Key Points
Suboptimal asparaginase exposure leads to an increased risk of relapse in non–high-risk ALL patients.
Therapeutic drug monitoring should be used to identify patients with no asparaginase enzyme activity to ensure treatment efficacy.
Abstract
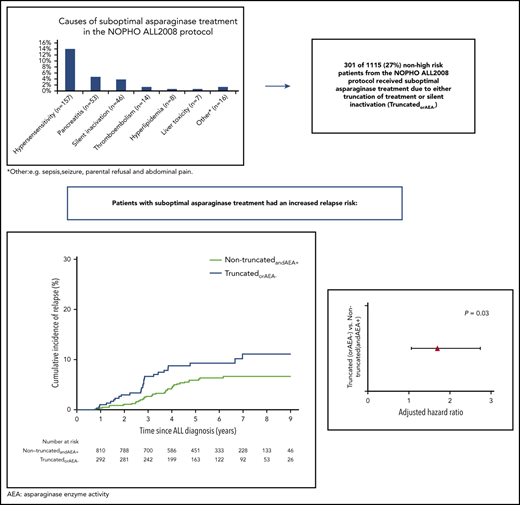
Truncation of asparaginase treatment due to asparaginase-related toxicities or silent inactivation (SI) is common and may increase relapse risk in acute lymphoblastic leukemia (ALL). We investigated relapse risk following suboptimal asparaginase exposure among 1401 children aged 1 to 17 years, diagnosed with ALL between July 2008 and February 2016, treated according to the Nordic Society of Pediatric Hematology and Oncology (NOPHO) ALL2008 protocol (including extended asparaginase exposure [1000 IU/m2 intramuscularly weeks 5-33]). Patients were included with delayed entry at their last administered asparaginase treatment, or detection of SI, and followed until relapse, death, secondary malignancy, or end of follow-up (median, 5.71 years; interquartile range, 4.02-7.64). In a multiple Cox model comparing patients with (n = 358) and without (n = 1043) truncated asparaginase treatment due to clinical toxicity, the adjusted relapse-specific hazard ratio (HR; aHR) was 1.33 (95% confidence interval [CI], 0.86-2.06; P = .20). In a substudy including only patients with information on enzyme activity (n = 1115), the 7-year cumulative incidence of relapse for the 301 patients with truncation of asparaginase treatment or SI (157 hypersensitivity, 53 pancreatitis, 14 thrombosis, 31 other, 46 SI) was 11.1% (95% CI, 6.9-15.4) vs 6.7% (95% CI, 4.7-8.6) for the 814 remaining patients. The relapse-specific aHR was 1.69 (95% CI, 1.05-2.74, P=.03). The unadjusted bone marrow relapse-specific HR was 1.83 (95% CI, 1.07-3.14, P=.03) and 1.86 (95% CI, 0.90- 3.87, P=.095) for any central nervous system relapse. These results emphasize the importance of therapeutic drug monitoring and appropriate adjustment of asparaginase therapy when feasible. This trial was registered at www.clinicaltrials.gov as #NCT03987542.
Introduction
The importance of asparaginase in the treatment of acute lymphoblastic leukemia (ALL) is well established.1-5 The overall survival for children with ALL is now above 90% in most contemporary protocols,6 but asparaginase-associated toxicities still constitute a significant problem as they, besides causing acute morbidity and mortality, may cause truncation of the treatment with a subsequent increased risk of relapse.3,7,8 Balancing the antileukemic effect and asparaginase toxicity is challenging, and, despite multiple studies, the optimal asparaginase schedule is still unknown.9,10
The most frequent toxicities causing asparaginase truncations are hypersensitivity, pancreatitis, and thromboembolism.3 Hypersensitivity especially constitutes a problem due to asparaginase inactivation, not only in patients with clinical hypersensitivity but also among those without clinical symptoms (silent inactivation [SI]).9,11 Patients with SI have an inferior outcome7 and thus asparaginase enzyme activity (AEA) levels are used to monitor the treatment with asparaginase in order to identify the patients without enzyme activity and change the asparaginase preparation.12
In this article, we present results from the Nordic Society of Pediatric Hematology and Oncology (NOPHO) ALL2008 protocol. The protocol included prospective asparaginase-associated toxicity registration and detailed registration of asparaginase treatment. In addition, samples for AEA measurements were collected before every asparaginase administration and analyzed retrospectively. First, we investigated whether patients with a truncation of asparaginase therapy had a different risk of relapse compared with patients without truncated treatment, while taking AEA measurements into account. Second, we explored whether the risk of relapse was different among subgroups based on the asparaginase treatment intensity defined as the total accumulated asparaginase given.
Methods
Patients
The NOPHO ALL2008 protocol opened in July 2008 and the study period ended on 28 February 2016. Children aged 1.0 to 17.9 years, diagnosed with Philadelphia chromosome–negative B-cell precursor (BCP) or T-cell ALL in Denmark, Estonia, Finland, Iceland, Lithuania, Norway, and Sweden, and treated according to the protocol were included in this study. The protocol, risk grouping, and stratification criteria have previously been described in detail.13-15 Definition and grouping of central nervous system (CNS) disease and minimal residual disease (MRD) are described in supplemental Material (available on the Blood Web site).
The protocol was approved by the National Medicines Agencies (EudraCT no. 2008-003235-20) and relevant ethical committees. Research was conducted in accordance with the Declaration of Helsinki.
Asparaginase in the NOPHO ALL2008 protocol
The standard asparaginase preparation in the NOPHO ALL2008 protocol was pegylated asparaginase (PEG-asparaginase) (1000 IU/m2 per dose) administered as intramuscular injections. From day 30, at the end of induction therapy, standard-risk (SR) and intermediate-risk (IR) patients received 5 doses of PEG-asparaginase at 2-week intervals during consolidation after which patients were invited to participate in a randomization between an additional 3 doses at 6-week intervals (experimental arm) or 10 doses at 2-week intervals (standard arm) during the delayed intensification and the first part of maintenance therapy. The results of the randomization showed no difference in disease-free survival between the 2 arms and significantly less toxicity in the experimental arm.10 High-risk patients received 7 or 9 doses of PEG-asparaginase, given as 1 dose in each treatment block and 2 additional doses in the delayed intensification. In case of clinical hypersensitivity, the PEG-asparaginase treatment was truncated and patients switched to Erwinia asparaginase (20 000 IU/m2 per dose): non–high-risk patients were scheduled to receive 3 Erwinia asparaginase doses per week for 2 weeks during delayed intensification, replacing only 1 dose of PEG-asparaginase. The plan for high-risk patients was to receive 3 Erwinia asparaginase doses per week in each of the subsequent treatment blocks, corresponding to half of the planned PEG-asparaginase treatment. An additional 2 weeks of Erwinia asparaginase treatment were planned for the delayed intensification. Thus, all planned PEG-asparaginase doses were not substituted and these patients were therefore defined as “truncated” even though they received all of the planned substitution doses.
The prospective registration of asparaginase-associated toxicities and detailed registration of the asparaginase treatment included: number of PEG-asparaginase dosages given prior to truncation, reason for truncation, and number of Erwinia asparaginase doses given.
In assessing the asparaginase treatment intensity, we defined the amount of asparaginase given to the individual patient as the length of asparaginase treatment as follows: (1) 6 Erwinia asparaginase doses counted as 1 dose of PEG-asparaginase, and (2) 1 dose of PEG-asparaginase corresponded to 18 days of asparaginase treatment (based on previous studies10,16 ) when not given continuously at 14-day intervals.
Asparaginase enzyme activity
Samples for AEA measurement were taken before every PEG-asparaginase injection and analyzed retrospectively. The results were not reported to the treating clinician and did therefore not lead to clinical intervention. Samples were stored at −80°C and analyzed retrospectively by either Nessler reagent or aspartic acid β-hydroxamate assay.16,17 High-risk patients received PEG-asparaginase at ∼1-month intervals. Because AEA levels were only measured prior to asparaginase administration, the levels in high-risk patients were not informative, due to their dispersed asparaginase therapy. Samples with no measurable AEA were only included in the present study when taken within 14 days of administration. This cutoff relied on several different pharmacokinetic studies.16,18 Based on the AEA measurements, SR and IR patients were divided into groups prior to any analysis: (1) normal enzyme activities: patients with measurable AEA in at least 2 samples and at least 1 sample above 100 IU/L; (2) fast metabolizers: patients with AEA in at least 2 samples but no sample above 100 IU/L; and (3) no measurable AEA: patients without enzyme activity in samples taken within 14 days of the latest PEG-asparaginase administration (this included patients with enzyme activity in 1 sample taken within 5 days after the first dose of asparaginase or with a single sample with enzyme activity <100 IU/L, but no measurable AEA in any other samples). Patients with hypersensitivity without any samples were also included in group iii, assuming that they did not have any enzyme activity.19
Remaining patients without samples, patients with samples without any enzyme activity taken at erroneous time points (>14 days since administration), and patients with only 1 sample were categorized as patients with undetermined enzyme activity. AEA measurements were evaluated at the end of asparaginase treatment, and patients were classified based on all available samples.
Outcome measures and asparaginase-exposure groups
Patients were followed from ALL diagnosis (with delayed entry at the end of asparaginase treatment) until relapse, death in complete remission (DCR1), or secondary malignancy (SMN), until they were lost to follow-up, or until 18 September 2018, whichever came first. The main outcome was the risk of relapse considering DCR1 and SMN as competing events. We secondarily assessed the risk of any bone marrow relapse and any CNS relapse separately, in each subanalysis also considering the other type of relapse as a competing event.
In the main cohort, we compared patients with truncated asparaginase therapy to the patients who did not have their asparaginase therapy truncated. In a substudy consisting of SR and IR patients with determined AEA, we compared patients with truncated asparaginase therapy or no enzyme activity (truncatedorAEA−) to the patients who did not have asparaginase therapy truncated and had normal enzyme activity or were fast metabolizers (nontruncatedandAEA+). Because the group of fast metabolizers was found too small for individual analysis, we combined it with the normal activity group because low AEA levels have been shown to ensure sufficient depletion.20,21 As sensitivity analyses, we repeated the analyses for the subcohort with exclusion of truncatedorAEA− patients who had their asparaginase treatment truncated due to pancreatitis although none were registered with any major treatment modifications in the NOPHO database.
Investigating asparaginase treatment intensity, we categorized the patients into 3 groups: (1) low treatment intensity (asparaginase truncated and <10 weeks of asparaginase treatment or no AEA); (2) medium treatment intensity (asparaginase truncated, but at least 10 weeks of asparaginase treatment and normal AEA/fast metabolizers); or (3) high treatment intensity (no truncation and normal AEA/fast metabolizers corresponding to nontruncatedandAEA+). Patients who were truncated due to hypersensitivity and were substituted according to protocol were categorized as truncated because the substitution was inadequate and did not replace each dose of PEG-asparaginase.
Statistics
The follow-up time was estimated using the reverse Kaplan-Meier method. Cumulative incidences of relapse were estimated by the Aalen-Johansen estimator, and the Cox proportional-hazards model was used to estimate unadjusted (hazard ratio [HR]) and adjusted relapse-specific hazard ratios (aHRs) with significance evaluated by Wald tests. The multiple models included: age group (<10 years or ≥10 years); CNS status at diagnosis (CNS1, CNS2, CNS3, defined in supplemental Material); MRD day 29, categorized into 4 groups; white blood cell (WBC) count at diagnosis (log2-transformed due to skewness; hence, estimates corresponds to a doubling); and also, for analysis of the main cohort, stratification by risk group. In a post hoc subanalysis, multiple Cox models included National Cancer Institute (NCI) risk groups defined as NCI–high risk (if age ≥10 years or WBCs ≥50 × 109/L) and NCI-SR (if age <10 years and WBCs <50 × 109/L) instead of the above-mentioned variables. Two-sided values of P < .05 were considered statistically significant. Statistical analyses were performed using R version 3.5.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics: main cohort
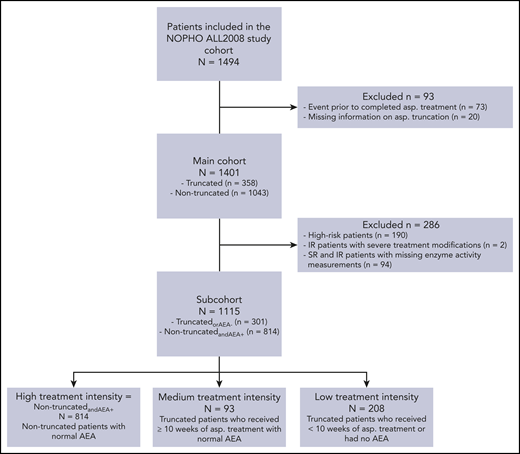
A total of 1494 patients were included in the NOPHO ALL2008 study cohort and were eligible for inclusion in this study. Following exclusion of patients with an event prior to completed asparaginase treatment and patients with missing information about asparaginase truncation, 1401 patients were included in the main cohort (Figure 1). Baseline characteristics of these patients are given in Table 1. There was an equal distribution of male patients (55%) and female patients (45%), median age at diagnosis was 4 years (interquartile range [IQR], 2-8 years), median WBCs was 10.9 × 109/L (IQR, 4.5-39.5), and 14% were high-risk patients. The majority of patients (87%) did not have CNS disease at time of diagnosis (CNS1), and 38% had undetectable bone marrow disease at day 29 (MRD group 4). Of the 1401 patients, 358 (25.6%) had their asparaginase treatment truncated. The median number of asparaginase doses given in the truncated group was 4 (IQR, 3-7) vs 15 (IQR, 8-15) in the nontruncated group. The most frequent reasons for truncation were clinical hypersensitivity (58.1%), pancreatitis (24.6%), and thromboembolism (6.7%) (Table 2).
Patient flow diagram of the included patients, subcohorts, and subgroups. AEA, asparaginase enzyme activity; asp., asparaginase; IR, intermediate risk; SR, standard risk.
Patient flow diagram of the included patients, subcohorts, and subgroups. AEA, asparaginase enzyme activity; asp., asparaginase; IR, intermediate risk; SR, standard risk.
Characteristics of the main cohort and the subsets based on asparaginase truncation
| Characteristics . | Main cohort . | Truncated . | Nontruncated . |
|---|---|---|---|
| No. of patients (%) | 1401 | 358 (25.6) | 1043 (74.4) |
| Age at diagnosis, y | |||
| Median [IQR] | 4 [2-8] | 4 [2-8.75] | 4 [2-8] |
| <10, no. (%) | 1118 (79.8) | 279 (77.9) | 839 (80.4) |
| ≥10, no. (%) | 283 (20.2) | 79 (28.3) | 204 (19.6) |
| Sex, no. (%) | |||
| Male | 765 (54.6) | 193 (53.9) | 572 (54.8) |
| Female | 636 (45.4) | 165 (46.1) | 471 (45.2) |
| NOPHO risk group, no. (%) | |||
| SR | 716 (51.1) | 173 (48.3) | 543 (52.1) |
| IR | 495 (35.3) | 123 (34.4) | 372 (35.7) |
| HR | 190 (13.6) | 62 (17.3) | 128 (12.3) |
| NCI risk group, no. (%) | |||
| SR | 884 (63.1) | 219 (61.2) | 665 (63.8) |
| HR | 517 (36.9) | 139 (38.8) | 378 (36.2) |
| CNS status at diagnosis, no. (%) | |||
| CNS 1 | 1225 (87.4) | 309 (86.3) | 916 (87.8) |
| CNS 2 | 113 (8.1) | 31 (8.7) | 82 (7.9) |
| CNS 3 | 61 (4.4) | 16 (4.5) | 45 (4.3) |
| Missing value | 2 (0.1) | 2 (0.6) | — (—) |
| MRD group, day 29, no. (%) | |||
| MRD 1 | 382 (27.3) | 107 (29.9) | 275 (26.4) |
| MRD 2 | 324 (23.1) | 78 (21.8) | 246 (23.6) |
| MRD 3 | 145 (10.3) | 39 (10.9) | 106 (10.2) |
| MRD 4 | 525 (37.5) | 127 (35.5) | 398 (38.2) |
| Missing value | 25 (1.8) | 7 (2.0) | 18 (1.7) |
| WBC at diagnosis, 109/L | |||
| Median [IQR] | 10.9 [4.5-39.5] | 13.0 [4.6-40.4] | 10.5 [4.5-37.5] |
| Asp. doses given* | |||
| Median [IQR] | 15 [8-15] | 4 [3-7] | 15 [8-15] |
| Characteristics . | Main cohort . | Truncated . | Nontruncated . |
|---|---|---|---|
| No. of patients (%) | 1401 | 358 (25.6) | 1043 (74.4) |
| Age at diagnosis, y | |||
| Median [IQR] | 4 [2-8] | 4 [2-8.75] | 4 [2-8] |
| <10, no. (%) | 1118 (79.8) | 279 (77.9) | 839 (80.4) |
| ≥10, no. (%) | 283 (20.2) | 79 (28.3) | 204 (19.6) |
| Sex, no. (%) | |||
| Male | 765 (54.6) | 193 (53.9) | 572 (54.8) |
| Female | 636 (45.4) | 165 (46.1) | 471 (45.2) |
| NOPHO risk group, no. (%) | |||
| SR | 716 (51.1) | 173 (48.3) | 543 (52.1) |
| IR | 495 (35.3) | 123 (34.4) | 372 (35.7) |
| HR | 190 (13.6) | 62 (17.3) | 128 (12.3) |
| NCI risk group, no. (%) | |||
| SR | 884 (63.1) | 219 (61.2) | 665 (63.8) |
| HR | 517 (36.9) | 139 (38.8) | 378 (36.2) |
| CNS status at diagnosis, no. (%) | |||
| CNS 1 | 1225 (87.4) | 309 (86.3) | 916 (87.8) |
| CNS 2 | 113 (8.1) | 31 (8.7) | 82 (7.9) |
| CNS 3 | 61 (4.4) | 16 (4.5) | 45 (4.3) |
| Missing value | 2 (0.1) | 2 (0.6) | — (—) |
| MRD group, day 29, no. (%) | |||
| MRD 1 | 382 (27.3) | 107 (29.9) | 275 (26.4) |
| MRD 2 | 324 (23.1) | 78 (21.8) | 246 (23.6) |
| MRD 3 | 145 (10.3) | 39 (10.9) | 106 (10.2) |
| MRD 4 | 525 (37.5) | 127 (35.5) | 398 (38.2) |
| Missing value | 25 (1.8) | 7 (2.0) | 18 (1.7) |
| WBC at diagnosis, 109/L | |||
| Median [IQR] | 10.9 [4.5-39.5] | 13.0 [4.6-40.4] | 10.5 [4.5-37.5] |
| Asp. doses given* | |||
| Median [IQR] | 15 [8-15] | 4 [3-7] | 15 [8-15] |
Asp., asparaginase; HR, high risk; IQR, interquartile range.
PEG-asparaginase doses including recalculated Erwinia asparaginase doses (6 doses of Erwinia asparaginase corresponds to 1 dose of PEG-asparaginase).
Reason for truncation of asparaginase in the main and subcohort
| . | Main cohort, n = 1401, n (% of the cohort) . | Subcohort, n = 1115, n (% of the cohort) . | No AEA,* n . |
|---|---|---|---|
| Clinical hypersensitivity | 208 (14.8) | 157 (14.1) | 139 |
| Pancreatitis | 88 (6.3) | 53 (4.8) | 1 |
| Thrombosis | 24 (1.7) | 14 (1.3) | — |
| Hyperlipidemia | 10 (0.7) | 8 (0.7) | — |
| Liver toxicity | 7 (0.5) | 7 (0.6) | — |
| Other† | 21 (1.5) | 16 (1.4) | — |
| Total no. of truncated patients | 358 (25.5) | 255 (22.9) | 140 |
| Including patients with silent inactivation (truncatedorAEA-) | — | 301 | 186 |
| . | Main cohort, n = 1401, n (% of the cohort) . | Subcohort, n = 1115, n (% of the cohort) . | No AEA,* n . |
|---|---|---|---|
| Clinical hypersensitivity | 208 (14.8) | 157 (14.1) | 139 |
| Pancreatitis | 88 (6.3) | 53 (4.8) | 1 |
| Thrombosis | 24 (1.7) | 14 (1.3) | — |
| Hyperlipidemia | 10 (0.7) | 8 (0.7) | — |
| Liver toxicity | 7 (0.5) | 7 (0.6) | — |
| Other† | 21 (1.5) | 16 (1.4) | — |
| Total no. of truncated patients | 358 (25.5) | 255 (22.9) | 140 |
| Including patients with silent inactivation (truncatedorAEA-) | — | 301 | 186 |
The “No AEA” column only applies to patients in the subcohort.
Other: for example, sepsis, seizure, parental refusal, and abdominal pain.
Cumulative incidences and relapse-specific HRs in the main cohort
With a median follow-up time of 5.7 years (IQR, 4.0-7.6), 13 patients died in DCR1, 11 developed a SMN, and 97 developed a relapse with a 7-year cumulative incidence of relapse of 8.5% (95% confidence interval [CI], 6.8% to 10.1%). The incidence of DCR1 and SMN were similar for the nontruncated and truncated patients: the 7-year cumulative incidence of DCR1 was 4.0% (95% CI, 0% to 10.2%) and 1.3% (95% CI, 0.03% to 2.7%), respectively; and the 7-year cumulative incidence of SMN was 0.7% (95% CI, 0.2% to 1.3%) and 1.2% (95% CI, 0.03% to 2.4%), respectively. The 7-year cumulative incidence of relapse was 7.5% (95% CI, 5.6% to 9.3%) for the nontruncated group and 10.5% (95% CI, 6.7% to 14.4%) for the truncated group with a relapse-specific risk-group–stratified aHR of 1.33 (95% CI, 0.86-2.06; P = .20).
Patient characteristics: subcohort
Following exclusion of high-risk patients and patients with undetermined or missing AEA measurements, 1115 patients remained in the subcohort (Figure 1). Overall, the baseline characteristics of the subcohort did not differ from the main cohort (Table 3).
Characteristics of the subcohort and the subsets based on asparaginase truncation and AEA
| Characteristics . | Subcohort . | TruncatedorAEA− . | Non-TruncatedandAEA+ . |
|---|---|---|---|
| No. of patients | 1115 | 301 (27.0) | 814 (73.0) |
| Age at diagnosis, y | |||
| Median [IQR] | 4 [2-7] | 4 [2-8] | 4 [2-7] |
| <10, no. (%) | 927 (83.1) | 244 (81.1) | 683 (83.9) |
| ≥10, no. (%) | 188 (16.9) | 57 (18.9) | 131 (16.1) |
| Sex, no. (%) | |||
| Male | 604 (54.2) | 164 (54.5) | 440 (54.1) |
| Female | 511 (45.8) | 137 (45.5) | 374 (45.9) |
| NOPHO risk group, no. (%) | |||
| SR | 671 (60.2) | 178 (59.1) | 493 (60.6) |
| IR | 444 (39.8) | 123 (40.9) | 321 (39.4) |
| NCI risk group, no. (%) | |||
| SR | 771 (69.1) | 207 (68.8) | 564 (69.3) |
| HR | 344 (30.9) | 94 (31.2) | 250 (30.7) |
| CNS status at diagnosis, no. (%) | |||
| CNS 1 | 980 (87.9) | 268 (89.0) | 712 (87.5) |
| CNS 2 | 86 (7.7) | 19 (6.3) | 67 (8.2) |
| CNS 3 | 47 (4.2) | 12 (4.0) | 35 (4.3) |
| Missing value | 2 (0.2) | 2 (0.7) | — (—) |
| MRD group, day 29, no. (%) | |||
| MRD 1 | 229 (20.5) | 72 (23.9) | 157 (19.3) |
| MRD 2 | 286 (25.7) | 70 (23.3) | 216 (26.5) |
| MRD 3 | 135 (12.1) | 38 (12.6) | 97 (11.9) |
| MRD 4 | 460 (41.3) | 119 (39.5) | 341 (41.9) |
| Missing value | 5 (0.4) | 2 (0.7) | 3 (0.4) |
| WBC at diagnosis, 109/L | |||
| Median [IQR] | 9.3 [4.4-31.9] | 9.4 [4.6-30.7] | 9.25 [4.3-32.1] |
| Asp. doses given* | |||
| Median [IQR] | 15 [8-15] | 6 [3-11] | 15 [8-15] |
| Characteristics . | Subcohort . | TruncatedorAEA− . | Non-TruncatedandAEA+ . |
|---|---|---|---|
| No. of patients | 1115 | 301 (27.0) | 814 (73.0) |
| Age at diagnosis, y | |||
| Median [IQR] | 4 [2-7] | 4 [2-8] | 4 [2-7] |
| <10, no. (%) | 927 (83.1) | 244 (81.1) | 683 (83.9) |
| ≥10, no. (%) | 188 (16.9) | 57 (18.9) | 131 (16.1) |
| Sex, no. (%) | |||
| Male | 604 (54.2) | 164 (54.5) | 440 (54.1) |
| Female | 511 (45.8) | 137 (45.5) | 374 (45.9) |
| NOPHO risk group, no. (%) | |||
| SR | 671 (60.2) | 178 (59.1) | 493 (60.6) |
| IR | 444 (39.8) | 123 (40.9) | 321 (39.4) |
| NCI risk group, no. (%) | |||
| SR | 771 (69.1) | 207 (68.8) | 564 (69.3) |
| HR | 344 (30.9) | 94 (31.2) | 250 (30.7) |
| CNS status at diagnosis, no. (%) | |||
| CNS 1 | 980 (87.9) | 268 (89.0) | 712 (87.5) |
| CNS 2 | 86 (7.7) | 19 (6.3) | 67 (8.2) |
| CNS 3 | 47 (4.2) | 12 (4.0) | 35 (4.3) |
| Missing value | 2 (0.2) | 2 (0.7) | — (—) |
| MRD group, day 29, no. (%) | |||
| MRD 1 | 229 (20.5) | 72 (23.9) | 157 (19.3) |
| MRD 2 | 286 (25.7) | 70 (23.3) | 216 (26.5) |
| MRD 3 | 135 (12.1) | 38 (12.6) | 97 (11.9) |
| MRD 4 | 460 (41.3) | 119 (39.5) | 341 (41.9) |
| Missing value | 5 (0.4) | 2 (0.7) | 3 (0.4) |
| WBC at diagnosis, 109/L | |||
| Median [IQR] | 9.3 [4.4-31.9] | 9.4 [4.6-30.7] | 9.25 [4.3-32.1] |
| Asp. doses given* | |||
| Median [IQR] | 15 [8-15] | 6 [3-11] | 15 [8-15] |
See Table 1 for expansion of abbreviations.
PEG-asparaginase doses including recalculated Erwinia asparaginase doses (6 doses of Erwinia asparaginase corresponds to 1 dose of PEG-asparaginase).
In the subcohort, 255 patients (22.9%) were registered with truncated asparaginase treatment. The most frequent causes of PEG-asparaginase truncation were clinical hypersensitivity (61.6%), pancreatitis (20.8%), and thrombosis (5.5%) (Table 2). Of the 157 patients with clinical hypersensitivity, 130 (82%) had at least 1 dose of Erwinia asparaginase administered. Of these 130 patients, 57 had follow-up AEA measurements done, and 55 of the 57 (96.5%) had sufficient AEA levels >100 IU/L. When assessing the AEA measurements in the subcohort, 910 patients (81.6%) had normal enzyme activity levels, 19 (1.7%) were fast metabolizers, and 186 (16.7%) had no enzyme activity. Of the 186, 46 were not registered with a truncation (Table 2); consequently, 301 patients were classified as truncatedorAEA− and 814 as nontruncatedandAEA+. The baseline characteristics of the truncatedorAEA− and the nontruncatedandAEA+ patients are shown in Table 3.
Cumulative incidences and relapse-specific HRs in the subcohort
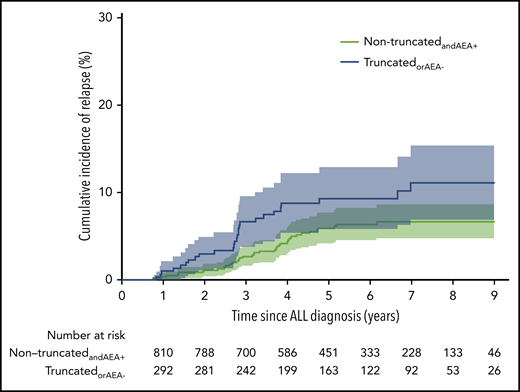
Median follow-up time was 5.6 years (IQR, 4.0-7.5 years), and a total of 81 events occurred: 2 DCR1, 8 SMN, and 71 relapses with a 7-year cumulative incidence of relapse of 7.9% (95% CI, 6.0% to 9.7%). For the nontruncatedandAEA+ patients, the 7-year cumulative incidence of relapse was 6.7% (95% CI, 4.7% to 8.6%); for the truncatedorAEA− patients, it was 11.1% (95% CI, 6.9% to 15.4%) (Figure 2). The relapse-specific HR comparing truncatedorAEA− with nontruncatedandAEA+ was 1.73 (96% CI, 1.07-2.80; P = .03), and the aHR was 1.69 (95% CI, 1.05-2.74; P = .03). Age ≥10 years, positive MRD (MRD group 1), and WBCs were significantly associated with the relapse-specific aHR (Table 4).
Cumulative incidence of relapse in the subcohort for the 2 groups based on asparaginase truncation and enzyme activity. TruncatedorAEA-: 11.1% (95% CI, 6.9-15.4), nontruncatedandAEA+: 6.7% (95% CI, 4.7-8.6). ALL, acute lymphoblastic leukemia.
Cumulative incidence of relapse in the subcohort for the 2 groups based on asparaginase truncation and enzyme activity. TruncatedorAEA-: 11.1% (95% CI, 6.9-15.4), nontruncatedandAEA+: 6.7% (95% CI, 4.7-8.6). ALL, acute lymphoblastic leukemia.
Multiple Cox model of time to relapse in the subcohort
| Parameter . | Relapse-specific HR . | 95% CI . | P . |
|---|---|---|---|
| TruncatedorAEA− vs nontruncatedandAEA+ | 1.69 | 1.05-2.74 | .03 |
| Age at diagnosis ≥10 y vs <10 y | 2.44 | 1.48-4.01 | <.001 |
| CNS status at diagnosis | |||
| CNS 2 vs CNS 1 | 0.69 | 0.25-1.92 | .48 |
| CNS 3 vs CNS 1 | 1.16 | 0.41-3.27 | .78 |
| MRD group, day 29 | |||
| MRD 3 vs MRD 4 | 1.05 | 0.42-2.61 | .92 |
| MRD 2 vs MRD 4 | 1.43 | 0.76-2.69 | .27 |
| MRD 1 vs MRD 4 | 2.85 | 1.58-5.15 | .001 |
| Doubling in WBC at diagnosis | 1.15 | 1.02-1.29 | .02 |
| Parameter . | Relapse-specific HR . | 95% CI . | P . |
|---|---|---|---|
| TruncatedorAEA− vs nontruncatedandAEA+ | 1.69 | 1.05-2.74 | .03 |
| Age at diagnosis ≥10 y vs <10 y | 2.44 | 1.48-4.01 | <.001 |
| CNS status at diagnosis | |||
| CNS 2 vs CNS 1 | 0.69 | 0.25-1.92 | .48 |
| CNS 3 vs CNS 1 | 1.16 | 0.41-3.27 | .78 |
| MRD group, day 29 | |||
| MRD 3 vs MRD 4 | 1.05 | 0.42-2.61 | .92 |
| MRD 2 vs MRD 4 | 1.43 | 0.76-2.69 | .27 |
| MRD 1 vs MRD 4 | 2.85 | 1.58-5.15 | .001 |
| Doubling in WBC at diagnosis | 1.15 | 1.02-1.29 | .02 |
The sensitivity analysis excluding the 53 patients with a truncation due to pancreatitis (6 relapses) resulted in a relapse-specific HR of 1.61 (95% CI, 0.96-2.72; P = .07) and an aHR of 1.63 (95% CI, 0.97-2.75; P = .07), indicating that the pancreatitis patients alone were not causing the observed relapse association.
The subanalysis comparing truncatedorAEA− with nontruncatedandAEA+ adjusted for NCI risk groups (344 NCI–high risk, 771 NCI-SR patients) yielded a relapse-specific aHR of 1.77 (95% CI, 1.09-2.85; P = .02) similar to the result adjusted for age, WBCs, MRD group, and CNS status.
Investigating the effect of immunophenotype, the 7-year cumulative incidence of relapse was lower for the patients with T-cell ALL: for 65 T-cell and nontruncatedandAEA+ patients, 4.6% (95% CI, 0% to 9.7%); for 749 BCP and nontruncatedandAEA+ patients, 6.8% (95% CI, 4.7% to 8.9%); for 279 BCP and truncatedorAEA− patients, 12.1% (95% CI, 7.5% to 16.6%). There were no relapses among the 22 patients with T-cell ALL in the truncatedorAEA− group; hence, it was not possible to explore whether the relapse-specific HR for truncatedorAEA− vs nontruncatedandAEA+ differed between patients with BCP and T-cell ALL in a multiple Cox model with interaction.
Type of relapse
Of the 71 relapses in the subcohort, 56 involved the bone marrow, 30 involved the CNS, and 15 involved both. The 7-year cumulative incidence of any bone marrow relapse for the truncatedorAEA− patients vs the nontruncatedandAEA+ patients was 8.8% (95% CI, 5.1% to 12.6%) and 5.4% (95% CI, 3.6-7.2), respectively, and the unadjusted bone marrow relapse-specific HR was 1.83 (95% CI, 1.07-3.14; P = .03). The 7-year cumulative incidence of any CNS relapse for the truncatedorAEA− group vs the nontruncatedandAEA+ group was 5.0% (95% CI, 2.1% to 7.9%) and 2.4% (95% CI, 1.3% to 3.5%), respectively, and the unadjusted CNS relapse-specific HR was 1.86 (95% CI, 0.90-3.87; P = .01).
Treatment intensity
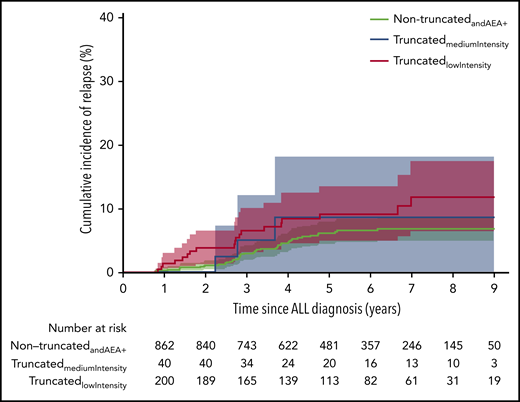
Based on truncation, AEA and length of asparaginase treatment, we placed the 301 truncatedorAEA− patients into a group of 208 patients with low treatment intensity (truncatedlowIntensity) and 93 patients with medium treatment intensity (truncatedmediumIntensity). The 814 nontruncatedandAEA+ patients were considered to have the highest treatment intensity.
The 7-year cumulative incidence of relapse was: for the truncatedlowIntensity patients, 11.9% (95% CI, 6.4% to 17.4%); for the truncatedmediumIntensity patients, 9.4% (95% CI, 3.2% to 15.7%); and for the nontruncatedandAEA+ patients, 6.7% (95% CI, 4.7% to 8.6%) (Figure 3). Comparing the truncatedmediumIntensity and the truncatedlowIntensity to the nontruncatedandAEA+ patients yielded an unadjusted relapse-specific HR = 1.63 (95% CI, 0.77-3.45; P = .206) and HR = 1.78 (95% CI, 1.04-3.05; P = .036), respectively. The multiple models showed similar results: aHR = 1.49 (95% CI, 0.69-3.18; P = .308) and aHR = 1.80 (95% CI, 1.05-3.09; P = .034), respectively.
Cumulative incidence of relapse in the subcohort for the 3 groups based on intensity of asparaginase treatment. NontruncatedandAEA+: 6.7% (95% CI, 6.0-9.7), truncatedmediumIntensity: 9.4% (95% CI, 3.2-15.7), truncatedlowIntensity: 11.9% (95% CI, 6.4-17.4).
Cumulative incidence of relapse in the subcohort for the 3 groups based on intensity of asparaginase treatment. NontruncatedandAEA+: 6.7% (95% CI, 6.0-9.7), truncatedmediumIntensity: 9.4% (95% CI, 3.2-15.7), truncatedlowIntensity: 11.9% (95% CI, 6.4-17.4).
Defining treatment intensity as number of doses per randomization arm and not considering AEA, we estimated the 7-year cumulative incidence of relapse for the dose-intensity groups with at least 3 relapses: 3, 4, and 15 doses in the standard arm with a total of 15 planned doses yielded 10.0%, 14.5%, and 6.2%, respectively, and 8 doses in the experimental arm with a total of 8 planned doses yielded 9.4%, but all with very wide CIs (supplemental Figure 2).
Discussion
This study investigated the outcome of children with ALL following suboptimal asparaginase exposure in the NOPHO ALL2008 protocol. We found a significantly increased risk of relapse in the truncatedorAEA− group compared with the nontruncatedandAEA+ group. We did several subanalyses, taking the heterogeneity of the truncated group into account and investigated subgroups receiving different amounts of asparaginase, but overall the increased HR remained the same.
A previous study investigating the event-free survival (EFS) in patients who had their asparaginase therapy truncated due to toxicities found decreased EFS among those who received <26 weeks of asparaginase treatment compared with those with ≥26 weeks of treatment, without taking AEA measurements into account.3 In the main cohort of our study, we analyzed the relapse-specific HR between truncated and nontruncated without taking enzyme activity levels into account, but as opposed to Silverman et al,3 we did not find an increased risk of relapse in the truncated group (aHR = 1.33; P = .20). Numerous differences between the 2 studies (including differences in asparaginase formulation and administration, protocols and substitution regimens) could be the cause of the divergent results. Several studies have found decreased EFS in patients with SI of PEG-asparaginase.7,22 Thus, we analyzed the subcohort including only patients with AEA measurements available. All patients treated suboptimally with asparaginase, that is, patients who had their asparaginase treatment truncated as well as patients with SI, were grouped together in these analyses. Our results showed a significantly increased risk of relapse in the truncatedorAEA− group (aHR = 1.69; P = .032), which is also in accordance with a recent study from the Children’s Oncology Group.23 Our results thus confirm that suboptimal asparaginase treatment leads to an increased risk of relapse. The discrepancy between the results in the main cohort and the subcohort can be due to several factors. The most obvious difference is the lack of AEA measurements as part of the classification in the main cohort, thus patients with SI were not identified. Moreover, it is possible that the truncation of asparaginase treatment in the high-risk group is not as crucial due to a more intensified treatment in these patients.
The optimal cumulative dose of asparaginase in the treatment of ALL is unknown, but has been widely debated. Some studies have shown that more intensified asparaginase treatment (eg, 20 additional weeks of asparaginase in reinduction) improves EFS,4,5,8,24 whereas others have found less intensified asparaginase treatment to have comparable outcomes.10,25,26 The results of the randomization of asparaginase in the NOPHO ALL2008 protocol showed that the disease-free survival did not differ between patients receiving 8 doses of PEG-asparaginase (5 doses at 2-week intervals and 3 doses at 6-week intervals) compared with patients receiving 15 doses of asparaginase (2-week intervals).10 Considering the results of the present study and the NOPHO ALL2008 randomized study, it is evident that non–high-risk patients can do well with less intensified asparaginase treatment, but discontinuation due to toxicities or SI potentially still leads to an increased risk of relapse.
Our study did not have the power to investigate how many doses of asparaginase would be needed to avoid the increased risk of relapse in the non–high-risk group, but in the subanalysis we divided patients into 3 groups based on truncation and weeks of asparaginase treatment. We found increasing cumulative incidence of relapse with a decreasing amount of asparaginase (Figure 3). In the multiple Cox regression analysis, we found a significantly increased risk of relapse in the truncatedlowIntensity group compared with the nontruncatedandAEA+ but not when comparing truncatedmediumIntensity to nontruncatedandAEA+. This could be due to lack of power, with only 93 patients in the medium-intensity group. It could also indicate that 10 weeks of asparaginase treatment in the non–high-risk group was sufficient. However, it is important to keep in mind that we predefined the cutoffs for the treatment-intensity groups. In addition, due to the randomization, there was an overlap of how many weeks of asparaginase treatment the patients had received in the truncatedmediumIntensity group and in the nontruncatedandAEA+ group: a patient with a truncation in the standard arm could have received more asparaginase compared with a patient not truncated in the experimental arm, but because the timing of the asparaginase administrations is thought to be of importance, we chose to keep the truncated and nontruncated patients in separate groups.
In the NOPHO ALL2008 protocol, Erwinia asparaginase was used in case of hypersensitivity to PEG-asparaginase. Yet, not all doses were replaced as non–high-risk patients received only 2 additional weeks of asparaginase treatment during the delayed intensification following hypersensitivity. Because most of these patients had no AEA during PEG-asparaginase treatment, they received very little effective asparaginase treatment in total. Based on current knowledge, it can seem irrational that the NOPHO ALL2008 protocol did not replace all PEG-asparaginase dosages in case of hypersensitivity; however, it merely reflects that the protocol was planned and was based on the knowledge and literature available at the time, and that new knowledge has since emerged. In February 2017, therapeutic drug monitoring (TDM) during PEG-asparaginase treatment became voluntary until closure of the NOPHO ALL2008. Patients identified without enzyme activity were switched to either Erwinia asparaginase or eryaspase (clinicaltrials.gov: NCT03267030). In the new ALLTogether protocol, TDM is standard of care to allow for the rational replacement of PEG-asparaginase in the case of SI and for assessment of allergic-like reactions.
Substituting all asparaginase doses in cases of hypersensitivity can seem like a simple recommendation. However, in recent years, the world has experienced a shortage of Erwinia asparaginase. To our knowledge, no doses of asparaginase were omitted due to Erwinia asparaginase shortage in the current study, but it is a critical issue that appears to be recurrent. This, combined with the results of our study, emphasizes the need for new asparaginase preparations.
We assumed that the increased risk of relapse in the truncatedorAEA− group was caused by the lack of asparaginase exposure, but other possible explanations cannot be ruled out. We probed this in a sensitivity analysis excluding the pancreatitis patients because pancreatitis can cause severe illness27,28 and thereby truncation or postponement of other elements of treatment. As our results did not change significantly, we conclude that the pancreatitis patients alone are not causing the increased aHR. It is, however, important to stress that we are studying a single drug in a long and complex treatment and the composition of the ALL treatment differs between the different study groups; thus, direct comparisons and recommendations can be difficult. To test whether the NCI criteria might yield different results, we performed a post hoc analysis adjusting for these risk groups; however, the results did not markedly change. We also made the assumption that SR and IR patients with hypersensitivity without any AEA measurements had no AEA and included these in the truncatedorAEA− group. However, this should not affect the results because all hypersensitivity patients in the NOPHO ALL2008 protocol were truncated due to lack of full asparaginase substitution.
In summary, we found that patients with truncated asparaginase treatment or no enzyme activity had a significantly increased risk of relapse compared with those with nontruncated therapy and measurable enzyme activity. This is in accordance with previously published studies. Ten weeks of efficient asparaginase treatment seemed to be sufficient in the NOPHO ALL2008 protocol. Our results confirm and emphasize the importance of TDM and substitution of all planned asparaginase doses to ensure treatment efficacy, which is even more essential now as new protocols are focusing on treatment reduction.
For original data, please e-mail the corresponding author.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors sincerely thank laboratory technician, Jane Hagelskjær Knudsen at the Pediatric Research Laboratory (Aarhus University Hospital) for excellent work conducting the enzyme analyses. Moreover, they kindly thank Jacob Bidstrup for cleaning the Erwinia asparaginase data.
This study was funded by the Danish Childhood Cancer Foundation (grants 2015-29, 2015-34, 2016-0186 and 2017-2031) and the Swedish Childhood Cancer Fund (grant KP2017-0008).
Authorship
Contribution: K.G., S.G.H., B.K.A., and K.S. analyzed and interpreted data; S.G.H. wrote the paper, which was critically reviewed by K.G., K.S., and B.K.A; and all authors conceived and designed the study, collected data, and approved the final manuscript.
Conflict-of-interest disclosure: B.K.A. is a sponsor for the investigator-initiated NOR-GRASPALL 2016 study (clinicaltrials.gov: NCT03267030). K.S. has received speaker and/or advisory board honoraria from Jazz Pharmaceuticals and Servier; a speaker fee from Amgen and Medscape; and an educational grant from Servier. The remaining authors declare no competing financial interests.
A complete list of the members of the Nordic Society of Pediatric Hematology and Oncology (NOPHO) ALL-2008 PI Working Group appears in the supplemental Appendix.
Correspondence: Birgitte Klug Albertsen, Aarhus University Hospital, Palle Juul-Jensens Blvd 99, 8200 Aarhus N, Denmark; e-mail: biralber@rm.dk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal