In this issue of Blood, Dufva et al systematically address a key issue in the exploding field of chimeric antigen receptor (CAR) T cells: how to improve their activity.1
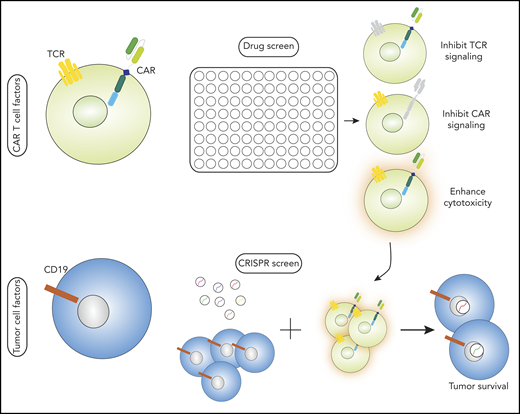
Complementary use of drug and small-molecule screen with CRISPR/Cas9 screen to identify factors that enhance the cytotoxicity of CAR T cells. This approach can provide new ways to identify mediators of CAR and tumor activity that are relevant across tumor types and CAR constructs for further investigation.
Complementary use of drug and small-molecule screen with CRISPR/Cas9 screen to identify factors that enhance the cytotoxicity of CAR T cells. This approach can provide new ways to identify mediators of CAR and tumor activity that are relevant across tumor types and CAR constructs for further investigation.
Although they have been used for less than a decade in the clinic, CAR T-cell therapies have revolutionized our approach to treating relapsed/refractory patients with B-cell malignancies. However, experience has shown us that despite high induction remission rates, approximately half the patients with B-cell acute lymphoblastic leukemia (B-ALL) who have been treated with CD19 or CD22 CAR T cells will eventually relapse,2,3 and treatment with CD19 CAR T cells will fail for approximately 60% of patients with B-cell lymphomas.4 It is widely accepted that improving efficacy of these therapies requires understanding CAR T cell–centric and tumor-centric mediators of CAR T-cell cytotoxicity.
In their article, Dufva et al used 2 complementary approaches to identify druggable pathways that enhance the cytotoxicity of CD19-directed CAR T cells. They evaluated factors related to CAR T cells themselves as well as factors related to tumor cells. By combining a drug and small-molecule screen with a genome-wide CRISPR-Cas9 loss-of-function screen, the authors identified pathways that influence the increased or attenuated CAR T-cell effect, established a robust resource of immunomodulatory properties of cancer drugs, and provided insight into genetic mediators of CAR T-cell cytotoxicity (see figure). Augmenting CAR T-cell efficacy by modulating tumor antigen expression has been shown to be effective in preclinical models of bryostatin-1 with a CD22 CAR T cell.5 In multiple myeloma, reports from both preclinical and clinical experiences suggest that combining a gamma secretase inhibitor with a B-cell maturation antigen (BCMA) to enhance the activity of CAR T cells seems promising6 (NCT035025777 ). So far, these reports have focused primarily on modulating the CAR T cell target on the tumor cells. In contrast, CAR T cell–specific immunomodulation is an open opportunity, particularly because of the complex differences in CAR T-cell activation compared with T-cell receptor (TCR) activation. Initial evaluations of immunomodulatory drug effects on cell-cell interactions8 is just beginning to be explored. The central question addressed in the article by Dufva et al is whether large-scale screens can be developed to identify potential targets for modulating CAR T-cell activity.
The authors used a drug and small-molecule screen to determine the effects of CD19-CD28z CAR T-cell cytotoxicity against B cells. To determine what targets were relevant to both CAR and TCR signaling vs targets modulating one or the other, they evaluated the top agents in a reporter T-cell line by discerning drugs that act by modulating TCR signaling vs CAR signaling. Interestingly, this approach provides a new avenue for understanding the biology of T-cell activation and may be of interest when modeling TCR vs CAR signaling in the future; the results could be translated to other CAR T-cell designs not evaluated here. Drugs identified in this screen as inhibitors of CAR T-cell activity, such as dasatinib, are currently under preclinical evaluation for controlling CAR T cell–mediated toxicities, which will validate the ability of this approach to identify biologically relevant drugs.9,10 The second mitochondrial-derived activator of caspases (SMAC) mimetic birinapant was identified as enhancing CAR T-cell cytotoxicity against primary B-ALL cells. It may be of interest to evaluate birinapant for potential clinical use in combination with CAR T cells. A clinical trial (NCT02587962) that uses birinapant combined with programmed cell death protein 1 (PD-1) blockade is currently underway and will provide a platform for understanding the immune effects of this drug. The authors note that the improvement in CAR T-cell activity in the setting of birinapant could be applied in other CAR T-cell therapies, but they also note that this will need further evaluation.
In a parallel approach to understanding the contributions of tumor-centric pathways to CAR T-cell cytotoxicity, the authors performed a genome-wide CRISPR screen on leukemia and lymphoma cell lines followed by exposure to CD19-CD28z CAR T cells with and without birinapant (the SMAC mimetic shown to enhance the T-cell response). This identified death receptor genes FADD and TNFRSF10B that, when disrupted in the tumor cells, resulted in decreased tumor sensitivity to CAR T-cell cytotoxicity. This suggests that despite approaches that amplify CAR T-cell cytotoxicity, tumor-intrinsic features can still evade their effect. Surprisingly, Dufva and colleagues did not find disruption of these genes to be uniform across different cancer cell lines, which suggests that heterogeneity among tumors could account for different mechanisms of resistance to CAR T-cell therapies.
Through their work, Dufva et al have established a robust model for identifying CAR T cell–centric and tumor cell–centric features that augment or suppress CAR T-cell cytotoxicity. These novel screening approaches provide tools for additional interrogation of the impact of drugs and genes in other CAR T cell constructs and across tumor types. Importantly, to attain an adequate scale of these screening modalities, they have been conducted in in vitro models and do not address in vivo effects of the drugs on CAR T-cell phenotype, persistence, and exhaustion. The authors note that this screening technique would be only the first step in identifying and validating potential drugs to combine with CAR T-cell therapies. As CAR T-cell therapy continues to evolve, researchers and clinicians will need a deeper understanding of CAR T-cell biology and rigorous analyses of potential combinatorial agents. Dufva and colleagues have provided a compelling platform for this understanding and a rich array of data to mine for such valuable information.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal