In this issue of Blood, Pan et al highlight their approach toward dual-antigen chimeric antigen receptor (CAR) T-cell targeting in an effort to reduce the risk of immune escape in pediatric B-cell acute lymphoblastic leukemia (B-ALL). They demonstrate the feasibility of sequentially administering CD19 CAR T cells followed by CD22 CAR T cells alongside the safety, toxicity, and efficacy of such a model.1 Their observation suggests that sequential CAR T-cell targeting strategies may be safe and effective and provides proof-of-concept of a potential strategy that may allow for dual-antigen CAR T-cell targeting.
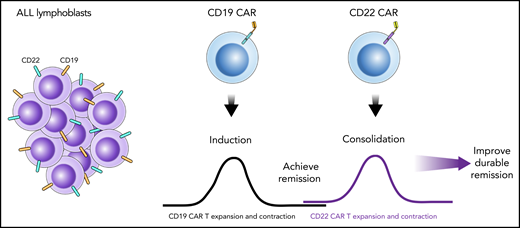
Schematic illustration of a sequential infusion strategy with CD19 CAR T-cell infusion followed by CD22 CAR T-cell infusion. In this model, the first CAR T-cell infusion provides a first attempt at disease-directed therapy, akin to ALL induction therapy. This is followed by a second CAR T-cell infusion that targets an alternative antigen, which provides consolidation-like therapy to further eradicate disease or help maintain remission. Collectively, the 2 sequential infusions help improve remission durability.
Schematic illustration of a sequential infusion strategy with CD19 CAR T-cell infusion followed by CD22 CAR T-cell infusion. In this model, the first CAR T-cell infusion provides a first attempt at disease-directed therapy, akin to ALL induction therapy. This is followed by a second CAR T-cell infusion that targets an alternative antigen, which provides consolidation-like therapy to further eradicate disease or help maintain remission. Collectively, the 2 sequential infusions help improve remission durability.
Based upon on the recognition that single-agent chemotherapy for ALL led to only transient remissions, combinatorial therapy quickly became the foundation upon which therapy for ALL was built.2 Induction followed by subsequent cycles of rationally designed combination therapy with drugs that have complimentary mechanisms of action provides a synergistic approach to eradicating disease, maintaining remission, and preventing relapse. This has led to the current highly effective paradigm in pediatric ALL therapy. Despite optimized combination chemotherapy strategies, patients who have multiple relapses or chemotherapy refractory disease and other high-risk subsets of patients need novel types of therapy.
Recent advances in immunotherapy have revolutionized the treatment of ALL by providing particularly effective strategies for those with chemotherapy-resistant disease. CD19-targeted strategies in particular, including both blinatumomab and CD19 CAR T cells, have been highly effective and have been approved by the US Food and Drug Administration for treatment of ALL in children and young adults.
Yet the lessons learned from the origin of ALL therapy still prevail. Indeed, despite the high remission induction rates, ALL cells can still evade effective single-antigen targeting strategies: loss of CD193,4 or CD225 serve as major mechanisms of immune escape after effective CAR T-cell–targeted treatment. Amid concerns for baseline heterogeneity in antigen expression and antigen loss after CD19- or CD22-targeted therapies, we arrive back at the fundamental principles of ALL therapy to test the hypothesis that combinatorial immunotherapies could synergize to improve upon single-antigen–targeted approaches and extend durable remissions.
Several preclinical6-8 and clinical efforts are underway to test novel dual-antigen–targeted CAR T cells that combine 2 single-chain variable fragments into a single vector and incorporate co-transduction models. Although these constructs provide a first-in-class approach to CAR T-cell–targeting strategies, developing a construct with dual functionality is no easy task.7 While we wait for the clinical data to mature on these translational efforts and watch for the ability of dual functionality to prevent antigen escape, Pan et al have taken a unique approach to achieving dual CAR T-cell targeting. Simply stated, if you can demonstrate the activity of single-antigen CD19- and CD22-targeted CAR T cells, can you give them sequentially and achieve the goals of combinatorial targeting?
Akin to ALL induction and consolidation, in this "one-two punch" strategy children with relapsed or refractory pre-B-ALL were enrolled in a clinical trial with a planned sequential infusion of previously tested CD19 CAR T cells9 followed by CD22 CAR T cells10 (see figure). Each construct contained a 4-1BB costimulatory domain, and lymphodepletion was performed before each CAR T-cell infusion. Timing for infusion of CD22 CAR T cells was based on loss of CD19 CAR T-cell persistence, as demonstrated by CAR T cells being undetectable in the peripheral blood by flow cytometry.
In the article by Pan et al, all 20 children achieved a minimal residual disease–negative (MRD–) complete remission by flow cytometry after the initial CD19 CAR T-cell infusion. Then, rather than waiting for potential disease recurrence, all patients were administered a CD22 CAR T-cell infusion while they were in an MRD– remission at a median of 1.65 months after the CD19 CAR T-cell infusion. Cytokine release syndrome was seen in the majority of patients after each CAR T-cell infusion, but it was generally mild (grades 1 to 2) and was limited at the second infusion when all patients were in remission. No patients received a consolidative allogeneic hematopoietic stem cell transplantation (HSCT). Even so, 17 (85%) of 20 were in an ongoing remission with a 1-year leukemia-free survival (LFS) of 79.5% and overall survival of 92.3%, which represents an improvement in remission durability over that seen with their single-antigen targeting treatments.9,10 These data support the safety and feasibility of sequential CAR T-cell infusion strategies, while demonstrating evidence for dual CAR T-cell expansion and the potential for clinical benefit, leading to an improved LFS without the necessity of a consolidative HSCT.
Notably, the immunophenotype of those with relapsed disease (n = 3) included CD19 loss (n = 2) and concurrent CD22 downregulation (n = 1), suggesting that this strategy may still need further optimization. Thus, there are still questions regarding whether additional targets are needed or whether dual functionality was achieved or adequate. Furthermore, given the critical role of CAR T-cell persistence to provide ongoing immune surveillance in preventing antigen-positive relapse, the limited CAR T-cell persistence seen in this trial, the interval lymphodepleting chemotherapy which may further eradicate previous CAR T cells, and the recovery of CD19+/CD22+ B cells in the majority of patients as a harbinger of impending antigen-positive relapse remain of concern. As such, longer follow-up is warranted. In addition, if loss of CAR T-cell persistence is required before a second CAR T-cell infusion, the generalizability of this approach may also be limited, particularly in settings in which the first CAR T cells may be more persistent. Finally, the cost-effectiveness of this treatment course raises some practical considerations and barriers, and discussing them may be warranted if future data strongly support this model for combinatorial targeting.
Nevertheless, B-ALL is a disease in which the paradigm of treatment is embedded in combinatorial treatment and antigen escape is the most common cause for treatment failure after single-antigen CAR T-cell targeting. Thus, developing combinatorial strategies is of the utmost importance in advancing the field. The work of Pan et al is particularly relevant because as CAR T-cell strategies go beyond ALL, issues of tumor heterogeneity and immune escape may be even more prevalent. Their work eloquently highlights the proof-of-concept of planned sequential CAR T-cell infusion strategy, which demonstrates a novel approach to optimizing CAR T-cell strategies with dual targeting and potentially broad applicability.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal