Key Points
Ibrutinib in the first-line setting is unlikely to be cost-effective for most patients with CLL, compared with its use in the third-line.
The monthly cost of ibrutinib would need to be decreased by at least 72% for first-line ibrutinib to be cost-effective.
Abstract
The ALLIANCE A041202 trial found that continuously administered ibrutinib in the first-line setting significantly prolonged progression-free survival compared with a fixed-duration treatment of rituximab and bendamustine in older adults with chronic lymphocytic leukemia (CLL). In this study, we created a Markov model to assess the cost-effectiveness of ibrutinib in the first-line setting, compared with a strategy of using ibrutinib in the third-line after failure of time-limited bendamustine and venetoclax-based regimens. We estimated transition probabilities from randomized trials using parametric survival modeling. Lifetime direct health care costs, quality-adjusted life years (QALYs), and incremental cost-effectiveness ratios (ICERs) were calculated from a US payer perspective. First-line ibrutinib was associated with an improvement of 0.26 QALYs and 0.40 life-years compared with using ibrutinib in the third-line setting. However, using ibrutinib in the first-line led to significantly higher health care costs (incremental cost of $612 700), resulting in an ICER of $2 350 041 per QALY. The monthly cost of ibrutinib would need to be decreased by 72% for first-line ibrutinib therapy to be cost-effective at a willingness-to-pay threshold of $150 000 per QALY. In a scenario analysis where ibrutinib was used in the second-line in the delayed ibrutinib arm, first-line ibrutinib had an incremental cost of $478 823, an incremental effectiveness of 0.05 QALYs, and an ICER of $9 810 360 per QALY when compared with second-line use. These data suggest that first-line ibrutinib for unselected older adults with CLL is unlikely to be cost-effective under current pricing. Delaying ibrutinib for most patients with CLL until later lines of therapy may be a reasonable strategy to limit health care costs without compromising clinical outcomes.
Introduction
Chronic lymphocytic leukemia (CLL) is the most common leukemia in adults, accounting for ∼30% of all leukemias in the United States.1 The median age of diagnosis is between 67 and 72 years,2 and the incidence of CLL is expected to increase given our aging population.3 Although CLL is generally incurable with standard therapies, many patients have been effectively managed with active surveillance punctuated by periods of fixed-duration chemoimmunotherapy, with historical CLL cohorts having a median overall survival of ∼10 years from time of diagnosis.4,5
Use of the once-daily, orally administered ibrutinib, an inhibitor of Bruton’s tyrosine kinase, has led to meaningful responses in CLL subgroups typically resistant to standard chemoimmunotherapy.6 Given the promising activity seen in high-risk CLL patients, ibrutinib has undergone testing in the first-line setting.7,8 A large phase III study (ALLIANCE A041202) randomized treatment-naïve patients 65 years or older to ibrutinib alone, ibrutinib in combination with rituximab, or standard chemoimmunotherapy with bendamustine plus rituximab (R-bendamustine).8 In this study, the ibrutinib-containing arms reduced the risk of disease progression by >60%, with 2-year progression-free survival (PFS) rates of 87%, 88%, and 74%, respectively.8 In contrast to the fixed duration of treatment in the R-bendamustine arm, patients in the ibrutinib arms received ibrutinib indefinitely until disease progression or intolerance, with ∼63% still receiving treatment at the time of data cutoff.8
Although ibrutinib used in the first-line setting reduces the risk of disease progression compared with fixed-duration chemoimmunotherapy, this continuous treatment comes at a significant cost. Priced at ∼$160 000 per year in the US,9 ibrutinib acquisition costs can be considerable for both patients and payers.10 Furthermore, recent studies have demonstrated that treatment with the oral Bcl-2 inhibitor venetoclax can lead to deep remissions in relapsed/refractory CLL with a finite treatment schedule rather than indefinite therapy.11 Compared with ibrutinib, fixed-duration regimens such as R-bendamustine and venetoclax plus rituximab (R-venetoclax) may be less costly because of the limited time on treatment, particularly when considering total health care costs over years of CLL management. Therefore, we hypothesized that ibrutinib as first-line therapy in older adults with CLL would not be a cost-effective strategy when compared with reserving its use until third-line therapy, after the failure of contemporary fixed-duration regimens of R-bendamustine and R-venetoclax.
Methods
Patients and intervention
We developed a cost-effectiveness model to compare the strategy of using ibrutinib in the first-line setting to reserving ibrutinib for the third-line setting after failure of 2 fixed-duration regimens: R-bendamustine followed by R-venetoclax. Patients entering our model mirrored the cohort of individuals in the phase III ALLIANCE trial comparing first-line ibrutinib therapy to R-bendamustine.8 This patient cohort had a median age of 71 years, 67% were male, 61% had an unmutated immunoglobulin heavy chain variable region (IGHV) gene, and 6% of all patients had a 17p deletion.8
Model construction
Our analysis was based on a memoryless Markov model (Figure 1). Readers seeking additional background on Markov modeling and its use in health economic analyses are referred to prior literature.12,13 Individuals entered the model requiring first-line therapy for CLL and received either ibrutinib or R-bendamustine. Individuals who relapsed in the ibrutinib arm received R-venetoclax, rituximab plus idelalisib (R-idelalisib), and ofatumumab as second-line, third-line, and fourth-line therapy, respectively. Individuals who relapsed in the R-bendamustine arm received R-venetoclax, ibrutinib, and R-idelalisib as second-line, third-line, and fourth-line therapy, respectively. Dosing for each line of treatment was based on the respective clinical trial.8,11,14,15 Patients were allowed to enter a best supportive care health state after relapsing from third or subsequent lines of therapy, with transition probabilities derived from prior studies.16
Diagram of Markov models. (A) Markov model for individuals who receive first-line ibrutinib therapy. (B) Markov model for individuals who receive delayed ibrutinib after failure of fixed-duration treatment.
Diagram of Markov models. (A) Markov model for individuals who receive first-line ibrutinib therapy. (B) Markov model for individuals who receive delayed ibrutinib after failure of fixed-duration treatment.
We used a 3-month Markov cycle and a lifetime horizon to estimate the costs and utilities associated with each CLL treatment strategy. The outputs of the model were used to calculate an incremental cost-effectiveness ratio (ICER) for each treatment strategy, which reflects the cost in 2019 US dollars for each additional quality-adjusted life year (QALY) gained because of treatment. Our analysis was performed from a US payer perspective, with both costs and utilities discounted at a rate of 3% annually.17 We used a willingness-to-pay threshold of $150 000 per QALY gained.18 The Markov model was constructed using TreeAge Pro (TreeAge Software, Williamstown, MA), and additional statistical analyses were performed using R (www.R-project.org) and STATA (StataCorp, College Station, TX).
Transition probabilities
Base-case estimates for transition probabilities are provided in Table 1. Progression rates for each line of therapy were derived from the respective clinical trial using standard extrapolation techniques.19 Briefly, individual patient-level data were recreated from Kaplan-Meier curves and at-risk tables of each trial.20 We then fit individual patient-level data with standard parametric models (exponential, Weibull, and Gompertz), and the parametric distribution that exhibited the best fit by the Akaike information criterion and Bayesian information criterion was selected for inclusion in the Markov model19 (supplemental Figures 1-6, available on the Blood Web site).
Model clinical parameters
| Result or transition . | Estimate . | Range . | References . |
|---|---|---|---|
| PFS for ibrutinib first-line therapy | Exponential: λ = 0.005904 | — | 8 |
| PFS for R-bendamustine, entire cohort | Gompertz: λ = 0.0089865, γ = 0.0257203 | — | 8 |
| PFS for R-bendamustine, IGHV mutated only | Gompertz: λ = 0.0038591, γ = 0.0315173 | — | 8 |
| PFS for R-bendamustine, IGHV unmutated only | Gompertz: λ = 0.0107668, γ = 0.0293043 | — | 8 |
| PFS for R-venetoclax | Gompertz: λ = 0.0044257, γ = 0.0399164 | — | 11 |
| PFS for ibrutinib third-line therapy | Exponential: λ = 0.015356 | — | 46 |
| PFS for R-idelalisib | Weibull: λ = 0.0050368, κ = 1.794209 | — | 15 |
| PFS for ofatumumab | Gompertz: λ = 0.0231548, γ = 0.3048745 | — | 14 |
| Time from progression to start of next therapy, mo | 10.3 | 9-12 | 21 |
| Probability of treatment discontinuation, ibrutinib first-line, yearly, % | 23 | ||
| Year 0-1 | 6.725 | 5.38-8.07 | |
| Year 1-2 | 5.798 | 4.64-6.96 | |
| Year 2-3 | 5.388 | 4.31-6.47 | |
| Year 3+ | 0 | ||
| Probability of treatment discontinuation, ibrutinib third-line, yearly, % | 23 | ||
| Year 0-1 | 6.728 | 5.38-8.07 | |
| Year 1-2 | 2.714 | 2.17-3.25 | |
| Year 2-3 | 1.999 | 1.60-2.40 | |
| Year 3+ | 2.599 | 2.07-3.12 | |
| Time from discontinuation because of toxicity to start of next therapy, ibrutinib, mo | 6.5 | 6-9 | 24 |
| Probability of treatment discontinuation, R-bendamustine, 6 cycles, % | 13.3 | 10.6-15.9 | 22 |
| Probability of treatment mortality because of R-bendamustine, 6 cycles, % | 1 | 0.8-2.0 | 8,22 |
| Probability of receiving pegfilgrastim during R-bendamustine, cycles 2-6, % | 14.3 | 11.4-17.2 | 35,36 |
| Probability of receiving best supportive care after progression from third-line treatment, % | 11.6 | 9.28-13.92 | 16 |
| Probability of background death | — | — | 26 |
| Probability of death from best supportive care state, yearly, % | 55 | 44-66 | 25 |
| Probability of receiving IV rituximab rather than SQ, % | 80 | 64-96 | Expert opinion |
| Discount rate | 0.03 | 0.015-0.06 | 17,54 |
| Median starting age of cohort, y | 71 | 65-77 | 8 |
| Result or transition . | Estimate . | Range . | References . |
|---|---|---|---|
| PFS for ibrutinib first-line therapy | Exponential: λ = 0.005904 | — | 8 |
| PFS for R-bendamustine, entire cohort | Gompertz: λ = 0.0089865, γ = 0.0257203 | — | 8 |
| PFS for R-bendamustine, IGHV mutated only | Gompertz: λ = 0.0038591, γ = 0.0315173 | — | 8 |
| PFS for R-bendamustine, IGHV unmutated only | Gompertz: λ = 0.0107668, γ = 0.0293043 | — | 8 |
| PFS for R-venetoclax | Gompertz: λ = 0.0044257, γ = 0.0399164 | — | 11 |
| PFS for ibrutinib third-line therapy | Exponential: λ = 0.015356 | — | 46 |
| PFS for R-idelalisib | Weibull: λ = 0.0050368, κ = 1.794209 | — | 15 |
| PFS for ofatumumab | Gompertz: λ = 0.0231548, γ = 0.3048745 | — | 14 |
| Time from progression to start of next therapy, mo | 10.3 | 9-12 | 21 |
| Probability of treatment discontinuation, ibrutinib first-line, yearly, % | 23 | ||
| Year 0-1 | 6.725 | 5.38-8.07 | |
| Year 1-2 | 5.798 | 4.64-6.96 | |
| Year 2-3 | 5.388 | 4.31-6.47 | |
| Year 3+ | 0 | ||
| Probability of treatment discontinuation, ibrutinib third-line, yearly, % | 23 | ||
| Year 0-1 | 6.728 | 5.38-8.07 | |
| Year 1-2 | 2.714 | 2.17-3.25 | |
| Year 2-3 | 1.999 | 1.60-2.40 | |
| Year 3+ | 2.599 | 2.07-3.12 | |
| Time from discontinuation because of toxicity to start of next therapy, ibrutinib, mo | 6.5 | 6-9 | 24 |
| Probability of treatment discontinuation, R-bendamustine, 6 cycles, % | 13.3 | 10.6-15.9 | 22 |
| Probability of treatment mortality because of R-bendamustine, 6 cycles, % | 1 | 0.8-2.0 | 8,22 |
| Probability of receiving pegfilgrastim during R-bendamustine, cycles 2-6, % | 14.3 | 11.4-17.2 | 35,36 |
| Probability of receiving best supportive care after progression from third-line treatment, % | 11.6 | 9.28-13.92 | 16 |
| Probability of background death | — | — | 26 |
| Probability of death from best supportive care state, yearly, % | 55 | 44-66 | 25 |
| Probability of receiving IV rituximab rather than SQ, % | 80 | 64-96 | Expert opinion |
| Discount rate | 0.03 | 0.015-0.06 | 17,54 |
| Median starting age of cohort, y | 71 | 65-77 | 8 |
—, not applicable.
Recognizing that a CLL progression event as reported on a clinical trial may not be a criterion to begin next line of therapy (ie, discrepancy between PFS and time-to-next treatment), our base-case model used 10.3 months as the average time from progression event to next line of CLL therapy.21 The exception were patients who experienced early disease progression during treatment with R-bendamustine (ie, within 6 months of treatment initiation) or R-venetoclax (ie, within 24 months of treatment initiation); these individuals immediately began next-line therapy. Furthermore, individuals who progressed after third-line or fourth-line treatment were modeled to immediately begin the subsequent line of therapy.
We also incorporated in the model the discontinuation of ibrutinib and R-bendamustine because of adverse events (AEs), with transition probabilities obtained from existing literature.22,23 Individuals who discontinued ibrutinib because of AEs began the next line of therapy after 6.5 months, informed by data from Hampel et al.24 Last, transition probabilities for death during each line of treatment were derived from US Life Tables, and the probability of death from the best supportive care state was estimated based on mortality data for relapsed/refractory CLL patients.25,26
Costs
Costs incorporated in the model are outlined in Table 2. The costs of IV or subcutaneous (SQ) medications, including bendamustine, rituximab, and ofatumumab, were obtained from the July 2019 Center for Medicare Services (CMS) average sales price files.27 We assumed a total body surface area of 1.7 m2, and accounted for drug wastage by rounding up to the next full single-use vial size available for each dose administered.28 Administration costs for chemotherapy infusions were based on the 2019 CMS Physician Fee Schedule.29 The length of infusion for each drug was determined based on the FDA prescribing information datasheets. In the base-case model, 80% of individuals were modeled to receive IV rituximab and 20% received the SQ form.
Model costs
| Costs . | Baseline (US$) . | Range (US$) . | Study or reference . |
|---|---|---|---|
| Bendamustine/treanda, 1 mg | 28.88 | — | J9033 |
| Rituximab IV, 10 mg | 94.97 | — | J9312 |
| Rituximab SQ, 10 mg | 44.32 | — | J9311 |
| Ofatumumab, 10 mg | 59.80 | — | J9302 |
| Pegfilgrastim, 6 mg | 4 528.31 | — | J2505 |
| Ibrutinib, 420 mg, monthly | 12 489.59 | — | CMS plan finder tool |
| Venetoclax, 400 mg, monthly | 11 482.76 | — | CMS plan finder tool |
| Idelalisib, 300 mg, monthly | 10 277.70 | — | CMS plan finder tool |
| Routine office visit | 112.80 | 105.32-152.91 | CPT 99215 |
| Chemotherapy IV infusion, first hour | 143.08 | 124.35-188.20 | CPT 96413 |
| Chemotherapy IV infusion, additional hour | 30.99 | 27.49-39.41 | CPT 96415 |
| Chemotherapy IV infusion, additional sequence | 69.20 | 60.46-90.25 | CPT 96417 |
| Preinfusion medication | 12.30 | — | 16 |
| Chemotherapy SQ injection | 80.73 | 70.32-105.51 | CPT 96401 |
| CBC with differential | 8.63 | — | CPT 85025 |
| Comprehensive metabolic panel | 11.74 | — | CPT 80053 |
| Best supportive care, monthly | 196.50 | 189.02-236.61 | 16 |
| End-of-life care | 83 053.18 | 56 467.50-214 892.37 | 37,38,39 |
| Costs . | Baseline (US$) . | Range (US$) . | Study or reference . |
|---|---|---|---|
| Bendamustine/treanda, 1 mg | 28.88 | — | J9033 |
| Rituximab IV, 10 mg | 94.97 | — | J9312 |
| Rituximab SQ, 10 mg | 44.32 | — | J9311 |
| Ofatumumab, 10 mg | 59.80 | — | J9302 |
| Pegfilgrastim, 6 mg | 4 528.31 | — | J2505 |
| Ibrutinib, 420 mg, monthly | 12 489.59 | — | CMS plan finder tool |
| Venetoclax, 400 mg, monthly | 11 482.76 | — | CMS plan finder tool |
| Idelalisib, 300 mg, monthly | 10 277.70 | — | CMS plan finder tool |
| Routine office visit | 112.80 | 105.32-152.91 | CPT 99215 |
| Chemotherapy IV infusion, first hour | 143.08 | 124.35-188.20 | CPT 96413 |
| Chemotherapy IV infusion, additional hour | 30.99 | 27.49-39.41 | CPT 96415 |
| Chemotherapy IV infusion, additional sequence | 69.20 | 60.46-90.25 | CPT 96417 |
| Preinfusion medication | 12.30 | — | 16 |
| Chemotherapy SQ injection | 80.73 | 70.32-105.51 | CPT 96401 |
| CBC with differential | 8.63 | — | CPT 85025 |
| Comprehensive metabolic panel | 11.74 | — | CPT 80053 |
| Best supportive care, monthly | 196.50 | 189.02-236.61 | 16 |
| End-of-life care | 83 053.18 | 56 467.50-214 892.37 | 37,38,39 |
—, not applicable.
Costs of oral medications, including ibrutinib, venetoclax, and idelalisib, were obtained from Medicare’s publicly available plan finder tool.30 Since our model perspective was from the US payer, and recent studies suggest industry-supported patient-assistance programs cover a majority of patient cost-sharing for high-cost oral cancer therapies,31,32 we did not include patient out-of-pocket costs in our oral treatment calculations. Rather, the costs of oral medications in our model reflect the amount covered by part D prescription plans and the amount reimbursable by Medicare when filling these oral medications.
During treatment with ibrutinib, idelalisib, or ofatumumab, individuals were assumed to receive routine follow-up monthly for the first 6 months of treatment, followed by every 3 months thereafter. During treatment with R-venetoclax, individuals were assumed to receive follow-up 3 times weekly during dose ramp-up, followed by monthly follow-up thereafter. Last, during treatment with R-bendamustine, individuals were assumed to receive follow-up twice monthly. The costs of follow-up included the cost of an office visit and routine laboratory tests, which were derived from the 2019 CMS Physician Fee Schedule and 2019 Q3 Medicare Clinical Laboratory Fee Schedule, respectively.29,33 The costs of grade 3 or 4 AEs were also incorporated in the model. Each severe AE was assumed to result in an inpatient admission, and costs were derived from 2019 Medicare diagnosis-related-group–based payments16 ,34 (supplemental Table 1). The model also included the cost of pegfilgrastim support during treatment with R-bendamustine, with the frequency and duration of treatment based on published reports.35,36 The cost of the best supportive care health state and end-of-life care was based on prior work.16,37-39 All costs were converted to 2019 US dollars using the medical care component of the Consumer Price Index.40
Utilities
Utility scores, which range from 0 (dead) to 1 (full health), reflect the value of the quality of life in a particular health state.41 Data on patient survival can be weighted based on utility estimates to produce QALYs, a health outcomes measure that combines information on morbidity and mortality into a single index.42 Our utility values were based on Kosmas et al,43 a study deriving CLL-specific health state utilities for the UK population. Based on this work, PFS states offered the greatest utility during earlier lines of therapy in our model (Table 3). For example, ibrutinib without progression in the first-line compared with the third-line setting was associated with utility of 0.71 and 0.55, respectively. In addition to these baseline utilities, we also adjusted for severe AEs for each line of treatment. Similar to a previous study,16 the monthly probability and duration of grade 3+ AEs was estimated from each respective randomized trial and the disutility of the AE from published literature (supplemental Tables 1 and 2).16,44
Model utilities
| Utilities . | Health states with assigned utility . | QALY . | Range . | Reference . |
|---|---|---|---|---|
| PFS, oral treatment | Ibrutinib 1L R-venetoclax | 0.71 | 0.67-0.75 | 43 |
| PFS, IV treatment | R-bendamustine | 0.67 | 0.63-0.71 | 43 |
| PFS, no treatment (after first-line) | After completion of R-bendamustine or discontinuation of ibrutinib 1L or R-bendamustine because of AE | 0.82 | 0.78-0.85 | 43 |
| PFS, no treatment (after second-line or later) | After completion of R-venetoclax or discontinuation of ibrutinib 3L because of AE | 0.71 | 0.66-0.75 | 43 |
| Progression after first-line therapy | After progression from R-bendamustine, before starting second-line therapy | 0.66 | 0.62-0.71 | 43 |
| Progression after second-line therapy | After progression from R-venetoclax, before starting third-line therapy | 0.59 | 0.55-0.64 | 43 |
| PFS, third-line therapy | Ibrutinib 3L R-idelalisib (first-line ibrutinib arm) | 0.55 | 0.50-0.60 | 43 |
| PFS, fourth-line therapy | R-idelalisib (delayed ibrutinib arm) Ofatumumab | 0.42 | 0.37-0.47 | 43 |
| Relapsed lines of treatment | Best supportive care | 0.42 | 0.37-0.47 | 43 |
| Utilities . | Health states with assigned utility . | QALY . | Range . | Reference . |
|---|---|---|---|---|
| PFS, oral treatment | Ibrutinib 1L R-venetoclax | 0.71 | 0.67-0.75 | 43 |
| PFS, IV treatment | R-bendamustine | 0.67 | 0.63-0.71 | 43 |
| PFS, no treatment (after first-line) | After completion of R-bendamustine or discontinuation of ibrutinib 1L or R-bendamustine because of AE | 0.82 | 0.78-0.85 | 43 |
| PFS, no treatment (after second-line or later) | After completion of R-venetoclax or discontinuation of ibrutinib 3L because of AE | 0.71 | 0.66-0.75 | 43 |
| Progression after first-line therapy | After progression from R-bendamustine, before starting second-line therapy | 0.66 | 0.62-0.71 | 43 |
| Progression after second-line therapy | After progression from R-venetoclax, before starting third-line therapy | 0.59 | 0.55-0.64 | 43 |
| PFS, third-line therapy | Ibrutinib 3L R-idelalisib (first-line ibrutinib arm) | 0.55 | 0.50-0.60 | 43 |
| PFS, fourth-line therapy | R-idelalisib (delayed ibrutinib arm) Ofatumumab | 0.42 | 0.37-0.47 | 43 |
| Relapsed lines of treatment | Best supportive care | 0.42 | 0.37-0.47 | 43 |
1L, first-line; 3L, third line.
Sensitivity analysis
We performed sensitivity analyses to evaluate uncertainty in our model. During 1-way sensitivity analyses, individual parameters were varied across a range to determine the impact on the ICER. These ranges are detailed in Tables 1,2-3. Utilities were varied across their 95% confidence intervals.43 Other transition probabilities were varied within a 20% range. During probabilistic sensitivity analysis (PSA), we performed 10 000 Monte Carlo simulations, each time randomly sampling from the distribution of model inputs. Costs were described by γ distributions, and probabilities and utilities were represented by β distributions.
To assess the robustness of our model conclusions, we also performed several scenario analyses. In the first, the prices of oral CLL therapies were decreased following patent expiration. Ibrutinib was modeled to go off-patent in June 2031, idelalisib in March 2030, and venetoclax in May 2030. The treatment start date was modeled as October 2019. Although there is considerable uncertainty about generic pricing of small molecular cancer therapies, we considered an off-patent price of 16% of current Medicare Part D pricing, similar to the discount currently observed for generic imatinib.9 In the second scenario analysis, the sequence of therapy in the delayed ibrutinib arm was switched such that ibrutinib was used in the second-line setting, followed by R-venetoclax in the third-line setting. This sequence more closely aligned with the ALLIANCE trial, which allowed crossover of patients from R-bendamustine to ibrutinib.8
In the third and fourth scenario analyses, we considered the cost-effectiveness of first-line vs delayed ibrutinib in patients exclusively with mutated IGHV and unmutated IGHV, respectively. Since patients with unmutated IGHV have significantly inferior PFS when treated with chemoimmunotherapy,45 these scenarios allowed us to determine if the cost-effectiveness of first-line ibrutinib is markedly affected by IGHV mutation status. In these scenarios, progression rates for patients receiving R-bendamustine were based on published PFS curves from the ALLIANCE trial that were stratified by IGHV mutation status8 (supplemental Figures 5 and 6). However, because patients with unmutated IGHV and mutated IGHV have similar rates of disease progression when treated with ibrutinib in clinical trials,45,46 transition probabilities for patients on first-line ibrutinib remained identical to those used in our base-case analysis.
Results
Base-case analysis
Use of first-line ibrutinib was associated with an improvement of 0.26 QALYs compared with the strategy of delaying ibrutinib to the third-line setting (6.85 vs 6.59 QALYs, respectively). However, first-line ibrutinib was associated with significantly greater health care costs ($1 367 275 vs $754 575, respectively), with an incremental cost of $612 700 (Table 4). The ICER of first-line ibrutinib therapy was $2 350 041 per QALY compared with using ibrutinib in the third-line setting after bendamustine and venetoclax-based fixed-duration regimens.
Base-case cost-effectiveness analysis
| Baseline model . | PSA model . | |||||
|---|---|---|---|---|---|---|
| Strategy . | Costs (US$) . | Incremental costs (US$) . | Effectiveness (QALY) . | Incremental effectiveness (QALY) . | ICER ($ per QALY) . | ICER 95% CI ($ per QALY) . |
| Ibrutinib first-line | $1 367 275 | $612 700 | 6.85 | 0.26 | $2 350 041 | $939 719-dominated |
| Delayed ibrutinib | $754 575 | — | 6.59 | — | — | — |
| Baseline model . | PSA model . | |||||
|---|---|---|---|---|---|---|
| Strategy . | Costs (US$) . | Incremental costs (US$) . | Effectiveness (QALY) . | Incremental effectiveness (QALY) . | ICER ($ per QALY) . | ICER 95% CI ($ per QALY) . |
| Ibrutinib first-line | $1 367 275 | $612 700 | 6.85 | 0.26 | $2 350 041 | $939 719-dominated |
| Delayed ibrutinib | $754 575 | — | 6.59 | — | — | — |
—, not applicable.
Sensitivity analyses
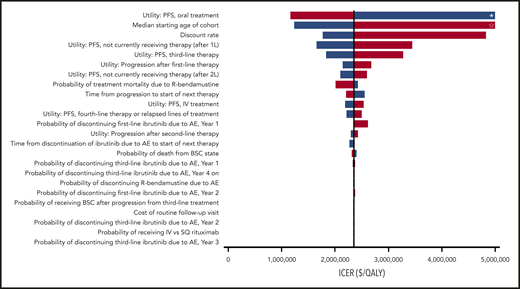
Our model was most sensitive to changes in the utility while taking orally administered CLL treatment in the first- or second-line setting; increasing the utility to 0.75 resulted in an ICER of $1 160 608 per QALY, whereas decreasing the utility to 0.67 caused the first-line ibrutinib treatment strategy to be dominated (Figure 2). Other parameters with significant impact on model results were the starting age of the cohort, discount rate, and the utility of progression-free state without active CLL treatment. However, all ICERs during 1-way sensitivity analyses remained above the willingness-to-pay threshold of $150 000 per QALY. Threshold analysis showed that the monthly cost of ibrutinib would need to be decreased by ∼72% to $3535 for first-line ibrutinib therapy to be cost-effective. During PSA, 100% of iterations produced ICERs greater than the willingness-to-pay threshold of $150 000 per QALY (Figure 3).
One-way sensitivity analysis. All model parameters were varied across the ranges indicated in Tables 1-3 to determine changes in the ICER of first-line ibrutinib. Only model parameters that produced a >$5000 per QALY change when evaluated across their entire range are included in the tornado diagram. *, Dominated. ☆, ICER exceeds $5 million per QALY. Blue bars represent the lower value in the range; red bars represent the higher value. 1L, first-line; 2L, second-line; BSC, best supportive care.
One-way sensitivity analysis. All model parameters were varied across the ranges indicated in Tables 1-3 to determine changes in the ICER of first-line ibrutinib. Only model parameters that produced a >$5000 per QALY change when evaluated across their entire range are included in the tornado diagram. *, Dominated. ☆, ICER exceeds $5 million per QALY. Blue bars represent the lower value in the range; red bars represent the higher value. 1L, first-line; 2L, second-line; BSC, best supportive care.
PSA. Results of the probabilistic sensitivity analyses are based on 10 000 iterations of the Markov model.
PSA. Results of the probabilistic sensitivity analyses are based on 10 000 iterations of the Markov model.
In our first scenario analysis, the price of oral CLL therapies were reduced to 16% of the on-patent price after patent expiration. This adjustment modestly reduced the ICER to $2 281 430 per QALY. In our second scenario analysis, ibrutinib was used in second-line therapy rather than third-line therapy in the delayed ibrutinib arm. Here, use of ibrutinib in the first-line setting was associated with an incremental cost of $478 823 and an incremental effectiveness of 0.05 QALYs, leading to an ICER of $9 810 360 per QALY. When the strategy of using ibrutinib in the second-line setting was compared with the third-line setting, the incremental cost was $133 878, with an incremental effectiveness of 0.21 QALYs, producing an ICER of $631 764 per QALY.
In the third and fourth scenario analyses, we determined whether the cost-effectiveness of first-line ibrutinib was significantly impacted by IGHV mutation status. When only considering patients with unmutated IGHV, first-line ibrutinib was associated with an incremental effectiveness of 0.43 QALYs and an incremental cost of $584 695, resulting in an ICER of $1 373 500 per QALY. In contrast, when only considering patients with mutated IGHV, the first-line ibrutinib strategy was dominated, with an incremental effectiveness of −0.12 QALYs and an incremental cost of $678 286. This was primarily because of differences in utility scores for first-line therapy, because our model ascribed a utility of 0.71 for continuous first-line ibrutinib and 0.82 during the treatment-free interval in those achieving remission after R-bendamustine.
Modeled clinical outcomes
In addition to estimating the total utility and costs for each CLL treatment strategy, we also used our base-case model to estimate long-term clinical outcomes for patients. Nonfuture-discounted overall survival favored first-line ibrutinib by an average of 0.40 years (12.31 years for first-line ibrutinib vs 11.91 years for the delayed ibrutinib arm; supplemental Figure 7). However, individuals who received first-line ibrutinib were on active CLL treatment for a longer total duration than those in the delayed ibrutinib arm, with average durations of 10.42 years and 5.34 years, respectively. The average duration of ibrutinib was 8.69 years when used as first-line treatment. This was longer than the average duration of third-line ibrutinib treatment, which was 3.85 years for those that reached this line of therapy before death.
Discussion
Although a recent randomized phase III trial found ibrutinib-based regimens to improve PFS compared with R-bendamustine,8 ibrutinib requires continuous therapy, and was not found to impact overall survival at a median follow up of 38 months. By incorporating findings from this and other contemporary CLL clinical trials, we developed a Markov model to estimate the cost-effectiveness of first-line ibrutinib. Under current US drug pricing where ibrutinib costs >$12 000 per month, first-line ibrutinib was not cost-effective when compared with a strategy of using ibrutinib in the third-line setting after failure of fixed-duration regimens, with an ICER of $2 350 041 per QALY. Our findings support a reduction in the price of ibrutinib used in the first-line setting to better align its cost to its clinical utility when compared with contemporary fixed-duration regimens.
Our study has important strengths. First, our model was based on results from a large, randomized trial directly comparing ibrutinib with R-bendamustine in the first-line setting. Second, our analysis included contemporary data to reflect recent advances in the treatment and outcomes of individuals with CLL, including the use of R-venetoclax in the relapsed/refractory setting.11 Third, our model adjusted for drug wastage by calculating drug costs based on single-use vials. This practice has been infrequently used in prior cost-effectiveness analyses, yet has the potential to significantly affect results.28 Fourth, we included AEs in the model, including discontinuation of first-line therapy because of AEs as well as disutility and costs associated with drug toxicity.
Our model was developed to be conservative, because we selected inputs that favored first-line ibrutinib when multiple reasonable options were available. For example, we elected to use results from the MURANO trial to inform outcomes for R-venetoclax for both the R-bendamustine and ibrutinib arms. However, B-cell receptor inhibitors were used infrequently by patients before they entered the MURANO study (only 2.6% of patients in the R-venetoclax arm), and available data suggest patients progressing on ibrutinib may have inferior outcomes compared with patients progressing after chemoimmunotherapy.47,48 Because of the uncertainty around outcomes in patients progressing after first-line ibrutinib, we conservatively assumed our arms would have similar post-progression outcomes. We also attributed higher health state utilities during earlier lines of therapy; therefore, ibrutinib used in the first-line setting had a baseline utility nearly 30% higher than when used in the third-line setting. Our model may also underestimate the toxicity of ibrutinib, because a number of real-world studies have reported significantly higher rates of AEs and treatment discontinuation compared with clinical trial data.49,50 Last, the ALLIANCE study that informed our model included a heterogenous group of CLL patients, including subtypes of CLL with inferior outcomes with chemoimmunotherapy compared with ibrutinib (ie, deletion 17p, deletion 11q, and IGHV unmutated). Thus, our base-case ICER of $2 350 041 per QALY is likely to represent a conservative estimate for first-line ibrutinib when considering patients with mutated IGHV and low-risk cytogenetics. This is supported by our scenario analysis which considered only IGHV mutated patients, in which the first-line ibrutinib treatment strategy had lower QALYs despite greater costs than the delayed ibrutinib strategy.
To our knowledge, there is only 1 other published study examining the cost-effectiveness of ibrutinib in the first-line setting in the United States.16 Barnes et al16 used a semi-Markov model to estimate the cost-effectiveness of ibrutinib compared with a theoretical treatment alternative with the effectiveness of chlorambucil alone but the costs and AEs of chlorambucil plus obinutuzumab. This study also found first-line ibrutinib was not cost-effective, but had a lower ICER ($189 000 per QALY) compared with our study. However, there are important differences between our present study and the report by Barnes et al. 16 First, the previous study was unable to compare ibrutinib directly to a standard of care because of the lack of available randomized control data, and instead compared ibrutinib to chlorambucil alone, which is currently a category 3 National Comprehensive Cancer Network recommendation for first-line treatment of CLL.51 In contrast, our model uses data from a phase III trial to directly compare ibrutinib to R-bendamustine, which is an accepted first-line chemoimmunotherapy regimen for CLL. Second, our study models second-line treatment with R-venetoclax, using randomized control data from the MURANO trial.11 Because R-venetoclax has been shown to result in significantly higher rates of PFS compared with chemoimmunotherapy in the relapsed setting, the inclusion of this treatment option reflects the most up-to-date advances in CLL therapy and increases the external validity of our cost-effectiveness analysis.
Although our study has notable strengths, there are limitations to consider. First, although most of our model is populated using data from large, randomized trials, there is uncertainty regarding the long-term outcomes of novel agents beyond the trial period. In our model, we used parametric survival modeling to extrapolate post-trial transition probabilities and identify distributions with the best fit. Second, we recognize that the treatment landscape is rapidly evolving in CLL, and there is compelling new data regarding the use of fixed-duration venetoclax-based combinations as first-line therapy in CLL52,53 which are absent from our model. However, direct comparison trials between ibrutinib and venetoclax in the first-line setting are not available, and we chose to avoid using indirect comparisons across currently available first-line trials. Third, although we varied the median starting age of the cohort and probability of treatment mortality during sensitivity analyses, our model does not directly assess the impact of comorbidities or frailty on the cost-effectiveness of first-line ibrutinib. Fourth, the ALLIANCE trial randomized patients with high-risk features, such as unmutated IGHV, to R-bendamustine despite data suggesting that these patients have a poorer response to chemoimmunotherapy.45,52 As a result, our model may not reflect the optimal treatment strategy in terms of improving PFS for this high-risk cohort. However, we did perform a scenario analysis considering exclusively IGHV unmutated patients and found that first-line ibrutinib therapy is still unlikely to be cost-effective compared with delaying ibrutinib until third-line therapy, with an ICER of $1 373 500 per QALY. Given the availability of fixed-duration first-line regimens with greater efficacy in this high-risk patient population, such as venetoclax and obinutuzumab,52 future studies will be helpful in elucidating the most cost-effective sequence of contemporary CLL therapies. Last, although we were able to assess the impact of IGHV mutation status on our model conclusions, we were not able to isolate other high-risk subgroups such as del17p, because the trial that informed our model had few such patients.8 However, the inclusion of a small number of del17p patients, who have improved clinical outcomes with ibrutinib, allows for a more conservative estimate of our base-case model comparing ibrutinib to R-bendamustine in the first-line setting.
Although a recent randomized study found that first-line ibrutinib reduced the risk of progression in older patients with CLL, drug acquisition costs for this continuous therapy are ∼$160 000 per year. With median survivals of ≥10 years for historical CLL cohorts managed in the chemotherapy era,4,5 the bar is relatively high for novel CLL therapies to improve long-term survival in contemporary CLL cohorts. Despite the clear improvement in PFS related to first-line ibrutinib compared with standard chemoimmunotherapy, our study suggests first-line ibrutinib is unlikely to be cost-effective for most older adults when compared with the strategy of delaying ibrutinib until third-line therapy following failure of contemporary fixed-duration regimens. Combined with available clinical trial data showing similar survival between patients randomized to ibrutinib or chemoimmunotherapy with crossover to ibrutinib at progression,8,46 our model provides evidence that delaying ibrutinib until later lines of therapy may be a reasonable strategy to limit health care costs without dramatically compromising patient outcomes, particularly in patients lacking risk factors for early chemoimmunotherapy failure. Alternatively, for ibrutinib to be used in the first-line setting for all older adults with CLL, our model predicts that considerable price reduction (72+%) would be required to produce more widely acceptable ICERs. Given the potential economic burden of CLL in the era of ibrutinib and other targeted therapies,10 these results emphasize the importance of incorporating cost-effectiveness into treatment recommendations and assessments of clinical value.
For original data, please contact the corresponding author, Scott Huntington (scott.huntington@yale.edu).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported by the American Society of Hematology Physician-Scientist Career Development Award (K.K.P.).
Authorship
Contribution: K.K.P. and S.F.H. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis; K.K.P. and S.F.H. are responsible for the concept and design of the study, acquiring, analyzing, and interpreting the data, and drafting the manuscript; K.K.P. performed statistical analysis and obtained funding; S.F.H. supervised the work; and all authors critically revised the manuscript for important intellectual content.
Conflict-of-interest disclosure: I.I. was a consultant for Celgene, Astra Zeneca, Novartis, and Kite. A.J.D. received research funding from Celgene and was a consultant for Amgen. C.P.G. received research funding from the National Comprehensive Cancer Network, Pfizer, J&J, and Flatiron Inc. S.F.H. was a consultant for Celgene, Bayer, Genentech, Pharmacyclics, and AbbVie, and received research funding from DTRM Biopharm, Celgene, and TG Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Scott F. Huntington, Department of Hematology/Oncology, Yale University School of Medicine, 333 Cedar St, PO Box 208028, New Haven, CT 06520; e-mail: scott.huntington@yale.edu.