Key Points
Combined anti-BMP6 antibody and EPO treatment improves systemic iron availability and reduces EPO needs for the treatment of ACD.
Modulation of ferroportin on erythroid precursors results in reduced free intracellular iron levels and maturation of the erythroid lineage.
Abstract
Recombinant erythropoietin (EPO) and iron substitution are a standard of care for treatment of anemias associated with chronic inflammation, including anemia of chronic kidney disease. A black box warning for EPO therapy and concerns about negative side effects related to high-dose iron supplementation as well as the significant proportion of patients becoming EPO resistant over time explains the medical need to define novel strategies to ameliorate anemia of chronic disease (ACD). As hepcidin is central to the iron-restrictive phenotype in ACD, therapeutic approaches targeting hepcidin were recently developed. We herein report the therapeutic effects of a fully human anti-BMP6 antibody (KY1070) either as monotherapy or in combination with Darbepoetin alfa on iron metabolism and anemia resolution in 2 different, well-established, and clinically relevant rodent models of ACD. In addition to counteracting hepcidin-driven iron limitation for erythropoiesis, we found that the combination of KY1070 and recombinant human EPO improved the erythroid response compared with either monotherapy in a qualitative and quantitative manner. Consequently, the combination of KY1070 and Darbepoetin alfa resulted in an EPO-sparing effect. Moreover, we found that suppression of hepcidin via KY1070 modulates ferroportin expression on erythroid precursor cells, thereby lowering potentially toxic-free intracellular iron levels and by accelerating erythroid output as reflected by increased maturation of erythrocyte progenitors. In summary, we conclude that treatment of ACD, as a highly complex disease, becomes more effective by a multifactorial therapeutic approach upon mobilization of endogenous iron deposits and stimulation of erythropoiesis.
Introduction
Erythropoietin (EPO), the master regulator of red blood cell (RBC) production, is known to act on several erythroid progenitor stages, as it promotes erythroid lineage commitment as well as survival and proliferation of late erythroid progenitors.1-4
Anemia of inflammation or anemia of chronic disease (ACD) is the most prevalent form of anemia in hospitalized patients and a common disease-related complication in rheumatoid arthritis, chronic kidney disease (CKD), inflammatory bowel disease, cancer, and infectious diseases.5-14 As anemia negatively impacts quality of life, patients’ cardiovascular performance, and metabolic activities, therapy is warranted but often challenging.15-17 Besides insufficient EPO production, especially in patients suffering from CKD, reduced EPO sensitivity of the erythron contributes to anemia.18 Taken together, this is often referred to as “EPO hyporesponsiveness.” Moreover, functional iron deficiency due to inflammation-driven high production of hepcidin is central to the pathophysiology of ACD.7,19-22 Hepcidin is a small liver-derived hormone controlling systemic iron availability. It binds to the only known iron exporter, ferroportin-1 (FPN), inducing its internalization, thus hampering cellular iron export. Accordingly, elevated hepcidin levels prevent iron uptake from the duodenum and withhold iron within the mononuclear phagocyte system, leading to hypoferremia and iron-restricted erythropoiesis.23 Moreover, it was found that erythroblasts and mature RBCs express FPN throughout erythroid maturation. Consequently, excess iron that is not used for heme synthesis can effectively be exported by these cells, and an intact FPN-hepcidin axis was found to be critical for erythropoiesis in mice and humans.24,25
Studies in patients with CKD have shown that EPO hyporesponsiveness can be ameliorated to a certain degree with concomitant administration of IV iron, highlighting the critical interplay of sufficient iron supply and EPO function for a sustained erythroid output.7,8,26-29 Higher IV iron doses that resulted in higher ferritin levels were more effective in achieving the desired reduction in EPO dose in anemic hemodialysis patients.30 Consequently, iron administration is an established strategy to enhance erythropoiesis stimulating agent (ESA)-related responses, as recommended by international guidelines (Kidney Disease: Improving Global Outcomes) in patients suffering from CKD.31 Thereby the beneficial effect of iron is primarily linked to the increase in transferrin saturation (Tf-Sat).32-34 Mechanistically, this may partly be linked to iron-mediated stimulation of EPO-receptor surface expression via transferrin receptor-2–dependent increase of Scribble formation.35 However, iron administration implicates the risk of secondary iron overload, causing generation of labile cellular iron accompanied by the risk of infection, oxidative stress, cardiovascular complications, and mortality.33,6-40 Moreover, clinical trials in patients with CKD emphasized possible adverse outcomes related to EPO therapy, including stroke and venous thromboembolic disease. In addition, in being a pleiotropic growth factor, EPO was linked to malignancy progression, which has made EPO therapy less appealing than it used to be.41-43
Therapeutic strategies targeting hepcidin expression or circulatory concentrations have been highlighted as a possible solution to ESA hyporesponsiveness as well as toxic iron overload.44 Both bone morphogenetic protein (BMP)2 and BMP6 critically control hepcidin transcription via the BMPR/SMAD pathway, making an anti-BMP6 treatment strategy a promising therapeutic approach.45-52 Moreover, the erythroid cell–derived peptide erythroferrone (ERFE) was shown to trap BMP6, consequently suppressing hepcidin formation in times of stimulated erythropoiesis, highlighting the importance of BMP6-mediated signaling for hepcidin induction.53
We herein report that a BMP6 targeted therapy (KY1070) reverses hepcidin-mediated iron restriction and improves systemic iron availability and anemia when used as a monotherapy in vivo. KY1070 in combination with EPO synergistically acts toward anemia correction. Therefore, KY1070 drastically reduces EPO need in the treatment of ACD in animal models. Mechanistically, we demonstrate that the more sustained and mature erythroid output is not solely mediated by increased systemic iron availability and erythroid cell proliferation, but also driven by an increased FPN expression on erythroid progenitor cells causing reduced concentrations of free, potentially toxic intracellular erythroid iron. Using an anti-BMP6 antibody is a novel approach to treat ACD, which synergizes with ESA as an efficient therapy.
Materials and methods
Animals
All animals had free access to food and water and were housed according to institutional and governmental guidelines in the animal facility of the Medical University of Innsbruck. All animal experiments were approved by the Austrian Federal Ministry of Science and Research (BMWF-66.011/0146-WF/V/3b/2017, BMWF-66.011/0101-WF/V/3b/2017, and BMWFW-66.011/0177-WF-/V/3b/2017). Additional details on the experimental procedures performed in mice and rats, including the different diets, are provided in the supplemental Data, available on the Blood Web site.
Antibody generation
KY1070 was generated using the Kymouse with a Bmp6 gene knockout containing human immunoglobulin genes producing human antibodies.54 More details are provided in the supplemental data.
In vitro testing of KY1070
All details on surface plasmon resonance binding, the HepG2 Hamp luciferase reporter gene assay, HepG2 cell culture experiments, and the receptor dimerization assay are provided in the supplemental Data.
Plasma biochemistry and complete blood count
Blood was collected via retroorbital puncture into heparinized tubes, and plasma was prepared. Details on iron, transferrin, hepcidin, EPO, and blood urea nitrogen determination are provided in the supplemental Data.
Complete blood count analysis was performed using a Vet–Animal Blood Counter (ABC) (Scil Animal Care Co. GmbH, Viernheim, Germany).
Flow cytometry analysis
For quantification of cellularity of late erythroid progenitors, bone marrow was flushed from femurs with phosphate-buffered saline (PBS). For determination of blood neutrophils, RBCs were removed by lysis with an ammonium-chloride-potassium lysis buffer. Cell suspensions were then stained with fluorochrome-conjugated antibodies. Further details and antibody specifications are provided in the supplemental Data. All data were acquired on a Gallios Flow Cytometer or CytoFLEX S (both by Beckman Coulter, Brea, CA) and analyzed with FlowJo software (Flow Jo LLC, Ashland, OR). For cell sorting, a FACSAria I device (Becton Dickinson, Franklin Lakes, NJ) was used.
Western blot analysis and tissue iron determination
RNA extraction and quantitative real-time polymerase chain reaction
Quantitative polymerase chain reaction was performed as described previously.55 Additional details on primer sequences and analysis are provided in the supplemental Data.
Statistics
Statistical analysis was performed using Prism software (version 7; GraphPad, La Jolla, CA) and R (packages tidyverse, stats, and models; https://www.r-project.org/). Results are expressed as means ± standard error of the mean (SEM). A value of P < .05 was considered statistically significant. All further details are provided in the supplemental Data.
Results
Characterization of the fully human, highly BMP6 specific antibody KY1070
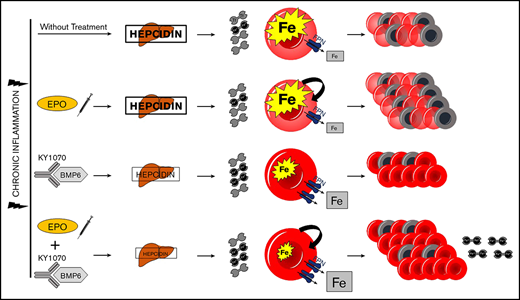
First, we investigated the binding kinetics of KY1070 to human BMP6 and other species via surface plasmon resonance. To obtain genuine dissociation constant (KD) values of a 1:1 interaction, monovalent Fab fragments were used giving a Kd value of 140 pM for binding to recombinant human BMP6. Next, cross-reactivity for human, mouse, and rat BMP6 was established using recombinant BMP6 of the respective species in a HepG2 Hamp luciferase reporter gene assay, which revealed that KY1070 was able to neutralize BMP6 from all 3 species with comparable efficacies (Figure 1A).
Specificity and activity of KY1070. (A) Cross-reactivity of KY1070 against human, rat, and mouse BMP6 was tested using a HepG2 Hamp luciferase reporter gene cell line. After assessing the assay window using a range of concentrations, a fixed concentration of recombinant human, rat, or mouse BMP6 of 10 nM was used. KY1070 inhibition curves were generated by titration starting at a final concentration of 600 nM. A human IgG4 isotype control was included in each assay. (B) KY1070 specificity was tested against human BMP5 and BMP7. HepG2 Hamp luciferase reporter gene cells were stimulated with a fixed concentration of recombinant human BMP5 or BMP7 of 10 nM. Inhibition curves for KY1070 were generated by titration starting at a final concentration of 600 nM. A titration of commercially available murine anti-BMP5 and anti-BMP7 antibodies as well as a human IgG4 isotype control was included. (C) BMPR1A/ BMPR2 dimerization assay performed in transfected U2OS cells. Dimerization was induced using human BMP6 (Peprotech 120-06) used at a fixed final concentration of 200 ng/mL. KY1070 inhibition curves were generated by titration starting at a final concentration of 600 nM. A human IgG4 isotype control was included. (D) Liver Hamp mRNA levels and plasma hepcidin levels of Bmp6 wild-type and Bmp6−/− mice determined 3 days after a single injection of IgG4 isotype antibody (3 mg/kg; n = 4 [Bmp6 Wt]; n = 8 [Bmp6−/−]) or KY1070 (3 mg/kg; n = 5 [Bmp6 wild type]; n = 9 [Bmp6−/−]). Unpaired Student t test was applied for comparison between genotypes for panel D. Data are presented as means ± SEM. **P < .01. RLU, relative light units.
Specificity and activity of KY1070. (A) Cross-reactivity of KY1070 against human, rat, and mouse BMP6 was tested using a HepG2 Hamp luciferase reporter gene cell line. After assessing the assay window using a range of concentrations, a fixed concentration of recombinant human, rat, or mouse BMP6 of 10 nM was used. KY1070 inhibition curves were generated by titration starting at a final concentration of 600 nM. A human IgG4 isotype control was included in each assay. (B) KY1070 specificity was tested against human BMP5 and BMP7. HepG2 Hamp luciferase reporter gene cells were stimulated with a fixed concentration of recombinant human BMP5 or BMP7 of 10 nM. Inhibition curves for KY1070 were generated by titration starting at a final concentration of 600 nM. A titration of commercially available murine anti-BMP5 and anti-BMP7 antibodies as well as a human IgG4 isotype control was included. (C) BMPR1A/ BMPR2 dimerization assay performed in transfected U2OS cells. Dimerization was induced using human BMP6 (Peprotech 120-06) used at a fixed final concentration of 200 ng/mL. KY1070 inhibition curves were generated by titration starting at a final concentration of 600 nM. A human IgG4 isotype control was included. (D) Liver Hamp mRNA levels and plasma hepcidin levels of Bmp6 wild-type and Bmp6−/− mice determined 3 days after a single injection of IgG4 isotype antibody (3 mg/kg; n = 4 [Bmp6 Wt]; n = 8 [Bmp6−/−]) or KY1070 (3 mg/kg; n = 5 [Bmp6 wild type]; n = 9 [Bmp6−/−]). Unpaired Student t test was applied for comparison between genotypes for panel D. Data are presented as means ± SEM. **P < .01. RLU, relative light units.
To exclude cross-reactivity against related BMP family members, binding of human BMP2, BMP4, BMP5, BMP7, and BMP9 was tested. No inhibitory effects of KY1070 on either of these BMP-induced Hamp promoter activiies at KY1070 concentrations up to 600 nM could be detected (Figure 1B; supplemental Figure 1A-C).
As heterodimerization of type I and type II BMP receptors (BMPR) is needed to mediate BMP-induced signaling, the impact of BMP6 inhibition of this receptor dimerization was characterized. Transfected U2OS cells carrying modified human BMPR1A (type I) and BMPR2 (type II), intracellularly tagged with inactive enzyme subunits, revealed that KY1070 effectively inhibited BMP6-induced heterodimerization (Figure 1C).
To verify the specificity of KY1070 also in vivo, we administered either a human immunoglobulin 4 (IgG4) Isotype antibody (Isotype) or KY1070 to healthy Bmp6-wt mice and Bmp6-deficient (Bmp6−/−) mice (supplemental Figure 2A). Although Bmp6-wt animals reacted to a single injection of KY1070 with a significant decrease in hepatic Hamp messenger RNA (mRNA) after 3 days, which was paralleled by an increase in plasma iron levels, neither hepatic Hamp mRNA nor plasma iron levels were changed in BMP6−/− mice (Figure 1D). Thus, in the absence of BMP6, KY1070 has no effect on hepatic hepcidin production and consequently has no effect on plasma iron levels.
KY1070 modulates Darbepoetin alfa (EPO)-induced erythroid response in healthy rodents
Next, we studied the effects of KY1070, Darbepoetin alfa (EPO), and the combination of both on iron homeostasis in healthy rodents (supplemental Figure 2B-C). To verify specificity of the reported antibody effects, we included a vehicle-treated control group (PBS) and an EPO single-treatment group in our first set of mouse experiments. Among all the analyzed parameters, treatment with a human IgG4 Isotype antibody did not cause any significant differences, so the PBS/Isotype-treated animals will be referred to as “control animals” and the EPO ± IgG4 Isotype-treated mice will be referred to as EPO group (Figure 2A-D).
KY1070 effectively suppresses hepcidin levels in healthy mice and rats and leads to an improved erythroid output in combination with Darbepoetin alfa over time. (A-D) Healthy C57BL/6N mice were treated with a single dose of either PBS (n = 5), IgG4 isotype control (3 mg/kg; n = 5), KY1070 (3 mg/kg; n = 5), Darbepoetin alfa (10 µg/kg; n = 5), Darbepoetin alfa and IgG4 isotype control (n = 5), or KY1070 and EPO (n = 5). Liver Hamp mRNA levels (A), plasma iron (B), Tf-Sat, hepatic tissue iron levels (C), and reticulocyte counts (D) (shown as %Thiazol Orangepos) were determined 3 days after indicated treatments. (E-G) Healthy Lewis rats were treated with IgG4 isotype control (3 mg/kg; n = 6), KY1070 (3 mg/kg; n = 7), Darbepoetin alfa (10 µg/kg; n = 7), or KY1070 and EPO (n = 7). Liver Hamp mRNA levels (E), hemoglobin levels (Hgb) (F), MCV, and hepatic tissue iron levels (C), and reticulocyte counts (G) (shown as %Thiazol Orangepos) were determined at day 21 after a single KY1070 treatment ± weekly EPO application. (H) Flow cytometric analysis of rat bone marrow erythropoiesis at the end of treatment in rats of all different treatment groups. Representative dot plots for Lineageneg cells with percentages of the different erythropoietic populations (I to IV) per femur are given; basophilic cells (I), polychromatic cells (II), orthochromatic cells and reticulocytes (III), and mature RBCs (IV); for gating strategy see supplemental Figure 3F. Two-way analysis of variance (ANOVA) with Tukey corrected post hoc Student t test for multiple comparisons between all groups was applied for panels A-G. Results are shown as means ± SEM. Significant levels are indicated for the Isotype-treated control group against all treatment groups and EPO vs KY1070 + EPO–treated rodents. *P < .05; **P < .01; ***P < .001. FSC-A, forward scatter area.
KY1070 effectively suppresses hepcidin levels in healthy mice and rats and leads to an improved erythroid output in combination with Darbepoetin alfa over time. (A-D) Healthy C57BL/6N mice were treated with a single dose of either PBS (n = 5), IgG4 isotype control (3 mg/kg; n = 5), KY1070 (3 mg/kg; n = 5), Darbepoetin alfa (10 µg/kg; n = 5), Darbepoetin alfa and IgG4 isotype control (n = 5), or KY1070 and EPO (n = 5). Liver Hamp mRNA levels (A), plasma iron (B), Tf-Sat, hepatic tissue iron levels (C), and reticulocyte counts (D) (shown as %Thiazol Orangepos) were determined 3 days after indicated treatments. (E-G) Healthy Lewis rats were treated with IgG4 isotype control (3 mg/kg; n = 6), KY1070 (3 mg/kg; n = 7), Darbepoetin alfa (10 µg/kg; n = 7), or KY1070 and EPO (n = 7). Liver Hamp mRNA levels (E), hemoglobin levels (Hgb) (F), MCV, and hepatic tissue iron levels (C), and reticulocyte counts (G) (shown as %Thiazol Orangepos) were determined at day 21 after a single KY1070 treatment ± weekly EPO application. (H) Flow cytometric analysis of rat bone marrow erythropoiesis at the end of treatment in rats of all different treatment groups. Representative dot plots for Lineageneg cells with percentages of the different erythropoietic populations (I to IV) per femur are given; basophilic cells (I), polychromatic cells (II), orthochromatic cells and reticulocytes (III), and mature RBCs (IV); for gating strategy see supplemental Figure 3F. Two-way analysis of variance (ANOVA) with Tukey corrected post hoc Student t test for multiple comparisons between all groups was applied for panels A-G. Results are shown as means ± SEM. Significant levels are indicated for the Isotype-treated control group against all treatment groups and EPO vs KY1070 + EPO–treated rodents. *P < .05; **P < .01; ***P < .001. FSC-A, forward scatter area.
In mice, a single application of KY1070, EPO, or a combination of both significantly reduced hepatic Hamp mRNA levels after 3 days. Interestingly, the combination regimen reduced Hamp expression to nearly undetectable levels (Figure 2A). Upon effective hepcidin suppression by EPO and KY1070, only KY1070-treated animals showed a concomitant increase in plasma iron levels, Tf-Sat, and hepatic iron content. In contrast, EPO-treated mice showed a trend toward decreased iron parameters but higher erythropoietic output as reflected by reticulocytosis, suggesting that EPO-mobilized iron is immediately consumed by the bone marrow for erythropoiesis. Notably, combination of KY1070 with EPO had a double-positive effect, resulting in an effective erythropoietic output together with a balanced iron metabolism (Figure 2B-D).
In rats, KY1070, EPO, and the combination treatment showed comparable effects to the murine data on plasma iron, hepcidin levels, Tf-Sat, and reticulocytes after 3 days (supplemental Figure 3A-C). In addition, we analyzed rats 21 days after a single KY1070 ± weekly EPO injection. Even 21 days after a single KY1070 administration, hepatic Hamp mRNA levels were still effectively suppressed, being lowest among animals receiving combination treatment (Figure 2E). Moreover, EPO and double-treated rats had higher Hgb and mean corpuscular volume (MCV) levels (Figure 2F). However, an increased reticulocyte count was only detectable in rats receiving EPO monotherapy, but not among rats receiving the combination therapy (Figure 2G). The latter observation suggests that the high MCV levels seen in EPO-treated mice is due to a washout of the erythroid progenitors/reticulocytes, which are larger in size, whereas the combination therapy results in higher MCV levels due to a higher iron content. This is further substantiated by results from flow cytometric analysis of erythroid progenitors in the bone marrow: EPO-treated rats had a shift toward significantly higher progenitor populations (population I to III) and less mature RBCs (population IV) as compared with rats receiving the combination therapy. Importantly, total cell numbers were equally increased among both treatment groups (Figure 2H; supplemental Figure 3D-E). In summary, combination treatment causes a more sustainable mature erythroid output over time.
KY1070 suppresses hepcidin levels during inflammation and synergizes with EPO in ACD treatment efficacy
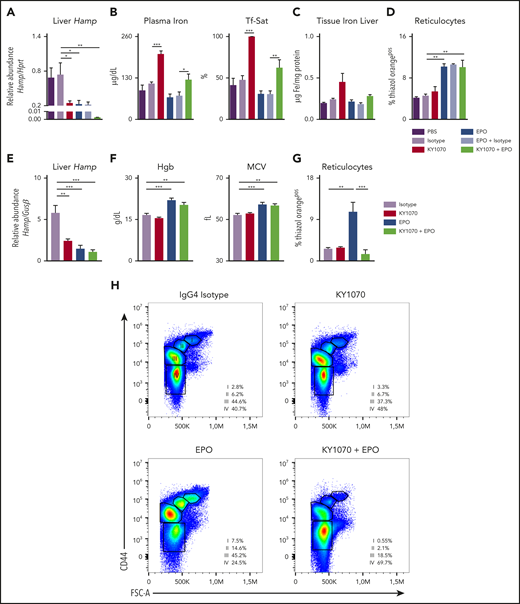
To evaluate the efficacy of our anti-BMP6 antibody in a therapeutic setting, we administered KY1070 in a well-established rat ACD model.20 Two weeks after group A streptococcal peptidoglycan-polysaccharide administration, exposed rats developed arthritis with concomitant inflammation and elevated hepcidin levels, characteristic of ACD (supplemental Figure 4A-C). Rats were randomized according to Hgb level at time point “week 0” and assigned to treatment with either Isotype, KY1070, EPO, or a combination thereof. Four weeks after start of treatment, Isotype-treated ACD rats developed anemia, and each monotherapy only moderately ameliorated Hgb levels. However, combination of KY1070 and EPO completely corrected anemia with Hgb levels and increased the number of RBCs compared with healthy control animals (Figure 3A). Furthermore, the MCV and mean corpuscular hemoglobin (MCH) were both increased in KY1070-treated rats, yet being only significant in animals treated with the combination therapy (Figure 3A). Again, higher MCV and MCH levels seen in these rats reflected higher erythroid iron content rather than a release of immature erythroid precursors, as reticulocyte numbers were not increased (supplemental Figure 4D). Alongside this sufficient iron mobilization in KY1070-treated animals, plasma hepcidin levels were substantially decreased, and Tf-Sat remained elevated throughout the whole study period, thereby having an even more pronounced and statistically significant effect seen when combined with EPO administration (Figure 3B-C; supplemental Figure 4E-F). Interestingly, despite massive erythroid iron consumption leading to improvement of RBC parameters, the combination of KY1070 and EPO kept Tf-Sat at levels seen in healthy animals.
KY1070 mobilizes iron for erythropoiesis in 2 rodent models of chronic anemias and has a synergistic effect on anemia resolution when combined with Darbepoetin alfa. (A-C) ACD in female Lewis rats was induced by intraperitoneal administration of group A streptococcal peptidoglycan-polysaccharide. ACD rats were treated with IgG4 isotype control (ACD; n = 8), KY1070 (3 mg/kg; n = 9), EPO (10 µg/kg; n = 8), or both (n = 7). Hgb, RBC, MCV, MCH (A), plasma hepcidin levels (B), plasma iron levels and Tf-Sat (C) were determined in ACD rats at the end of treatment (week 4; see also supplemental Figure 4A). (D-G) CKD in male C57BL/6N mice was induced by a diet containing 0.2% adenine. CKD mice were either treated with IgG4 isotype control (CKD; n = 5), KY1070 (3 mg/kg; n = 6), Darbepoetin alfa (10 µg/kg; n = 6), or both (n = 6). (D) Hematological parameters (Hgb, RBC, MCH, and MCV) were determined in CKD mice at the end of treatment (week 4; see also supplemental Figure 7A). Plasma EPO levels (E), plasma iron levels, Tf-Sat (F), liver Hamp mRNA and plasma hepcidin levels (G) of indicated mice following treatment (week 4; see also supplemental Figure 7A). A naive control group was included in all experiments (n = 5 [rats], n = 7 [mice]). Two-way ANOVA with Tukey corrected post hoc Student t test for multiple comparisons between ACD and CKD animals was applied. Results are shown as means ± SEM. *P < .05; **P < .01; ***P < .001.
KY1070 mobilizes iron for erythropoiesis in 2 rodent models of chronic anemias and has a synergistic effect on anemia resolution when combined with Darbepoetin alfa. (A-C) ACD in female Lewis rats was induced by intraperitoneal administration of group A streptococcal peptidoglycan-polysaccharide. ACD rats were treated with IgG4 isotype control (ACD; n = 8), KY1070 (3 mg/kg; n = 9), EPO (10 µg/kg; n = 8), or both (n = 7). Hgb, RBC, MCV, MCH (A), plasma hepcidin levels (B), plasma iron levels and Tf-Sat (C) were determined in ACD rats at the end of treatment (week 4; see also supplemental Figure 4A). (D-G) CKD in male C57BL/6N mice was induced by a diet containing 0.2% adenine. CKD mice were either treated with IgG4 isotype control (CKD; n = 5), KY1070 (3 mg/kg; n = 6), Darbepoetin alfa (10 µg/kg; n = 6), or both (n = 6). (D) Hematological parameters (Hgb, RBC, MCH, and MCV) were determined in CKD mice at the end of treatment (week 4; see also supplemental Figure 7A). Plasma EPO levels (E), plasma iron levels, Tf-Sat (F), liver Hamp mRNA and plasma hepcidin levels (G) of indicated mice following treatment (week 4; see also supplemental Figure 7A). A naive control group was included in all experiments (n = 5 [rats], n = 7 [mice]). Two-way ANOVA with Tukey corrected post hoc Student t test for multiple comparisons between ACD and CKD animals was applied. Results are shown as means ± SEM. *P < .05; **P < .01; ***P < .001.
To gain insight into the mechanism of action, we analyzed pSMAD 1/5/9 levels in the livers of ACD rats, which have either been treated once with Isotype antibody or KY1070 after 3 days. As shown in supplemental Figure 5, pSMAD 1/5/9 levels were increased in ACD rats with high Hamp levels and normalized if KY1070 was applied.
As EPO represents a standard of care in the treatment of anemia in CKD, we next sought to corroborate the synergistic effects of the combination therapy observed in the rat arthritis model in a CKD mouse model, representing another form of ACD57 (supplemental Figure 6A-I). In this model, the combination of KY1070 and EPO resulted in normalization of Hgb levels, RBC count, MCV, and MCH. Monotherapy with either drug also ameliorated Hgb levels, which was however not comparable to the effects seen in animals treated with combination therapy (Figure 3D; supplemental 7B). Of note, we could not discern any effects of combined therapy on systemic or local inflammatory hallmarks, such as blood granulocyte levels or cytokine gene expression in the kidney (supplemental Figure 7C-D).
Interestingly, as shown in Figure 3E, endogenous EPO levels were highest in CKD animals, normalized in KY1070- and EPO-treated animals, but significantly decreased in mice exposed to the combination therapy. As EPO production is mainly driven by tissue hypoxia, these results suggest a quantitative and qualitative improvement in erythropoiesis leading to improved oxygen tissue supply under the combination therapy. Of importance, injected recombinant human Darbepoetin alfa is not detected with this enzyme-linked immunosorbent assay. Higher EPO levels in CKD mice may seem to be contrary to expectations. However, comparison of EPO levels in phlebotomized mice, having the same Hgb values, show that EPO levels in untreated CKD mice were inappropriately low in relation to the observed anemia (supplemental Figure 6J-K).
Plasma hepcidin levels, iron, and Tf-Sat in treated CKD mice paralleled the results seen in ACD rats, underlining our hypothesis that the combination of EPO and KY1070 acts in a superadditive manner on erythroid output: combination therapy caused the strongest increase in plasma iron levels and a significant increase in Tf-Sat (Figure 3F). This confirmed the results obtained in healthy C57BL/6N mice; the addition of an isotype to EPO treatment did not lead to any significant differences in CKD mice (supplemental Figure 8A-E). Intriguingly, analysis of CKD mice 3 days after the last injection revealed that EPO monotherapy is also sufficient to lower hepcidin levels, which is in agreement with previous reports52,58,59 (supplemental Figure 8F-G), but levels were again lowest in double-treated animals. Thus, EPO-mediated hepcidin suppression is only transient, whereas an anti-BMP6 antibody-mediated effect is long lasting, especially when combined with EPO.
Inhibition of hepcidin by KY1070 is independent of ERFE
KY1070 targets BMP6, which is also sequestered by ERFE for EPO-mediated hepcidin suppression.53 To gain insight if therapy with KY1070 alters ERFE-mediated hepcidin suppression, we measured ERFE levels in the plasma of our CKD mice by enzyme-linked immunosorbent assay at 2 different time points (at day 3 and 1 week after last treatment). Although ERFE was upregulated in wild-type mice 12 hours after a single EPO dose, ERFE was no longer detectable in the plasma of CKD mice (supplemental Figure 9A-B). In parallel, in vitro experiments, using HepG2 cells, did not show any interference between the effects of KY1070 and ERFE on Hamp mRNA levels (supplemental Figure 9C). Based on these in vitro and in vivo data, we conclude that the long-lasting effect of KY1070-mediated hepcidin suppression in combination with EPO is independent of ERFE.
Combining EPO and KY1070 leads to an EPO-sparing effect in a rat arthritis model for ACD
Based on our observations that rats treated with a combination of KY1070 and EPO displayed Hgb levels, which exceeded those seen in healthy rats (Figure 3A), we next wanted to test the hypothesis that addition of KY1070 to an EPO treatment regimen helps to effectively reduce EPO doses needed to correct anemia. As EPO monotherapy displayed limited efficacy under inflammation in our rat arthritis model, EPO hyporesponsiveness can be postulated. To examine such an EPO-sparing effect, experiments were designed to mirror ACD patient evaluation and treatment. In detail, treatment (EPO alone or in combination) was started only if Hgb values dropped below a predefined value (<13.5 g/dL, normal Hgb levels in healthy rats ∼16 g/dL), and Hgb values were then weekly reevaluated. Once treatment started, additional EPO was only administered if Hgb values were below the average level of healthy control rats (for details see supplemental “ACD rats EPO-sparing protocol” and supplemental Figure 10). First, KY1070 in combination with EPO again dramatically boosted Hgb levels, normalized MCV and MCH, and triggered changes in iron homeostasis accordingly (supplemental Figure 11A-B). Second, the number of additional EPO doses at week 1 to 4 given to rats treated with EPO alone was higher at every week compared with rats receiving the combination therapy. Taken together, these weekly differences in EPO requirement in total resulted in an ∼65% reduction of the total cumulative EPO dose (Figure 4A-C).
KY1070 leads to an EPO-sparing effect. Female ACD rats were used to evaluate an EPO-sparing effect of KY1070 combination therapy (for further details on the study protocol, please see supplemental Figure 10A-C and supplemental “ACD rats EPO-sparing protocol”). (A) The weekly number of Darbepoetin alfa (EPO) applications needed for anemia correction in ACD rats with an EPO monotherapy (left panel; n = 10) and KY1070 + EPO combination (right panel; n = 11) is shown. (B) Percent of applied Darbepoetin alfa doses in total (week 1 to 4) and consequent reduction of EPO doses needed (in %) is indicated. (C) Number of possible vs indeed consumed EPO doses in total (week 1 to 4) is shown. (D) Differences (Δ) in hemoglobin (ΔHgb) for CKD mice treated with indicated combinations of KY1070 and Darbopoietin alfa. Pooled data from 5 separate experiments are plotted. Δ values refer to the difference in the parameter value between the given combination treatment group and IgG4 isotype control-treated CKD group in each experiment. Results are shown as means ± SEM, and n, numbers, are presented. Surface of plotted circles is proportional to the Δ value. Statistical significance was assessed using Fisher's exact test (C) and with a 2-sided Student t test with Benjamini-Hochberg correction for multiple testing. **P < .01. Colored circles indicate statistical significant Δ values (P < .05); gray circles indicate insignificant Δ values. Comb, combination; ns, not significant.
KY1070 leads to an EPO-sparing effect. Female ACD rats were used to evaluate an EPO-sparing effect of KY1070 combination therapy (for further details on the study protocol, please see supplemental Figure 10A-C and supplemental “ACD rats EPO-sparing protocol”). (A) The weekly number of Darbepoetin alfa (EPO) applications needed for anemia correction in ACD rats with an EPO monotherapy (left panel; n = 10) and KY1070 + EPO combination (right panel; n = 11) is shown. (B) Percent of applied Darbepoetin alfa doses in total (week 1 to 4) and consequent reduction of EPO doses needed (in %) is indicated. (C) Number of possible vs indeed consumed EPO doses in total (week 1 to 4) is shown. (D) Differences (Δ) in hemoglobin (ΔHgb) for CKD mice treated with indicated combinations of KY1070 and Darbopoietin alfa. Pooled data from 5 separate experiments are plotted. Δ values refer to the difference in the parameter value between the given combination treatment group and IgG4 isotype control-treated CKD group in each experiment. Results are shown as means ± SEM, and n, numbers, are presented. Surface of plotted circles is proportional to the Δ value. Statistical significance was assessed using Fisher's exact test (C) and with a 2-sided Student t test with Benjamini-Hochberg correction for multiple testing. **P < .01. Colored circles indicate statistical significant Δ values (P < .05); gray circles indicate insignificant Δ values. Comb, combination; ns, not significant.
KY1070 increases the sensitivity to EPO in the murine CKD-associated anemia model
Next, different concentrations of KY1070 (0.1 to 3 mg/kg) and EPO (0.01 to 10 µg/kg) were combined to treat the murine CKD-associated anemia. Hematological responses were evaluated and compared with Isotype-treated animals. In this setting, 1 µg/kg EPO, combined with any tested KY1070 concentration, proved to be effective in rising Hgb values, whereas 1 µg/kg EPO as a monotherapy had no effect on Hgb levels. In other words, addition of KY1070 made an ineffective EPO dose effective. Strikingly, a further 10 times lower EPO dose (0.1 µg/kg) combined with 1 mg/kg KY1070 was sufficient to significantly augment Hgb levels (Figure 4D).
Based on clinical practice, where physicians evaluate the efficacy of anemia treatment by an increase in Hgb levels, we fed the data presented in Figure 4D to a first-order linear model to quantify therapeutic efficacy of each drug alone and their interaction on Hgb response (supplemental Figure 12). Thereby, effects of each individual drug and their interaction were highly significant, suggesting superadditive effects of the combination treatment. Applying the modeling results, we then calculated the amounts of EPO and KY1070 required to increase Hgb by 2 g/dL. To achieve such a therapeutic effect, high EPO (4.4 µg/kg) and high KY1070 doses (7 mg/kg) as a monotherapy would be needed (supplemental Figure 12B). Combined treatment, however, can effectively lower the required EPO dose. For example, inclusion of 3 mg/kg KY1070 in the treatment protocol allows reduction of EPO by 73% down to 1.2 µg/kg.
KY1070 treatment increases FPN expression among erythroid progenitor cells and effectively reverses high intracellular iron levels
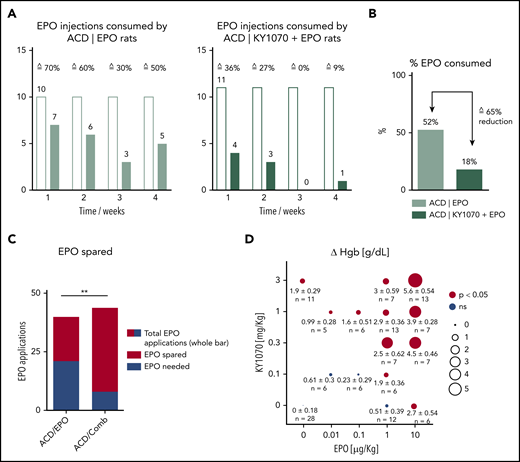
Recent studies indicate that intracellular iron levels, which are influenced by FPN expression on erythroid progenitor cells, are a critical regulator of oxidative stress during erythroid development.25 In addition, these cells actively contribute to systemic iron homeostasis and are decisive for anemia development.24,25 Since we identified an improved erythroid output together with higher Tf-Sat and suppressed hepcidin levels in our rodent models of ACD receiving combination therapy, we wanted to test the hypothesis that KY1070 upregulates FPN levels on erythroid progenitor cells allowing iron to be exported. To this end, we triggered high hepcidin levels via daily iron applications in C57BL/6N mice and concomitantly treated them with Isotype, EPO, KY1070, or a combination of the 2 latter (supplemental Figure 13A). The combination of iron and EPO mimics the standard approach in CKD anemia management. KY1070 treatment, irrespective of EPO therapy, lowered hepcidin levels comparable to those of PBS-treated control mice, an effect that could not be achieved by EPO monotherapy (Figure 5A). Accordingly, western blot analysis of FPN on magnetic-activated cell sorting–separated bone marrow erythroid progenitor cells revealed the lowest FPN protein levels after EPO treatment, higher levels in KY1070 treated mice, and normalized levels in double-treated animals (Figure 5B; supplemental 13B). Consistent with the differences seen in FPN protein levels, intracellular iron levels (quantitated by a Calcein-AM–based flow cytometric approach60 ) were highest in iron plus EPO-treated mice at all erythroid developmental stages (Figure 5C). In contrast, high-intracellular iron levels among erythroid progenitor cells can effectively be reduced by KY1070-mediated hepcidin suppression.
KY1070 in combination with EPO increases FPN expression on erythroid progenitor cells in the bone marrow and reduces high intracellular free iron levels. (A) Liver Hamp mRNA levels in iron-treated mice receiving either IgG4 isotype control (3 mg/kg n = 7), KY1070 (3 mg/kg; n = 8), EPO (10 µg/kg; n = 6), or both (n = 6) twice. (B) Densitometric quantification of FPN protein expression of erythroid precursor cells in the bone marrow. (C) Calcein fluorescence in erythroid bone marrow progenitors was measured by flow cytometry. Graphs show MFI (mean fluorescence intensity) values of individual erythroid precursor populations (basophilic cells [I], polychromatic cells [II], and orthochromatic cells and reticulocytes [III]; for gating strategy; see supplemental Figure 13C). Two-way ANOVA with Tukey corrected post hoc Student t test for multiple comparisons between all groups was applied. Results are shown as means ± SEM. Significant levels are indicated for the iron + Isotype–treated control group against all treatment groups and iron + EPO vs iron + KY1070 + EPO–treated rodents. *P < .05; **P < .01; ***P < .001.
KY1070 in combination with EPO increases FPN expression on erythroid progenitor cells in the bone marrow and reduces high intracellular free iron levels. (A) Liver Hamp mRNA levels in iron-treated mice receiving either IgG4 isotype control (3 mg/kg n = 7), KY1070 (3 mg/kg; n = 8), EPO (10 µg/kg; n = 6), or both (n = 6) twice. (B) Densitometric quantification of FPN protein expression of erythroid precursor cells in the bone marrow. (C) Calcein fluorescence in erythroid bone marrow progenitors was measured by flow cytometry. Graphs show MFI (mean fluorescence intensity) values of individual erythroid precursor populations (basophilic cells [I], polychromatic cells [II], and orthochromatic cells and reticulocytes [III]; for gating strategy; see supplemental Figure 13C). Two-way ANOVA with Tukey corrected post hoc Student t test for multiple comparisons between all groups was applied. Results are shown as means ± SEM. Significant levels are indicated for the iron + Isotype–treated control group against all treatment groups and iron + EPO vs iron + KY1070 + EPO–treated rodents. *P < .05; **P < .01; ***P < .001.
Consistent with these findings, we found the lowest bone marrow iron content and significantly increased Fpn protein levels in the bone marrows and RBCs of CKD mice receiving the combination therapy (supplemental Figure 14A-E). Moreover, western blot analysis of sorted erythroid progenitor cell populations (population III and IV; supplemental Figure 13C) also revealed higher FPN protein levels in the respective population (supplemental Figure 14F). Taken together, these findings unravel a new mechanistic insight for effective erythroid output in the context of ACD (see visual abstract).
Discussion
Despite treatment of the underlying disease, anemia management remains a major challenge in many chronic diseases.14 EPO and IV iron replacement therapy are integral parts of ACD therapy at present.16,61,62 However, both were associated with possible negative side effects, leading to the development of alternative anemia treatment approaches such as hypoxia-inducible factor prolyl hydroxylase inhibitors (HIF-PHIs) and anti–hepcidin compounds.50,63-67 Herein, we systematically evaluated the therapeutic efficacy of an anti-BMP6 antibody, which has been developed to counteract BMP6-induced hepcidin expression for the treatment of ACD.
In line with previous studies, employing alternative anti–hepcidin treatments or hepcidin knockout mice, we could show that decreasing hepcidin levels via an anti-BMP6 antibody could improve anemia caused by inflammation to some extent, as seen with EPO treatment.49,66,68,69 Importantly, we found that the combination of both EPO and KY1070 led not only to improvement of multiple RBC parameters (Hgb, MCV, MCH, and RBC) in a superadditive manner but also to increased Tf-Sat compared with EPO monotherapy. Although we could not find any interplay of KY1070 therapy with ERFE in our models, we could trace this effect back to augmented FPN levels among erythroid precursor cells due to reduced hepcidin levels. In light of published literature and based on our own results, we assume that high FPN expression on erythroid cells prevents accumulation of potentially toxic labile iron and catalysis of reactive oxygen species ultimately leading to insufficient erythroid output.24,25 Consequently, exported iron from erythroblasts is no longer detrimental for developing erythroid cells but available for erythropoiesis. Thus, besides EPO’s proliferative effect, KY1070-mediated hepcidin suppression leading to improved iron mobilization is needed, and either therapy alone is not as effective as if combined (see visual abstract). Although ROS formation leads to oxidative stress and apoptosis, which represents a well-established pathomechanism in β-thalassemia, such a phenomenon is yet to be described in ACD.70-72 Therefore, combining KY1070 with other anemia treatment strategies can deliver an enhanced response in ACD patients, by reverting putative negative effects of iron-mediated oxidative stress within erythroid progenitors.
Furthermore, we could provide compelling evidence that application of KY1070 resulted in a significant reduction of EPO levels required to achieve the desired hematological response. Thus, hypoproliferative anemia could effectively be reverted via ensuring a sufficient iron supply via repression of hepcidin activity. This is of clinical importance, as EPO hyporesponsiveness resulting in subsequent use of high EPO doses in patients with CKD was associated with an adverse clinical outcome, even though the underlying mechanisms explaining higher morbidity and mortality rates remain elusive thus far.31,41,42,73 However, published data suggest that the required dose of EPO and not Hgb per se or the iron doses were pivotal in determining outcomes.74,75
Thus, our alternative approach aiming to lower the EPO requirement may eventually circumvent these adverse effects. Indeed, application of KY1070 increases EPO sensitivity during inflammation in murine CKD. This is reflected by a reduction of EPO dosing to 10% of the amounts required for EPO monotherapy to achieve anemia correction when combined with KY1070. This effect is indicative that EPO hyporesponsiveness could be overcome, and thus, the use of high recombinant EPO doses could be avoided in this murine CKD anemia model.
Such a dose reduction has been proposed to be one of the benefits of the before-mentioned HIF-PHIs. Application of these drugs has been shown to result in lower circulating EPO levels rather than the high-peak concentrations seen after recombinant EPO treatment.76 In addition, HIF-PHIs are reported to increase, at least partially, iron availability, resulting in a significant reduction in iron supplementation needs.76,77 However, this effect cannot be ascribed to a direct inhibition of hepcidin, as is the case for KY1070.78 Of importance, HIF-PHIs have potential adverse effects, including higher VEGF expression and the potential induction of pulmonary artery hypertension or tumor growth.63
Some clinical trial data suggest that physicians nowadays, by using higher IV iron doses, may trade off lower EPO doses against the potential toxic iron overload, causing parenchymal iron toxicity over time.32 Several clinical studies point to an increased risk of infection and reduced overall survival in iron-treated patients who exceed certain ferritin cutoff levels; however, this was not seen in other studies.79-82
In summary, we could show the potential of KY1070 to reduce EPO need in animal models of ACD without the need of additional iron administration, making it an attractive opportunity in the treatment of patients avoiding the undesired and potentially life-debilitating effects of high dosages of EPO and/or IV iron. Mechanistically, combination therapy increases erythroid cell proliferation and RBC hemoglobinization and also improves the maturation of RBCs by reducing free cytotoxic iron in developing erythroid cells.
For original data, please email the corresponding author.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the doctoral college project W1253 HOROS (V.P., S.D., C.V., G.W.), the Austrian Research Funds (FWF) Project P 28302 (I.T.), the “Verein zur Förderung von Forschung und Weiterbildung in Infektiologie und Immunologie, Innsbruck” (G.W.), the Christian Doppler Society, Austria (L.V.d.S., A.H., G.W.), Krebshilfe Tirol project 15024 (P.T.), and a research grant of the Austrian Society of Hematology and Medical Oncology (OeGHO) (V.P.).
Authorship
Contribution: V.P., P.T., V.G., and I.T. conceived the project, designed and performed experiments, analyzed and interpreted data, and wrote the manuscript; M.A., M.S., N.B., L.V.d.S., R.H., P.G., E.D., S.D., C.V., S.B., F.B., A.H., L.v.R., and C.P.-O. performed experiments in mice and rats and analyzed experiments; J.C., J.P., C.D., L.B., and M.S.W. have been involved in the generation of KY1070 and in vitro testing of the antibody; S.A. and A.B. provided the BMP6 knockout mice and gave input for the respective experiments; S.S. performed sorting experiments; L.K. and P.P. provided the recombinant ERFE and gave input on experiments performed in connection therewith; and M.N., D.W., G.W., and V.G. provided intellectual input and edited the manuscript.
Conflict-of-interest disclosure: J.C., J.P., C.D., L.B., M.S.W., and V.G. are employees of Kymab Ltd. I.T. and G.W. received project funding and consultancy fees from Kymab Ltd. The remaining authors declare no competing financial interests.
Correspondence: Igor Theurl, Department of Internal Medicine II, Medical University of Innsbruck, Anichstr 35, A-6020 Innsbruck, Austria; e-mail: igor.theurl@i-med.ac.at.

![Specificity and activity of KY1070. (A) Cross-reactivity of KY1070 against human, rat, and mouse BMP6 was tested using a HepG2 Hamp luciferase reporter gene cell line. After assessing the assay window using a range of concentrations, a fixed concentration of recombinant human, rat, or mouse BMP6 of 10 nM was used. KY1070 inhibition curves were generated by titration starting at a final concentration of 600 nM. A human IgG4 isotype control was included in each assay. (B) KY1070 specificity was tested against human BMP5 and BMP7. HepG2 Hamp luciferase reporter gene cells were stimulated with a fixed concentration of recombinant human BMP5 or BMP7 of 10 nM. Inhibition curves for KY1070 were generated by titration starting at a final concentration of 600 nM. A titration of commercially available murine anti-BMP5 and anti-BMP7 antibodies as well as a human IgG4 isotype control was included. (C) BMPR1A/ BMPR2 dimerization assay performed in transfected U2OS cells. Dimerization was induced using human BMP6 (Peprotech 120-06) used at a fixed final concentration of 200 ng/mL. KY1070 inhibition curves were generated by titration starting at a final concentration of 600 nM. A human IgG4 isotype control was included. (D) Liver Hamp mRNA levels and plasma hepcidin levels of Bmp6 wild-type and Bmp6−/− mice determined 3 days after a single injection of IgG4 isotype antibody (3 mg/kg; n = 4 [Bmp6 Wt]; n = 8 [Bmp6−/−]) or KY1070 (3 mg/kg; n = 5 [Bmp6 wild type]; n = 9 [Bmp6−/−]). Unpaired Student t test was applied for comparison between genotypes for panel D. Data are presented as means ± SEM. **P < .01. RLU, relative light units.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/136/9/10.1182_blood.2019004653/2/m_bloodbld2019004653f1.png?Expires=1770184605&Signature=yuRrrKAksWwkg0ima4O4h-LELvS4iB-GiLEigPa~vB6gAMyIet4eXUddTwPQh17JlyLg~rYl39F40JzNNECnezTib-4feqhJBhL32NiPY1bJMem86ZCCL~3P2KzNnyMGjdemCnJmiExKpkbNa181AKXBFciycI170nGt1Ed1YtzG43mz7Mf4jgi2~NezIQqKNQpPDPbcr9JwleqEJ3jovjfL2Gt9GFIdck1TxtXrc5qltfVtBMtgFUci-xyAXHdWCxUmUXcG5yfprgpTwNLBxnwfge6RgkLqnbwjVcGrUgMOtRLG9VZv4LjO8qy1rmm0LAYwcQzpBHGey26fzvWvug__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![KY1070 mobilizes iron for erythropoiesis in 2 rodent models of chronic anemias and has a synergistic effect on anemia resolution when combined with Darbepoetin alfa. (A-C) ACD in female Lewis rats was induced by intraperitoneal administration of group A streptococcal peptidoglycan-polysaccharide. ACD rats were treated with IgG4 isotype control (ACD; n = 8), KY1070 (3 mg/kg; n = 9), EPO (10 µg/kg; n = 8), or both (n = 7). Hgb, RBC, MCV, MCH (A), plasma hepcidin levels (B), plasma iron levels and Tf-Sat (C) were determined in ACD rats at the end of treatment (week 4; see also supplemental Figure 4A). (D-G) CKD in male C57BL/6N mice was induced by a diet containing 0.2% adenine. CKD mice were either treated with IgG4 isotype control (CKD; n = 5), KY1070 (3 mg/kg; n = 6), Darbepoetin alfa (10 µg/kg; n = 6), or both (n = 6). (D) Hematological parameters (Hgb, RBC, MCH, and MCV) were determined in CKD mice at the end of treatment (week 4; see also supplemental Figure 7A). Plasma EPO levels (E), plasma iron levels, Tf-Sat (F), liver Hamp mRNA and plasma hepcidin levels (G) of indicated mice following treatment (week 4; see also supplemental Figure 7A). A naive control group was included in all experiments (n = 5 [rats], n = 7 [mice]). Two-way ANOVA with Tukey corrected post hoc Student t test for multiple comparisons between ACD and CKD animals was applied. Results are shown as means ± SEM. *P < .05; **P < .01; ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/136/9/10.1182_blood.2019004653/2/m_bloodbld2019004653f3.png?Expires=1770184605&Signature=TBIFXHI9OL88fbaLTdRJ-0TNHOWzk5PTsn2ECV0zGPIXq9HC0DXIRs180Yg9f0WzPcRDOM7eKstlCiILXaVRmnsLZoldHuANDJ~nBPl3b1cafsUbB0V9ecdh1O0TUvyrAip9q~doAfxNb2rJQ6f50ZUqJEoqMP6hDg~tzW7j6RZitt2dB6rbkS2IPWIW-xbRQbcCQ46ZWSEdJaK0S7LKTGhKS8IfPpNShu-lYUaQOm~ycArsvMs66Wz7zwKVUfWLuHZ036sAAzdU~PChBiqdbADzZ0cwSv-pdzcusCjqjSI8HRoZSv5pO1tDSGItJhskwcVoKa3aNdvBIfSq9IoWcw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![KY1070 in combination with EPO increases FPN expression on erythroid progenitor cells in the bone marrow and reduces high intracellular free iron levels. (A) Liver Hamp mRNA levels in iron-treated mice receiving either IgG4 isotype control (3 mg/kg n = 7), KY1070 (3 mg/kg; n = 8), EPO (10 µg/kg; n = 6), or both (n = 6) twice. (B) Densitometric quantification of FPN protein expression of erythroid precursor cells in the bone marrow. (C) Calcein fluorescence in erythroid bone marrow progenitors was measured by flow cytometry. Graphs show MFI (mean fluorescence intensity) values of individual erythroid precursor populations (basophilic cells [I], polychromatic cells [II], and orthochromatic cells and reticulocytes [III]; for gating strategy; see supplemental Figure 13C). Two-way ANOVA with Tukey corrected post hoc Student t test for multiple comparisons between all groups was applied. Results are shown as means ± SEM. Significant levels are indicated for the iron + Isotype–treated control group against all treatment groups and iron + EPO vs iron + KY1070 + EPO–treated rodents. *P < .05; **P < .01; ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/136/9/10.1182_blood.2019004653/2/m_bloodbld2019004653f5.png?Expires=1770184605&Signature=EOu58t~BzOSSR~hHxGajuoH10PmF31itpb--fw0S0YXE-BW7mlu52a4mZaPvKQCNSQr6JxGaY3CUFHC5G0h5S9i-xu71-unxnf-F9u36LVx6AVPLE4Te2an4e5K-gfnVYkef0uHfdNmKdkH1ScX-VxelVrqSvifnkzkf2yuOdD-VCZVt6~~AVP0YSn4jabsDdQcToY~snNlwnZpmssgU5AUlPcJ4ls06IgymBj8C7qV3lEKL~NUYhXQiNTTVBhoSMKcwpEXFwT7iDeggLPpt-BzugsGYt6eIu9~eruizgYMjZ8PAL8QdOp6FnDTV8OqCVYOmjvGkbY9uWKxLvS25qA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal