In this issue of Blood, Vinanica et al explore a novel strategy to enhance the efficacy of adoptive T-cell therapy through the expression of an erythropoietin (Epo) receptor capable of delivering a signal that boosts the survival and function of chimeric antigen receptor (CAR) T cells.1
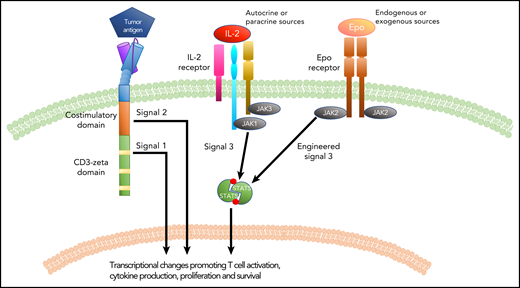
Signal transduction in a CAR T cell. Similar to IL-2 signaling, the introduction of an Epo receptor to a CAR T cell provides signal 3, which synergizes with signals 1 and 2 generated by the CAR molecule to promote CAR T-cell function and efficacy.
Signal transduction in a CAR T cell. Similar to IL-2 signaling, the introduction of an Epo receptor to a CAR T cell provides signal 3, which synergizes with signals 1 and 2 generated by the CAR molecule to promote CAR T-cell function and efficacy.
Over the last 10 years, great strides have been made in the application of adoptive cellular therapy to treat malignant diseases. Through technological advances that enable the robust ex vivo expansion of T cells collected from peripheral blood as well as efficient redirection of T-cell specificity through CARs or engineered T-cell receptors (TCRs), it is now possible to manufacture and deliver sufficient numbers of leukemia-reactive T cells to treat, and even eradicate, chemotherapy refractory cancers. The most successful application of this strategy has been with CAR T cells targeting B-lineage antigens, which can induce complete remissions in 70% to 90% of patients with acute lymphoblastic leukemia (ALL) and 50% of patients with lymphoma.2 Despite these promising results, a significant number of ALL patients will relapse after CAR T-cell therapy, and approximately half of patients with lymphoma will not achieve a complete remission, which highlights the limitations of our current CAR T-cell therapies and emphasizes the necessity for improvement.3
The CAR is a synthetic receptor that allows T cells to be activated in response to an antigen on the surface of a leukemia or tumor cell. The earliest CARs incorporated a single signaling domain (derived from the ζ chain of the TCR complex) designed to replicate the “signal 1” a T cell receives upon TCR activation. Subsequently, signaling domains from costimulatory molecules were incorporated into CAR designs resulting in second-generation CARs, capable of initiating both "signal 1" and "signal 2" within a T cell, resulting in enhanced in vivo function and persistence of the T cells and improved efficacy in both preclinical and clinical settings.4,5 Nuances in the biology of T cells expressing CARs that contain either a CD28- or a 4-1BB–costimulatory domain provide clues into the impact of details in CAR structure on functionality.6 On the basis of such observations, there is enthusiasm for identifying structural modifications or additional receptors capable of generating a signal that can enhance CAR T-cell function and ultimately CAR T-cell efficacy in patients. The authors of the Vinanica et al study demonstrate that expression of an Epo receptor (EpoR) in T cells is capable of replicating "signal 3" (see figure). Introduction of either the full-length or a truncated form of EpoR (EpoRm, a naturally occurring form that drives erythrocytosis7 ) results in signaling through the Janus kinases (JAKs) and signal transducer and activator of transcription (STAT) signaling axis, including activation of STAT5, a driver of T-cell proliferation and survival in response to cytokines such as interleukin-2 (IL-2). The authors demonstrate that signaling through EpoRm enhanced in vitro T-cell expansion and persistence in the absence of exogenous IL-2, and they extend these observations to T cells coexpressing a CAR. EpoRm-expressing CAR T cells also demonstrated enhanced in vivo persistence in both the presence and absence of antigen and were more efficacious at clearing leukemia in xenograft models. Furthermore, the authors demonstrate that signaling through the EpoRm can be altered either positively through the addition of exogenous Epo or negatively through the use of JAK inhibitors, thus making this strategy for enhancing CAR T-cell function, expansion, and persistence titratable with agents that are readily available in the clinic.
This study has several implications. Persistence of CAR T cells has been associated with a decreased risk of antigen-positive relapse in ALL. Thus, by providing a means of enhancing CAR T-cell persistence in vivo, this model represents a strategy with the potential to reduce relapses after CAR T-cell therapy. Because of the effect of the EpoRm in the absence of antigen, this strategy may also improve CAR T-cell expansion and persistence in patients with limited leukemic burden (and thus, limited CAR antigen) at the time of infusion, an increasingly common scenario because CAR T cells are being used earlier in therapy. Although not evaluated in these studies, it would be interesting to establish whether EpoRm could rescue expansion in a patient with poor-quality T cells, which have been linked to CAR T-cell failures.8 It is becoming evident that despite the effectiveness of CAR T cells at targeting CD19, there are characteristics of CAR T-cell biology that must be addressed to improve this therapy.9 The Vinanica study illustrates a novel means of invoking a T-cell–relevant signaling pathway that synergizes with CAR signaling to enhance the in vivo biology of T cells. This observation opens the door for additional opportunities to think beyond the tools typically available for modifying the function of T cells that express synthetic receptors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal