathogenesis in influenza A, H5N1, and H1N1, with Plg−/− mice exhibiting significantly reduced lung inflammation after viral exposure.75 Plasmin can also cleave the Spike protein of coronaviruses (and of SARS-CoV in vitro; data on SARS-CoV-2 are unknown), increasing their infectivity and enabling viral entry by binding to angiotensin-converting enzyme-2 receptors on the host cell surface.76
The CNS
Curious findings from the late 1940s by Fantl and Simon revealed that electroconvulsive therapy could increase fibrinolytic activity in blood.77 This prompted subsequent studies on human brain tissue. Fantl and Fitpatrick revealed that brain extracts contained a fibrinolytic activity when combined with human serum (akin to streptokinase and staphylokinase).78 The cellular source of the brain-derived plasminogen-activating activity (most certainly t-PA) was initially reported to be neuronal and epithelial,79 but t-PA is now known to exist in various cell types within the CNS, including astrocytes80 and microglia.81 t-PA was also described to exist within sympathetic nerve terminals,82 whereas plasminogen itself is expressed within the CNS.83
Over the ensuing years, much effort was devoted to understanding the role of plasminogen and the plasminogen-activating system in the CNS. Key among these include roles as a neuronal modulator,84 promotion of synaptic plasticity, the addictive response,85 and blood-brain barrier permeability.86 Not all of these effects are plasmin dependent,87,88 but many are.89 Overproduction of plasmin in the CNS may have deleterious consequences in conditions associated with increased amyloid and fibrin deposition that may promote excessive plasmin generation. On the other hand, fibrin(ogen) deposition in some CNS disorders is fundamental to the neuroinflammatory response, and fibrin-targeted therapies or antifibrinolytic agents could offer novel treatments for these conditions.53,90
New opportunities for plasmin blockade or plasminogen supplementation
Congenital plasminogen deficiency is ultrarare (frequency ∼1.6 in 1 000 000).91 Affected individuals often display pseudomembranes on the inside of the eyelid (ligneous conjunctivitis), although other tissues can be affected.92 Acquired ligneous conjunctivitis is rare but has been reported after TXA treatment.93 Interestingly, thrombosis risk is not increased in these individuals, although it may act as an additional risk factor.14 However, there is no evidence to suggest that any other phenotype exists (ie, related to immune function or inflammation). Although plasminogen levels (or activity) are significantly reduced, some plasminogen remains,94 which may be sufficient to maintain fibrinolysis in major vessels. However, given the rarity of this condition, significant knowledge gaps remain. It is interesting to note that an international registry (HISTORY) has been created of individuals with plasminogen deficiency to improve the understanding of the disease.91
Nonetheless, the question remains as to whether the therapeutic modulation of plasmin(ogen) (supplementation or inhibition) has any clinical sequelae in conditions unrelated to thrombosis or to excessive bleeding. It should be mentioned that, for ∼40 years, TXA has been used for cosmetic skin-whitening purposes and more recently as a means to reduce UV-induced hyperpigmentation in melasma95 by blocking the ability of plasmin to increase melanin production. On the other hand, a truncated plasminogen variant (ie, microplasmin “ocriplasmin”) has been in clinical use in the context of vitreomacular adhesion,96 where plasmin works to remove proteins involved in cell-cell interactions. Hence, it is not entirely new that inhibition or increases in plasmin generation have consequences distinct from its hemostatic effect. However, recent findings (some already mentioned) have provided a strong rationale to justify the modulation of plasmin(ogen) for clinical benefit beyond current indications.
Infection control
That plasmin can modulate immunosuppression and inflammation in mice raised the question as to whether this occurs in humans and, if so, whether antifibrinolytic agents might reverse this and improve immunity. This was evaluated by Draxler et al in human volunteers and in patients undergoing cardiac surgery.97 TXA reduced levels of tumor necrosis factor, interleukin-10, and interleukin-6 in volunteers within 2 hours of administration and enhanced the expression of key immune-activating markers (CD83) while reducing the expression of immunosuppressive markers (PD-L1 and LAP). In the patient cohorts, cytokine levels were profoundly increased because of surgery; however, in contrast to the effect seen in volunteers, TXA was without effect. However, TXA treatment still increased CD-83 and decreased PD-L1 and LAP expression, as seen in the volunteers. Of interest, TXA resulted in a 40% decrease in superficial surgical site infection rates.97 Curiously, TXA had no effect on infection rates or immune marker expression in patients with diabetes. Overall, the hemostatic effect of TXA was also weaker in the diabetic group for reasons that are still unclear, because it would be anticipated that such patients would be hypofibrinolytic anyway, in part as a result of glycated plasminogen.64 It is possible that the diabetic condition impairs the immune response in a general sense that could include plasminogen receptor signaling dysfunction, but this remains speculative.
These findings further support the fibrin-independent effect of TXA on immune function and reinforce the notion of plasmin’s immune-regulatory effects, even in the absence of surgery or other comorbidities, as revealed from the studies on volunteers. It was recently reported that TXA can reduce infection rates in other surgical procedures.98
It remains to be determined whether these effects of TXA in nondiabetic subjects is due to a reduction in plasmin or occurs via another mechanism (ie, by blocking plasminogen receptor binding to key immune cells). However, 1 clinical study comparing the effects of TXA and aprotinin (a direct plasmin inhibitor) on the levels of inflammatory cytokines in patients undergoing cardiac surgery revealed that aprotinin, but not TXA, significantly reduced the levels of tumor necrosis factor and MCP-1.99 That TXA had no effect of these cytokines postsurgery is consistent with the Draxler et al study.97 It is not clear whether aprotinin was more effective than TXA at modulating cytokine levels, as reflected by greater potency against plasmin, or mediated this effect by blocking other serine proteases (ie, kallikrein). It would be interesting to know the effect of aprotinin on immune markers and on infection rates. The capability of TXA to reduce infection rates as a primary outcome is being evaluated in patients undergoing gastrointestinal surgery (“TRIGS” trial; NCT04192435).
Viral infection
Recent studies have implicated elevated levels of plasmin(ogen) as a common risk factor in patients who are susceptible to the SARS-CoV-2 infection that causes COVID-19.100 Lower plasminogen concentrations have also been noted on admission in COVID-19 patients needing hospitalization and/or intensive care support101 compared with those who do not, which is suggestive of plasminogen consumption. The earlier finding of a role for plasmin at facilitating viral infectivity suggested that antiprotease treatments may be beneficial in patients with COVID-19.102,103 However, other investigators have raised caution about targeting plasmin,104 because an intact plasminogen-activating system is needed to remove fibrin and necrotic tissue occurring in late-stage disease. Hence, the timing of treatment with TXA would be critical105 and is more likely to be beneficial (if at all) in the very early course of the disease or even as a prophylactic measure.105 Interestingly, researchers in the United States are evaluating TXA in outpatients recently diagnosed with COVID-19 (NCT04338074), whereas another trial using aprotinin in combination with anticoagulants has been initiated.106
Trauma
TXA has been evaluated in trauma107 and traumatic brain injury (TBI),108 with an overall beneficial outcome of decreased mortality when it is administered early. Whether TXA influences the immune response and improves infection rates in these conditions (as seen in cardiac surgery) is an idea worth considering. One recent study demonstrated that TXA reduced plasmin-mediated complement activation in trauma patients,109 whereas another trauma (non-TBI) study reported only minimal immune-modulatory effects.110 However, the use of TXA in trauma is vastly different from its use in surgery; for trauma, TXA is administered after injury, when inflammatory and immune defense mechanisms have already been activated, whereas in surgery it is given prophylactically prior to any challenge. Nonetheless, recent animal models of spinal cord injury showed that short-term (3-day) TXA treatment was beneficial, whereas prolonged treatment was deleterious,111 possibly as a result of impaired tissue remodeling. In mouse models of TBI, early TXA administration reduced blood-brain barrier breakdown and improved neurological recovery, but this was only seen in male mice; TXA appeared to be deleterious in female mice.112 The influence of sex on the effect of TXA in human TBI patients was not reported in the recent CRASH-3 trial, and >80% of the patients recruited were male.108 However, further studies are required to confirm these findings and to investigate whether there is a genuine sex-dependent effect of TXA use in patients with TBI.
Plasminogen supplementation
Until now, the only consideration for plasminogen supplementation has been in patients with ligneous conjunctivitis due to plasminogen deficiency.113 In all other conditions requiring rapid increases in plasmin generation (ie, in thromboembolic conditions, including acute ischemic stroke [AIS]), it is achieved by administration of t-PA or u-PA. It is of interest that, in a mouse model of AIS, merely increasing plasminogen levels twofold improved outcome, even in the absence of thrombolysis,114 suggesting that the substrate, not the plasminogen activator, is limiting in AIS. It is also of interest that supplementation of patients with severe COVID-19 with nebulized plasminogen also improved outcome.115 Although this needs to be confirmed, it has raised interest in the repurposing of plasminogen for SARS-CoV-2116 to restore fibrinolytic activity within the lung parenchyma. It would be interesting to see whether increasing levels of the substrate (ie, plasminogen), rather than the thrombolytic agent (ie, t-PA), in these scenarios, especially in AIS, would carry less risk for symptomatic bleeding rates.
Plasminogen supplementation, locally or systemically, is under consideration to improve wound healing rates, which would be particularly important in patients with chronic wounds (ie, diabetes)42 and radiation-induced skin injury,67 as mentioned earlier, for which current remedies are limited or nonexistent. Increasing plasmin activity in the context of bone repair may prevent soft tissue calcification without adversely affecting bone physiology and muscle and bone regeneration following injury.
Plasminogen receptor targeting
Plasminogen implements many of its pleiotropic actions via 1 of many cell surface receptors. This has prompted recent calls to target plasminogen receptors to improve outcome in malignant disease.117
Diagnostic approaches
As mentioned earlier, there is marked variation in the capacity of healthy individuals to respond to plasminogen-activating agents ex vivo.9 This may be important in patients with low plasmin-generating capacity requiring thrombolysis.118 An approach to evaluate fibrinolytic capacity would be useful as a screening tool to identify those patients who are more likely to respond to thrombolysis. However, the challenge is to devise a reliable test that can be used rapidly for clinical utility.
Conclusions
The biological importance of plasminogen and the plasminogen-activating system clearly extends beyond the realm of thrombosis and hemostasis. Although this has been a widely held view, the impact of this in the clinical arena is now becoming clearer, and new opportunities are arising as a result (Figure 1). These broad consequences of plasminogen need to gain traction within the wider field to harness the full therapeutic potential of increasing or decreasing plasmin(ogen) beyond the current indications of clotting and bleeding, respectively.
Acknowledgments
R.L.M. received funding from the National Health and Medical Research Council of Australia (grant APP1156506). C.B.K. received a research training program scholarship from Monash University.
Authorship
Contribution: C.B.K. and R.L.M. wrote, reviewed, and edited the manuscript
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Robert L. Medcalf, Molecular Neurotrauma and Haemostasis, Australian Centre for Blood Diseases, Monash University, Level 1, Walkway via the Alfred Centre, 99 Commercial Rd, Melbourne, VIC 3004, Australia; e-mail: robert.medcalf@monash.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal