TO THE EDITOR:
Trials with CD19 or CD22 chimeric antigen receptor (CAR) T-cell therapy have shown 70% to 90% complete remission (CR) rate in patients with refractory or relapsed B acute lymphoblastic leukemia (r/r B-ALL).1-4 However, a large proportion of patients with CR relapsed within 1 year.5,6 It is critical to develop new strategies to improve the durability of remission after CAR T-cell therapy, especially when patients cannot be bridged to allogeneic hematopoietic cell transplantation (allo-HCT).2 Loss or mutation of CD19 was frequently observed and considered to be a major mechanism of relapse.1,4-6 Diminished CD22 site density has been observed to be associated with relapse after CD22 CAR T-cell therapy.3 Both CD19/CD22-bispecific CAR T cells and infusion of a cocktail of CD19 and CD22 CAR T-cells have been shown to prevent leukemia antigen loss.7-12 A phase 1 trial showed that 3 of 4 patients treated with a cocktail of CD19 and CD22 CAR T-cell infusion had a relapse within 1 year.9 Another early report showed that CD19/CD22-bispecific CAR T cells were well tolerated, and a study is ongoing to assess whether this strategy can improve long-term outcomes.8 In a recent study, combined CD19 and CD22 CAR T-cell therapy for r/r B-ALL resulted in a median progression-free survival of 12 months, but long-term outcome was not determined.10 The duration of CAR T-cell persistence in vivo is another important determinant of sustained remission, and relapse is also frequently associated with the loss of CAR T-cell surveillance.4,13 However, clinical strategies for improving CAR T-cell persistence in vivo have not been developed. Here, we hypothesized that sequential administration of a second CAR T product targeting a different antigen but before the possibility of relapse may extend CAR T-cell persistence to improve long-term outcomes, and we conducted a phase 1 trial of sequential CD19/CD22 CAR T-cell treatments in pediatric patients with r/r B-ALL. This trial has been registered at www.chictr.org.cn as ChiCTR-OIB-17013670. Details of the protocol are found in supplemental Methods, available on the Blood Web site.
Enrolled in this trial were 20 patients, aged 1 to 16 (median, 6) years, including 14 (70%) patients with hematologic relapse (HR) and 6 (30%) patients with refractory disease who were found positive by flow cytometry for measurable residual disease (FCM-MRD+) (patient characteristics are shown in supplemental Table 1). All patients received lymphodepleting chemotherapy before CD19 CAR T-cell infusion (cycle 1), and CD22 CAR T cells were subsequently infused when CD19 CAR T cells became undetectable by FCM in peripheral blood (PB; cycle 2); the median interval between the 2 cycles of infusion was 1.65 months (range, 1.1-5.2; Figure 1A-B). The median dose of CD19 CAR T-cells was 10 (3.3-42.8) × 105/kg, and the median dose of CD22 CAR T-cells was 10 (0.25-47.4) × 105/kg (supplemental Table 3). There was no significant difference between the 2 types of CAR T-cell doses (supplemental Figure 1).
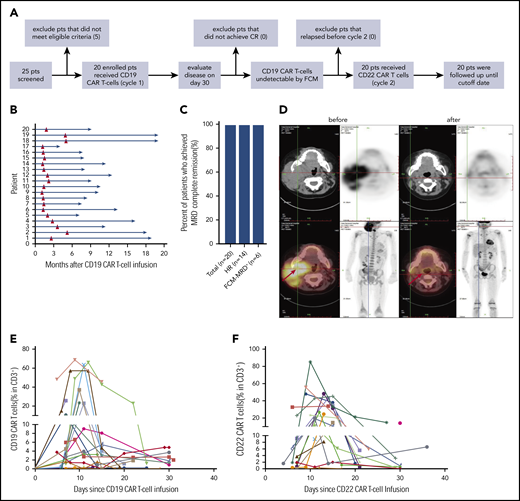
Response to CAR T-cell therapy. (A) The flowchart of the trial. (B) Time course of 2 cycles of CAR T-cell therapy in 20 individuals. (C) The rate of MRD− CR in all patients, those with hematological relapse, and those with refractory FCM-MRD+ disease. The total number of patients and those in the different groups are indicated in brackets. (D) Leukemia lesions in a patient before and after CAR-T-cell therapy, detected by whole-body position-emission tomography (PET)/computed tomography (CT). (E) CAR T-cell expansion in PB of 20 patients after CD19 (E) and CD22 (F) CAR T-cell infusion.
Response to CAR T-cell therapy. (A) The flowchart of the trial. (B) Time course of 2 cycles of CAR T-cell therapy in 20 individuals. (C) The rate of MRD− CR in all patients, those with hematological relapse, and those with refractory FCM-MRD+ disease. The total number of patients and those in the different groups are indicated in brackets. (D) Leukemia lesions in a patient before and after CAR-T-cell therapy, detected by whole-body position-emission tomography (PET)/computed tomography (CT). (E) CAR T-cell expansion in PB of 20 patients after CD19 (E) and CD22 (F) CAR T-cell infusion.
All 20 patients (100%) achieved CR and MRD− on day 30 after CD19 CAR T-cell infusion (Figure 1C-D). Before CD22 CAR T-cell infusion, all patients had remained in CR and MRD−. Efficient expansion of CD19 and CD22 CAR T-cells was detected in PB (Figure 1E-F; supplemental Figure 1). There was no correlation of CD19 and CD22 CAR T-cell expansion, viability, and transduction efficiency in the same patient (r = −0.029, r = 0.251, and r = −0.226). CD19 and CD22 CAR T cells were detectable in the cerebrospinal fluid of the patients who were specifically examined (supplemental Table 3). Serum cytokine markers, including TNF-α and interleukin-6 and -10, were elevated during the 2 cycles of infusion (supplemental Figure 2).
Details of adverse effects are shown in supplemental Table 2. The median onset of cytokine release syndrome (CRS) occurred on days 4 (range, 1-7) and 5 (range, 1-21) in cycles 1 and 2, respectively. CRS occurred in 18 of 20 patients (90%) in cycle 1 and was mild or moderate (grade 1-2) in 17 patients. Possibly due to the leukemia-free status, 4 of 20 patients had no CRS after CD22 CAR T-cell infusion, and mild or moderate (grades 1-2) CRS was observed in 15 of 20 patients. Grade 1 neurotoxicity occurred in 3 of 20 patients in both cycles 1 and 2. Only one patient developed grade 3 neurotoxicity (in cycle 1). These results indicate that sequential infusion of 2 CAR products is a safe strategy.
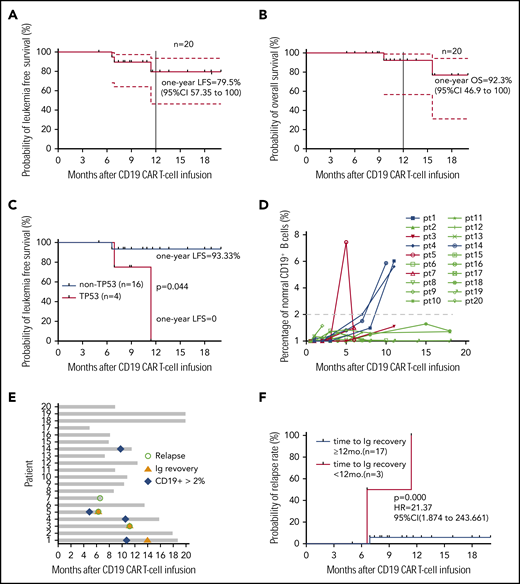
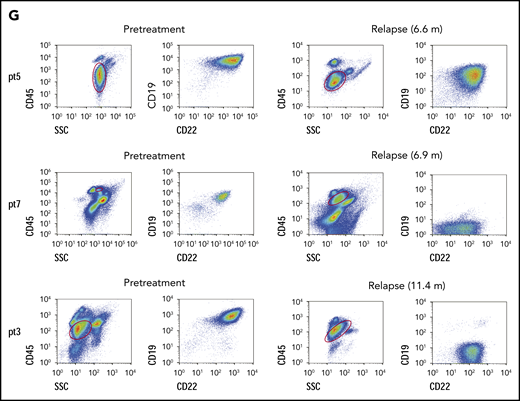
All 20 patients were followed up, and no allo-HCT consolidation was applied. At the study end point, the median overall survival (OS) and leukemia-free survival (LFS) were not reached. Seventeen patients remained in CR, and 3 had a relapse at 6.6, 6.9, and 11.4 months (median, 6.9 months) after CAR T-cell infusion, resulting in 1-year LFS and OS rates of 79.5% and 92.3%, respectively (Figure 2A-B). The patients who had the TP53 mutation had very poor LFS (Figure 2C), and they may not have benefited from this strategy (P = .044). Most patients had low percentages of normal CD19+ and CD22+ B cells (≤ 2%) in bone marrow (BM) within 1 year (Figure 2D; supplemental Figure 3). Three patients had immunoglobulin recovery, indicating the loss of CAR T-cell surveillance, and of them, 2 had a relapse (Figure 2E). Indeed, the patients who had early immunoglobulin recovery (<12 months) had a higher relapse rate (P = .000; hazards ratio = 21.37; 95% confidence interval, 1.874-243.661; Figure 2F). One patient with relapse exhibited CD22 downregulation on blasts, and CD19 antigen loss was observed in 2 with relapse (Figure 2G).
Long-term outcome. (A) LFS of all patients who received sequential CAR T-cell therapy. (B) Overall survival of patients who received sequential CAR T-cell therapy. (C) LFS comparison after CAR T-cell therapy between patients without or with the TP53 mutation. (D) Normal CD19+ B cells in every patient’s BM after sequential CD19 and CD22 therapy. The red lines indicate patients with relapse, and the last time point represents the diagnosis of relapse. (E) Recovery of immunoglobulin and normal B cells in BM among 20 patients, determined by immunoturbidimetry and FCM, respectively. (F) The probability of relapse rate associated with immunoglobulin recovery. (G) Leukemic CD19 and CD22 expression in the 3 patients with relapse, determined by FCM. In panels A and B, dashed lines indicate 95% confidence intervals. In all panels, the tick marks indicate the time of data censored at the last follow-up.
Long-term outcome. (A) LFS of all patients who received sequential CAR T-cell therapy. (B) Overall survival of patients who received sequential CAR T-cell therapy. (C) LFS comparison after CAR T-cell therapy between patients without or with the TP53 mutation. (D) Normal CD19+ B cells in every patient’s BM after sequential CD19 and CD22 therapy. The red lines indicate patients with relapse, and the last time point represents the diagnosis of relapse. (E) Recovery of immunoglobulin and normal B cells in BM among 20 patients, determined by immunoturbidimetry and FCM, respectively. (F) The probability of relapse rate associated with immunoglobulin recovery. (G) Leukemic CD19 and CD22 expression in the 3 patients with relapse, determined by FCM. In panels A and B, dashed lines indicate 95% confidence intervals. In all panels, the tick marks indicate the time of data censored at the last follow-up.
In our previous trials, without bridging to allo-HCT, 9 of 18 patients after CD19 CAR therapy had relapsed with a median time to relapse of 64 days,2 whereas 4 of 7 patients after CD22 CAR therapy had a median time to relapse of 3.4 months.13 In the current study, of 20 patients, 17 remained in remission at the cutoff date, resulting in an LFS rate of 79.5% at 12 and 18 months. Although the outcome of our cohort could not be directly compared with that reported by other groups, because of differences in patients, protocol, and CAR vector, the long-term efficacy of our sequential CAR strategy was encouraging, much better than single CD19 or CD22 CAR therapy in our hospital.2,13 Of the 20 treated patients, only 2 with relapse exhibited loss of CD19 on their blasts, suggesting that the risk of relapse associated with antigen escape was greatly reduced.
Normal B cells and serum immunoglobulin have been considered measures of CAR T-cell function.11,14-17 In our previous CD22 CAR therapy, patients in remission had no normal B cells, but normal B cells were detectable in 3 of the 4 patients with relapse.13 In the current study, 2 of the 3 patients with relapse displayed immunoglobulin recovery at 5 and 11.4 months, but only 1 of 17 patients who achieved CR exhibited immunoglobulin recovery at 1 year after infusion, suggesting that the improved outcome in our cohort may be partly attributable to prolonged CAR T-cell persistence with sequential strategy. Bridging to allo-HCT improved long-term outcomes after CD19 or CD22 CAR T-cell therapy in our group.2,13 However, for patients who lack suitable donors or are not willing to undergo allo-HCT, sequential CAR T-cell therapy can serve as a valuable treatment.
In summary, our study demonstrates that sequential infusion of CD19 and CD22 CAR T cells is effective and safe in treating r/r B-ALL patients and can improve the durability of remission in the long-term, possibly through preventing antigen escape and extending the time of CAR T-cell persistence. Future studies will determine the value of this strategy in different clinical settings and patient cohorts.
For original data, please contact the corresponding author Jing Pan.
The online version of this article contains a data supplement.
Acknowledgments
The authors thank Qiaoling Wen for data collection.
This work was supported by the National Key R&D Program for Precision Medicine (2019YFA0110204), the National Key Basic Research Program of China (No. 2016YFC1303403 and No. 2016YFC1303400), National Natural Science Foundation of China (81272325, 81670107, and 81421002) and Youth Project (31501082), the CAMS Innovation Fund for Medical Sciences (CIFMS, 2016-I2M-1-003), and the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (2019-RC-HL-013).
Authorship
Contribution: J.P., A.H.C., X.F., and C.T. contributed to the study design; J.P., Z.L., W.S., J.X., J.D., and Z.W. managed the clinical protocol; A.H.C. and B.D. produced the CAR T-cells; J.P., S.Z., X.X., Q.Z., X.Y., C.L., and X.F. contributed to data analysis and interpretation; J.P. and X.F. reviewed the data and wrote the manuscript; all authors take responsibility for the accuracy and completeness of the data; and all authors reviewed and approved the final manuscript.
Conflict-of-interest disclosure: A.H.C. is a founding member of Shanghai YaKe Biotechnology, Ltd. The remaining authors declare no competing financial interests.
Correspondence: Jing Pan, State Key Laboratory of Experimental Hematology, Beijing Boren Hospital, No. 6, South Zhengwangfen, Fengtai District, Beijing 100070, China; e-mail: panj@borenhospital.com; Alex H. Chang, Clinical Translational Research Center, Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai 200433, China; e-mail: alexhchang@yahoo.com; Xiaoming Feng, State Key Laboratory of Experimental Hematology, National Clinical Research Center for Blood Diseases, Institute of Hematology & Blood Diseases Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, 288 Nanjing Road, Tianjin 300020, China, and Central Laboratory, Fujian Medical University Union Hospital, Fuzhou 350001, China; e-mail: fengxiaoming@ihcams.ac.cn; and Chunrong Tong, Department of Hematology, Beijing Boren Hospital, No. 6, South Zhengwangfen, Fengtai District, Beijing 100070, China; e-mail: tongcr@borenhospital.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal