In this issue of Blood, Patel and colleagues have calculated that the price of ibrutinib should be reduced by 72% to be cost-effective as first-line therapy for patients with chronic lymphocytic leukemia (CLL).1
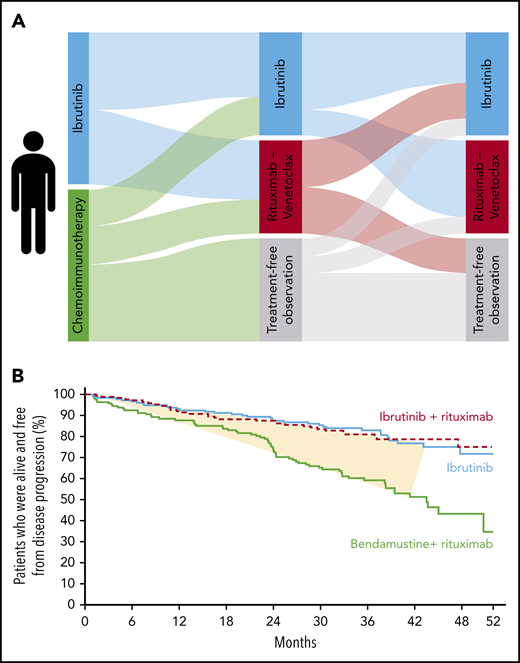
Treatment trajectories and outcome for patients with CLL. (A) The possible treatment paths for a patient with CLL meeting the criteria for treatment. (B) An example Kaplan-Meier curve for progression-free survival from the study forming the basis for cost-effectiveness analyses by Patel et al; the fraction of patients truly benefitting from targeted therapy is shaded in yellow.
Treatment trajectories and outcome for patients with CLL. (A) The possible treatment paths for a patient with CLL meeting the criteria for treatment. (B) An example Kaplan-Meier curve for progression-free survival from the study forming the basis for cost-effectiveness analyses by Patel et al; the fraction of patients truly benefitting from targeted therapy is shaded in yellow.
Targeted therapy has become a mainstay of CLL treatment. An abundance of treatment options, including combination approaches, are being tested in clinical trials. Thus, an evaluation of the cost-effectiveness of the different sequences of currently approved therapies is warranted. The cost of an extra quality-adjusted life-year was modeled based on published data from the phase 3 ALLIANCE study.2 Although superiority, in terms of progression-free survival, was demonstrated for ibrutinib-based therapy vs bendamustine plus rituximab, the cost of 1 additional quality-adjusted life-year was calculated to be $2 350 041. When restricting the use of ibrutinib as first-line therapy to patients with more aggressive disease in terms of IGHV-unmutated status, the price tag was $1 373 500 per quality-adjusted life-year.
Results from 4 pivotal clinical trials in CLL, comparing targeted therapy vs chemoimmunotherapy in the front-line setting, were published in 2019. The iLLUMINATE trial3 and the CLL14 trial4 compared ibrutinib plus obinutuzumab and venetoclax plus obinutuzumab, respectively, vs chlorambucil plus obinutuzumab for patients with significant comorbidities. The ALLIANCE study2 compared ibrutinib, with or without rituximab, vs bendamustine plus rituximab for patients older than 65 years of age, whereas the E1912 study5 compared ibrutinib plus rituximab vs fludarabine, cyclophosphamide, and rituximab for patients younger than 70 years of age. All 4 trials met their primary outcome and demonstrated superiority in terms of longer progression-free survival for targeted therapy of CLL in the front-line setting. Thus, the decision on front-line treatment in CLL might be considered apparent and easy when discussing your patient’s treatment path among the options illustrated (see figure): targeted therapy rather than chemoimmunotherapy. This is what most current clinical guidelines recommend.
However, the answer may not be that straightforward. As clearly demonstrated by Patel and colleagues, the cost for a gained quality-adjusted life-year may be higher than what is acceptable for our society. As they put it, “The monthly cost of ibrutinib would need to be decreased by at least 72% for first-line ibrutinib to be cost-effective.” Medical ethical standards differ between countries; however, in most countries, those standards do not permit the socioeconomic standing of the patient to impact (ie, limit) equal access to treatment. Ensuring the fair distribution of health resources is part of the responsibility of medical providers. This pinpoints the importance of developing new structures for price setting of pharmacological treatment, in general, and antineoplastic treatment, in particular. The lack of correlation between monthly treatment costs and clinical benefit for approved antineoplastic treatment emphasizes the need for a new price structure.6 Thus, health care payers, whether privately or publicly based, and pharmaceutical companies should join forces to address this issue.
A second issue concerning cost-effectiveness is indirectly addressed by Patel and colleagues: only a minority of patients actually benefit from targeted therapy. The patients in the yellow-shaded area between the 2 graphs are the ones experiencing an improved outcome upon targeted therapy vs chemoimmunotherapy (see figure). The patients below the yellow-shaded part did well independent of treatment path, whereas the patients above the yellow-shaded part did not benefit from either treatment, because they progressed within the first 3 years. Thus, we need personalized treatment. Essentially, we should give the right treatment to the right patient at the right time. The price tag per quality-adjusted life-year could be lowered to $1 373 500 by restricting targeted front-line therapy to patients with more aggressive CLL in terms of IGHV-unmutated status. Although still far away from the willingness-to-pay limit of $150 000 per quality-adjusted life-year, this would be a first step toward personalized treatment for CLL in the front-line setting. More than half of patients with CLL and IGHV-mutated status experience long-lasting remissions and, perhaps, even a cure.7 This is reflected by a negative quality-adjusted life-year gain upon ibrutinib vs chemoimmunotherapy for this patient group.
To smartly use targeted therapy approaches in CLL, we should combine molecular and genetic omics data with data assembled in electronic health care records. These so-called “big data” could improve the identification of patients at the highest chance of benefitting from a specific treatment approach. The impact of recurrent mutations in CLL has been detailed since the publishing of 2 landmark papers on the genetic landscape of CLL in 2015.8,9 The utilization of data from electronic health care records has recently been applied to identify newly diagnosed patients with CLL who are at high risk for infection or early need of treatment by an ensemble machine learning approach.10 By combining such approaches, we can improve cost-effectiveness and treatment decisions, paving the path toward truly personalized treatment.
Patel and colleagues have informed the discussion of cost-effectiveness for targeted therapy of CLL. In summary, they demonstrate that the price setting for ibrutinib should be lowered by 72% to reach the willingness-to-pay threshold of $150 000 per quality-adjusted life-year.
Furthermore, their analyses emphasize the need for personalized treatment, because patients with IGHV-mutated CLL demonstrated a loss of quality-adjusted life-years upon treatment with ibrutinib vs chemoimmunotherapy in the front-line setting. Thus, stringent cost-effectiveness analyses based on published clinical trial data with subgroup analyses defined by omics and “big data” should be encouraged by health authorities and the scientific community.
Conflict-of-interest disclosure: C.U.N. has received research grants/funding from AbbVie, AstraZeneca, Janssen, the Danish Cancer Society, and the Novo Nordisk Foundation and has received consultancy fees and/or travel grants from Janssen, AbbVie, Novartis, Roche, Sunesis, Gilead Sciences, AstraZeneca, and CSL Behring.